The possible application of a bacterial strain – Bacillus subtilis R1, isolated from an oil contaminated desert site in India, as biocontrol agent and its biosurfactant in microbial enhanced oil recovery are discussed. The biosurfactant production in minimal medium was carried out at different temperatures and salt concentrations, where it produced an efficient biosurfactant at 30–45°C and in presence of up to 7% salt. It significantly reduced the surface tension from 66±1.25mN/m to 29±0.85mN/m within 24h. In order to enhance the biosurfactant production, random mutagenesis of B. subtilis R1 was performed using chemical mutagen – ethyl methanesulfonate. Majority of the isolated 42 mutants showed biosurfactant production, but the difference was statistically insignificant as compared with parent strain R1. Therefore none of the mutants were selected for further study, and only parent strain R1 was studied. The biosurfactant was quite stable under harsh conditions for up to 10 days. The biosurfactant was extracted and characterized as similar to the lipopeptide group – surfactins and fengycin. The crude oil displacement experiments using biosurfactant broth in sand pack glass columns showed 33±1.25% additional oil recovery. The strain also showed inhibition of various plant pathogenic fungi on potato dextrose agar medium.
Chemical compounds are extensively used in petroleum industries for different operations, mainly in enhanced oil recovery (EOR), like surfactants and polymers, which are generally toxic and recalcitrant leading to disposal problems for oil field produced water, ground water contamination, and obvious health risks to humans. Biosurfactants are one of the environmental friendly alternatives to the currently used chemical surfactants. Biosurfactants are amphiphilic compounds produced by microorganisms, similar to chemical counterparts, but with better environmental compatibility, activity, stability, lower toxicity, and versatile applications in health, food and agriculture. Even though biosurfactants have many advantages over chemical surfactants, widespread applications are weighed down by the high costs of production. Researchers have reported several ways to reduce the costs of biosurfactant production like, selection of cheaper raw material or agro-industrial waste products, application of statistical methods to optimize the production, and mutation of the strains to enhance the production.1–5 Nevertheless the biosurfactant market is growing rapidly, and according to a recent report, global biosurfactant market was worth USD 1.7 billion in 2011, which is expected to reach USD 2.2 billion in 2018, growing at an average annual growth rate of 3.5% from 2011 to 2018 (http://www.prweb.com/releases/2012/11/prweb10158903.htm – as accessed on 12th April, 2015). Amongst different types of biosurfactants, smaller molecules like lipopeptides produced by Bacilli sp. are reported to be the most active in reducing the surface tension and interfacial tension between oil and water, leading to enhanced oil recovery. Those lipopeptides are also reported to inhibit microbial growth, and thus also have potential applications in plant protection and health applications. Several types of biosurfactants are reported to have applications in environmental bioremediation and in microbial enhanced oil recovery (MEOR). MEOR is one of the tertiary recovery techniques for enhancing or improving the recovery of crude oil from declining oil reservoirs, which is a well-anticipated technology, to be widely applied in the oil fields.6–9 We have isolated Bacillus subtilis R1 from oil contaminated site, identified and screened for biosurfactant production in glucose based minimal salt media, at different salt concentration and temperatures. The isolate was treated with chemical alkylating mutagen – ethyl methanesulfonate (EMS), for random mutagenesis to enhance biosurfactant production. The isolate produced a potent biosurfactant which was extracted, structurally characterized, and studied further for critical micelle concentration (CMC), stability under harsh conditions (high temperature, pH and salinity), MEOR studies by glass sand-pack models, and antifungal activity on agar plates.
Materials and methodsIsolation and identification of microorganismSeveral microorganisms were isolated as described previously, from Kutch desert (Gujarat, India) and screened on blood agar plate.2 One of the selected isolate R1 was used for all experiments. The isolate was maintained aerobically on Luria-Bartani (LB) agar plates and was regularly transferred into fresh LB medium for short-term storage. Stock cultures of pure isolate were prepared in 40% glycerol and stored below −80°C, for long-term preservation. The isolate was studied morphologically, different biochemical tests and 16S rRNA sequencing.
