Removal of bacterial biofilm from the root canal system is essential for the management of endodontic disease. Here we evaluated the antibacterial effect of N-acetylcysteine (NAC), a potent antioxidant and mucolytic agent, against mature multispecies endodontic biofilms consisting of Actinomyces naeslundii, Lactobacillus salivarius, Streptococcus mutans and Enterococcus faecalis on sterile human dentin blocks. The biofilms were exposed to NAC (25, 50 and 100mg/mL), saturated calcium hydroxide or 2% chlorhexidine solution for 7 days, then examined by scanning electron microscopy. The biofilm viability was measured by viable cell counts and ATP-bioluminescence assay. NAC showed greater efficacy in biofilm cell removal and killing than the other root canal medicaments. Furthermore, 100mg/mL NAC disrupted the mature multispecies endodontic biofilms completely. These results demonstrate the potential use of NAC in root canal treatment.
Biofilm can be defined as a sessile, multi-cellular, microbial community characterized by cells that are firmly attached to a surface and enmeshed in a self-produced matrix of extracellular polymeric substance (EPS).1,2 Microbial communities in biofilms are remarkably difficult to eradicate with antimicrobial agents, and microorganisms in mature biofilms can be notoriously resistant; microorganisms grown in biofilms can be 2–1000-fold more resistant than the corresponding planktonic forms.3 Endodontic disease is a biofilm-mediated infection, and the primary aim in the management of endodontic disease is the elimination of bacterial biofilm from the root canal system.4 Although the majority of bacteria are removed by mechanical and chemical methods during endodontic treatment, it is difficult to eradicate them completely from the root canal system. Hence, the use of intracanal medicaments to disrupt biofilms and thereby eradicate residual bacterial infections within root canals has been advocated to enhance the success of root canal treatment.5,6 For such purposes, chlorhexidine (CHX) and calcium hydroxide (CH) have been widely used because of their excellent antimicrobial and biological activities.7 However, CHX is inactivated by physiological salts and has a limited ability to penetrate the deep layer of biofilms.8,9 CH has low diffusibility, and its antimicrobial efficacy is compromised by the buffering effect of dentin and the resistance of Enterococcus faecalis to the hydroxyl ion.10–12
N-acetylcysteine (NAC), a derivative of the amino acid l-cysteine, is a potent thiol-containing antioxidant that serves as a precursor of glutathione synthesis.13 Stimulation of glutathione synthesis following administration of NAC results in greater availability of glutathione for the detoxification of oxygen-derived free radicals.14 Several studies have shown that NAC inhibits the production and/or release of proinflammatory cytokines such as NF-κB, TNF-α, matrix metalloproteinase-9, IL-8, IL-6 and IL-1β.15–17 In addition, NAC has been reported to exhibit antibacterial activity against a variety of medically important bacteria such as Staphylococcus epidermidis,18Escherichia coli,19Pseudomonas aeruginosa20 and Klebsiella pneumoniae.21 The antibacterial activity of NAC is likely to be achieved by inhibition of cysteine utilization in bacteria or by reaction of its thiol group (–SH) with bacterial cell proteins, thereby inducing irreversible damage to the essential proteins.19–21 Meanwhile, depending on bacterial strains, NAC reduces the production of EPS, consequently reducing bacterial adhesion to surfaces and disrupting mature biofilms.18,19,22 NAC has been used widely in the treatment of patients with obstructive pulmonary disease, as it breaks disulfide bonds in the mucus and inhibits bacterial biofilm formation, resulting in significant reductions in bacterial infections.21–23
In terms of oral diseases, it has been proposed that NAC exerts anti-inflammatory activity by inhibiting the expression of LPS-induced inflammatory mediators (IL-1β, -6 and -8) in phagocytic cells and gingival fibroblasts.24–26 Recent investigations have demonstrated that NAC has antibacterial and antibiofilm effects against oral pathogens such as Prevotella intermedia27 and E. faecalis,28 which are often found in endodontic infections. These characteristics indicate the possibility of using NAC for dental disease. Endodontic infection is the infection of the dental root canal system, and polymicrobial biofilms develop on the canal dentin walls. To evaluate the possibility of using NAC as an alternative intracanal medicament, the present study assessed the antibacterial effect of NAC against mature multispecies endodontic biofilms consisting of Actinomyces naeslundii, Lactobacillus salivarius, Streptococcus mutans and E. faecalis using a dentin infection model.