Colony PCR and 16S rDNA amplificationThe genomic DNA was extracted directly from colonies grown on LB agar and used as the template for polymerase chain reaction (PCR) as previously described.10 Isolated pure colonies (2–3 colonies) were suspended in 20–30μL sterile distilled water, heated at 99°C for 25min and centrifuged at 10,000rpm for 3min. The supernatant was used as a template DNA in the PCR system. The 16S rRNA gene fragment was amplified using universal eubacterial primers 27F (5′-GAG AGT TTG ATC CTG GCT CAG-3′) and 1107R (5′-GCT CGT TGC GGG ACT TAA CC-3′). The amount of DNA taken for amplification was ∼10ng. The PCR components and conditions (for 50μL system) used for amplification were as follows (μL): Target DNA (100ng), 15.0; Taq polymerase buffer (10×), 5.0; dNTPs (total 10mM with 2.5mM each), 2.0; forward primer (27F) 20pmoles, 1.0; reverse primer (1107R) 20pmoles, 1.0; Taq polymerase 3units/μL, 0.5; PCR water, 25.5. PCR program (Thermal Cycler, Applied Biosystems) was as follows: Lid temperature, 105°C; Step 1: Initial Denaturation, 94°C – 60s; Step 2: Denaturation, 94°C – 45s; Step 3: Primer annealing, 56°C – 30s; Step 4: Primer extension, 72°C – 1.5min; Steps 2, 3, 4 repeated for 30 cycles; Step 5: Final extension, 72°C – 10min. The purity and size of each PCR product was examined by gel electrophoresis on 0.8% agarose gel in 0.5× TBE buffer. The 100bp ladder (molecular weight marker) was used for comparison of size of the PCR products. The gel was visualized in UV transilluminator and photographed subsequently. All the PCR reagents, primers, Taq polymerase and other molecular biology reagents were obtained from Bangalore Genei Pvt. Ltd, India and used as per manufacturer's instructions. The amplified 16S rDNA gene was sequenced at Bangalore Genei Pvt. Ltd, India, and the nucleotide sequence was deposited in NCBI GenBank database.
Biosurfactant production studiesFor preparation of inoculum LB broth was used as a seed medium, where the loopful of bacteria were transferred and incubated at 30°C for 8–10h (OD600 – 0.8–0.9). For production of biosurfactant, 2% (v/v) inoculum was transferred into 50mL sterile glucose based minimal media in 250-mL Erlenmeyer flasks. The medium composition was (g/L): Glucose, 20; NaNO3, 4; MgSO4, 0.4; KCl, 0.2; CaCl2, 0.1; H3PO4, 0.5mL, trace elements (mg/L): H3BO4, 1.53; CuSO4, 0.284; MnSO4, 1.71; Na2MoO4, 0.7; ZnSO4, 2.9; FeSO4, 4.3; CoCl2, 0.1; EDTA, 200. The minimal medium pH was adjusted to 7.2 using 1.0N NaOH. The flasks were incubated at different temperatures (30°C, 40°C, 45°C, 50°C and 80°C) and to study the effect of salinity, different concentrations of NaCl (0%, 5%, 7%) were added in to the minimal media. Samples were collected at every 24h for growth (OD600) and biosurfactant production (Surface tension) analysis till 96h. Surface tension (ST) was measured by Du-Nouy tensiometer by ring detachment method using distilled water (72±0.50mN/m) and uninoculated media (66±1.25mN/m) as abiotic negative controls. All measurements were performed in triplicates using cell free broth.
Random mutagenesis for enhanced biosurfactant productionAlkalyting agent–ethyl methanesulfonate (EMS, Sigma–Aldrich, USA) was used for random mutagenesis of B. subtilis R1. The culture was grown in LB broth till OD600 reached 0.8–0.9, then centrifuged and the pellet was suspended in sterile normal-saline (0.85% NaCl) and EMS (15μL/mL) was added and incubated in cold condition. After incubation, cells were washed twice by saline, centrifuged to remove EMS and suspended in sterile normal-saline. Appropriate dilutions were spread on LB agar plates and incubated at 30°C for 24h. The well isolated mutant colonies on LB agar plates were further transferred on 10% blood agar plates for screening putative mutants. Culture showing zone of hemolysis were further used for production studies wherein they were grown in 2% glucose based minimal media and ST was measured after 72h.
Stability studiesStability studies were carried out using cell free broth, obtained by centrifuging the cultures at 11,292×g for 20min, as reported previously.2,11 For temperature stability study, tightly closed bottles with 50mL broth were incubated at 30–80°C for 10 days and cooled at room temperature before ST measurement. The pH stability was studied by adjusting the pH to different values (2.0, 4.0, 6.0, 8.0, 10.0 and 12.0) with 1N NaOH or 1N HCl, and salt stability was determined by adding different concentrations of salt (NaCl – 5%, 7%, 10%, 15%, and 20% (w/v)) to the cell free broth followed by incubation for 10 days at 30°C. The ST was measured from all the samples at different intervals till 10th day.
Biosurfactant extractionThe method based on acid precipitation was used for extraction and partial purification of biosurfactant.12 The culture broth was centrifuged at 11,292×g for 20min to get cell free broth. Biosurfactant was precipitated by adjusting the pH of the cell free broth to 2.0 using 6N HCl and keeping it at 4°C overnight. After decanting off the supernatant, the precipitate was collected and suspended in distilled water (pH 8.0) and lyophilized. The lyophilized product was weighed to find out the yield of biosurfactant and used for further characterization.
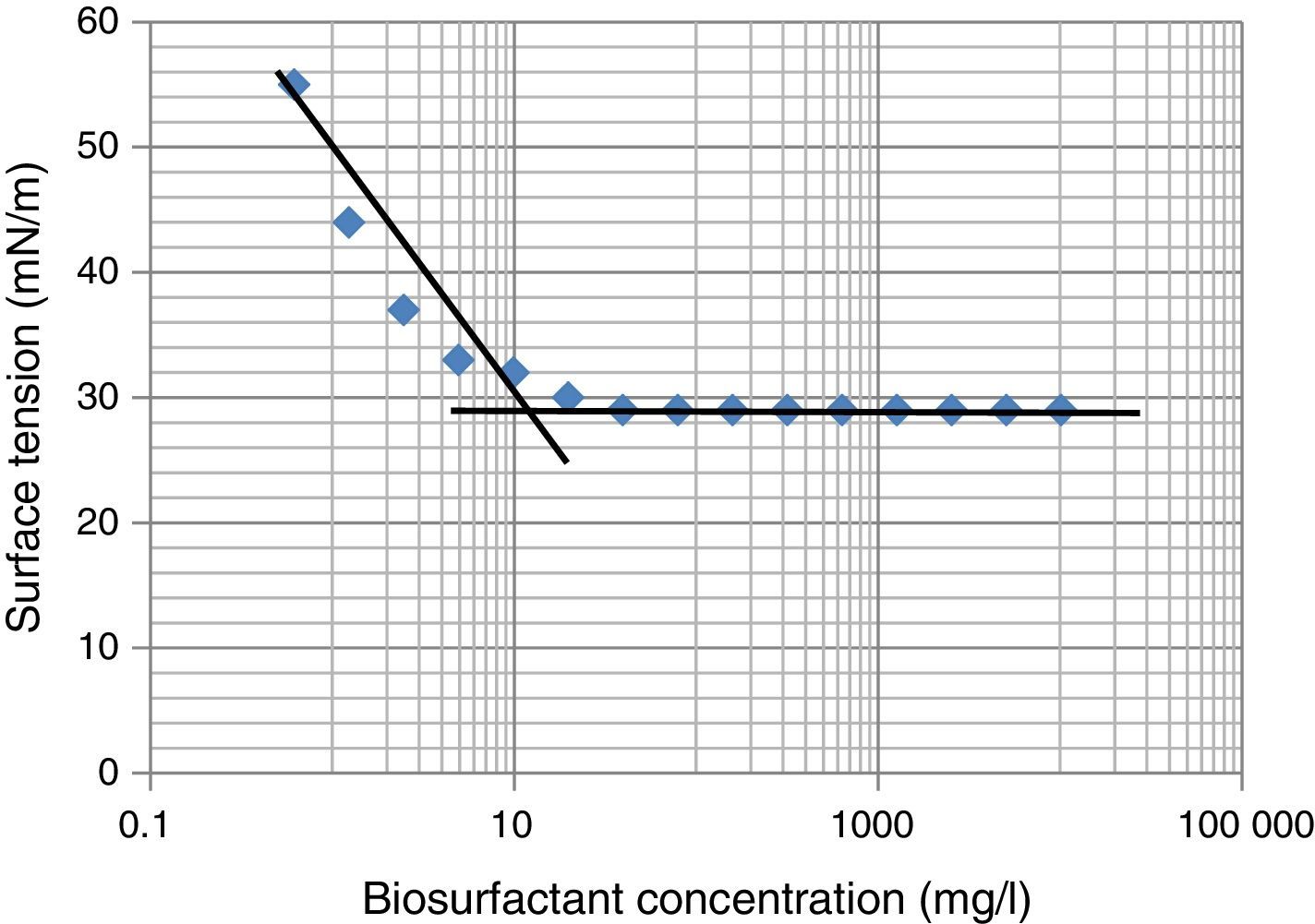
Critical micelle concentration (CMC)Critical micelle concentration (CMC) is defined as the concentration of the surfactant necessary to initiate micelle formation. Upon reaching the CMC, the ST does not continue to decrease even after increasing the surfactant concentration. The CMC was determined by plotting the surface tension as a function of the biosurfactant concentration. The concentrations ranging from 0.6 to 10,000mg/L were prepared with distilled water and ST was determined using Du-Nouy's ring Tensiometer at room temperature (30±2°C).