This study was carried out under the approved guidelines of the Review Board of Kyung Hee University, Seoul, Korea (KHD IRB-1106-02). We collected 27 single-rooted human premolar teeth with fully formed apices and caries-free crowns from patients undergoing tooth extraction for orthodontic reasons. Dentin blocks were prepared as described previously.29 Briefly, the dentin blocks were cut from the middle one-third of each root and the thickness was adjusted to approximately 4mm×4mm×1mm. The smear layer was removed by immersing the specimens in an ultrasonic bath of 17% EDTA (pH 7.2), followed by 2.5% sodium hypochlorite, for 5min each. After neutralization with a 5% sodium thiosulfate solution, the dentin blocks were thoroughly rinsed with distilled water and then sterilized by autoclaving for 15min at 121°C. The sterile dentin blocks were incubated in brain heart infusion broth (Difco Laboratories, Detroit, MI, USA) at 37°C for 24h to ensure no bacterial contamination.
It has been shown that 3-week-old mature biofilms are more resistant to disinfecting agents than planktonic bacteria and young biofilms.30 To examine the effect of NAC on 3-week-old biofilms, preformed mature multispecies endodontic biofilms were established on the dentin blocks as described previously,5 with some modifications. Briefly, peptone–yeast extract–glucose (PYG) was dissolved in 10mM potassium phosphate buffer, pH 7.5, and each bacterial species was grown in this medium at 37°C anaerobically (80% N2, 10% H2, 10% CO2). The bacterial culture was grown to exponential phase, then diluted to an optical density at 600nm (OD600) of approximately 0.1 in fresh medium. To prepare the multispecies suspension, four OD-adjusted bacterial cultures were mixed in equal proportions. Sterile dentin blocks were immersed in the multispecies suspension and then incubated at 37°C for 3 weeks, with PYG medium being changed every 2 or 3 days. Biofilm formation and bacterial penetration into the dentinal tubules were confirmed using scanning electron microscopy (SEM).
After the 3-week incubation, the dentin blocks were gently washed with saline to remove unattached bacteria, then treated with 100μL of one of the following medicaments: sterile saline (control), NAC (25, 50 and 100mg/mL, Sigma Chemical Co., St. Louis, MO, USA), saturated CH solution (Sigma), or 2% CHX (Sigma). Adenosine triphosphate (ATP) bioluminescence measurements in relative light units (RLU) has been utilized as a quantitative assay to evaluate viable bacteria in different biological samples, including mixed biofilm populations such as dental plaque.31 At 7 days after exposure to the medicaments, the viability of the bacterial cells in the biofilms was evaluated by quantifying ATP-bioluminescence using BacTiter-Glo™ Microbial Cell Viability Assay Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Results were expressed as the percentage of RLU and calculated as shown below. Percentage of RLU in the saline treated biofilm was assumed as 100. Relative viability (% RLU)=(RLU of medicament treated biofilm−background RLU)/(RLU of saline treated biofilm−background RLU)×100%. Colony forming unit (CFU) counting, the most commonly used conventional viable bacterial quantification, was also performed. Briefly, biofilm bacterial cells were harvested from the dentin blocks by sonication as described previously,30 then diluted serially in PYG medium and subsequently enumerated by plating them on agar plates which were incubated for 48h at 37°C. The biofilm morphology was assessed using SEM.