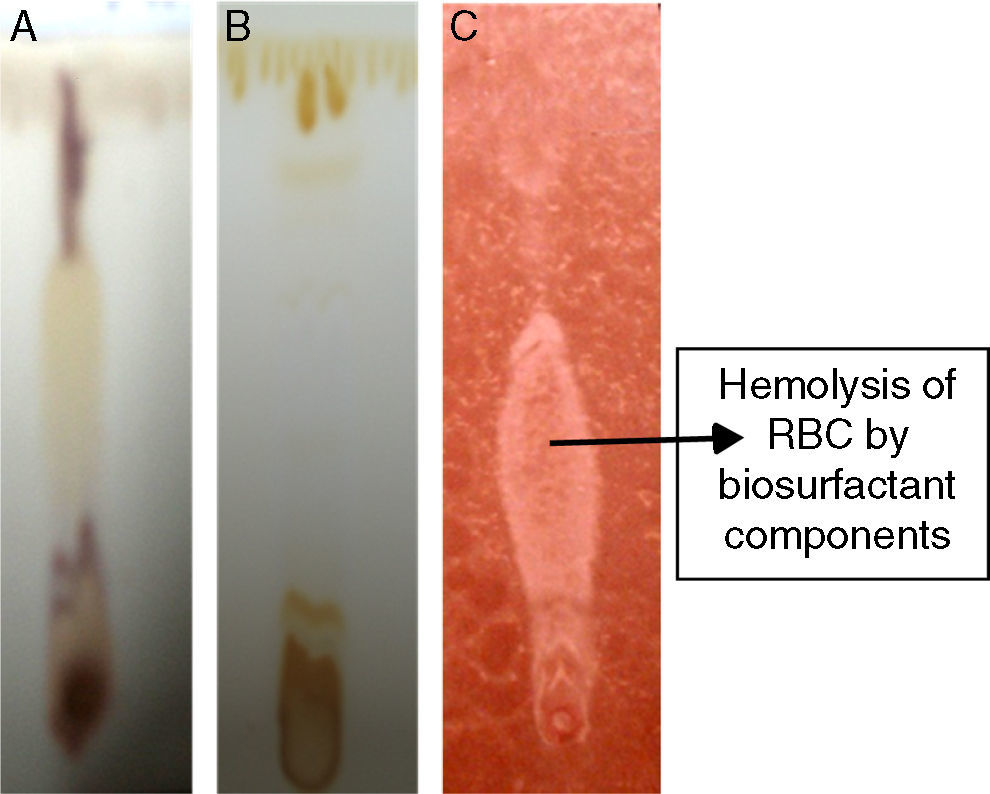
Analytical techniquesThin layer chromatography (TLC)TLC analysis of partially purified biosurfactant was performed using Silica gel 60 (Merck) TLC plates, where 20μL samples were spotted on TLC plates and developed in a chamber saturated with solvent system – chloroform:methanol:water (65:25:4). The separated components were detected with three different methods – saturation of iodine vapors; spraying with methanol:sulphuric acid (95:5) reagent, followed by heating at 100°C for 30min; and spraying the plates with blood reagent.