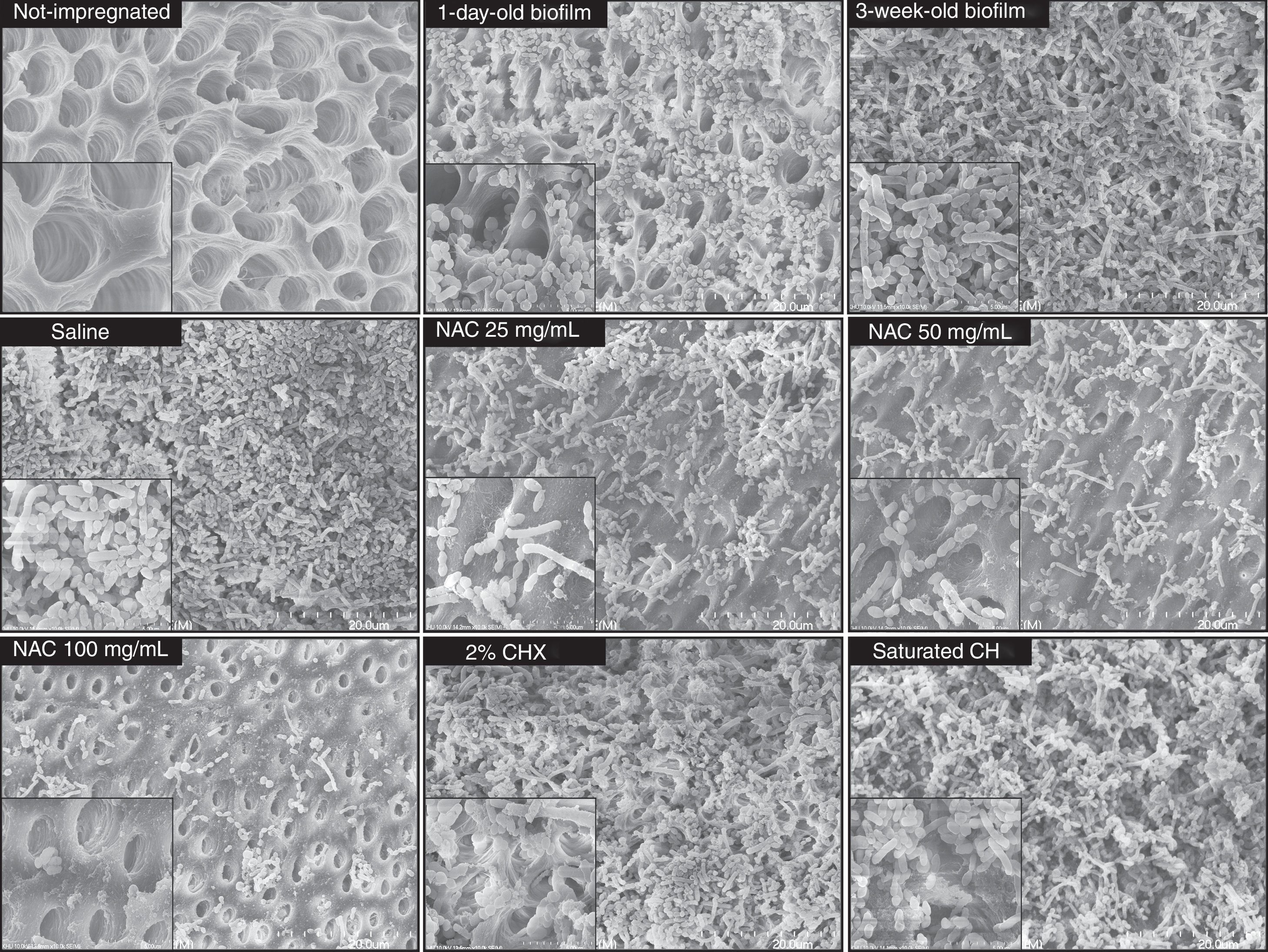
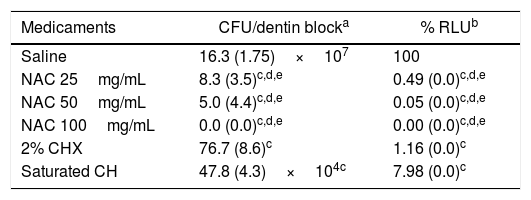
After incubation for 1 day, the multispecies bacteria had become successfully impregnated into the interior of the dentin blocks, and bacterial biofilm-like structures consisting mostly of cocci were observed on the surface of the dentin blocks (Fig. 1). The mature 3-week-old biofilms were composed of rods and coccal cells, forming a layered structure of bacterial aggregates covering the dentin surface. No obvious structural disruption was observed in the biofilms treated with saline for 7 days. Treatment of the biofilms with either CH or CHX for 7 days resulted in partial disruption of the layered structure, whereas 25 and 50mg/mL NAC resulted in obvious disruption of the biofilms with clearly exposed dentin surface. Compared with the saline treated biofilm, significant reduction (over 99%) in CFUs counts and % RLU was observed in the biofilms treated with 25 and 50mg/mL NAC. Furthermore, NAC (≥25mg/mL) showed statistically higher bactericidal effect than CH and CHX. In the 100mg/mL NAC treatment group, biofilm structure completely disappeared (Fig. 1) and viable cells were not detected (Table 1). These results clearly indicate that 100mg/mL NAC completely disrupted the mature multispecies endodontic biofilms consisting of A. naeslundii, L. salivarius, S. mutans and E. faecalis, probably via affecting EPS produced by the bacterial cells. Small pieces of cell debris and a very limited number of dead cells observed on the dentin surface without biofilm structure may be related to physicochemical linkages between cells and dentin surface because cell viability has limited influence on attachment, as suggested by previous studies.27,32 It seems that NAC at the concentrations from 25 to 50mg/mL can remove the mature multispecies biofilm partially, but was still able to effectively kill the bacteria located in the remaining biofilm that was at most a few cell layers thick.
Scanning electron microscope (SEM) images of multispecies endodontic biofilms. Multispecies bacteria were successfully impregnated into the interior of the sterile dentin blocks after incubation for 1 day. After the 3-week incubation, the mature biofilm exhibited a layered structure of bacterial aggregates covering the dentin surface. The mature multispecies biofilms were treated with saline, saturated calcium hydroxide solution (CH), 2% chlorhexidine solution (CHX) or NAC at the indicated concentrations for 7 days and then observed using SEM at a magnification of 20,000× and 100,000× (inset) operating at 10kV.
Viability of the bacterial cells in the biofilms exposed to medicaments for 7 days.
| Medicaments | CFU/dentin blocka | % RLUb |
|---|---|---|
| Saline | 16.3 (1.75)×107 | 100 |
| NAC 25mg/mL | 8.3 (3.5)c,d,e | 0.49 (0.0)c,d,e |
| NAC 50mg/mL | 5.0 (4.4)c,d,e | 0.05 (0.0)c,d,e |
| NAC 100mg/mL | 0.0 (0.0)c,d,e | 0.00 (0.0)c,d,e |
| 2% CHX | 76.7 (8.6)c | 1.16 (0.0)c |
| Saturated CH | 47.8 (4.3)×104c | 7.98 (0.0)c |
ATP-based luminescence quantification was assessed in at least three independent experiments and was expressed as the mean value (SD) percent relative light units (RLU) vs. saline-treated biofilms (control).
The data were analyzed by use of 1-way ANOVA, followed by the Dunnett T3 as a post hoc test. All statistical analyses were performed using IBM SPSS version 22 statistical software (IBM SPSS, Chicago, IL, USA).
Collectively, NAC exhibited strong biofilm removal and killing activities against the mature multispecies endodontic biofilms formed on the dentin surfaces. It is noteworthy that NAC has also been proposed as a substitute for ibuprofen for post-endodontic pain,33 and exerts anti-inflammatory activity through its ability to inhibit the expression and release of a variety of proinflammatory cytokines.34,35 All these observations demonstrate that NAC has the potential to be developed as an alternative intracanal medicament.
Conflicts of interestThe authors have no conflicts of interest relevant to this article.
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare (HI13C1945) and by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A2A01054588).