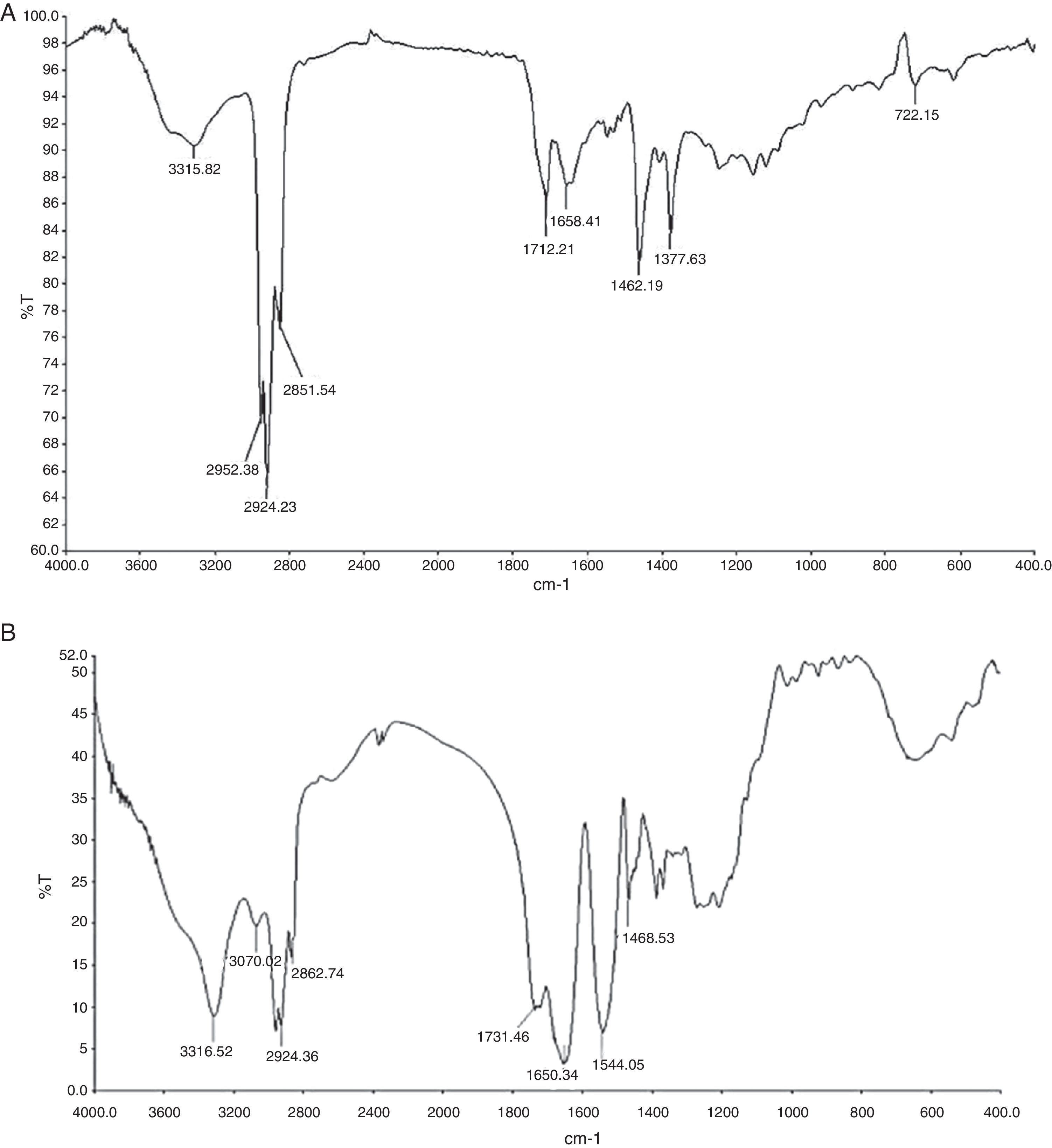
Fourier transform infrared spectroscopy (FTIR)FTIR (Perkin Elmer, Spectrum GX, FT-IR system) spectrum of partially purified biosurfactant was performed at Sophisticated Instrumentation Centre for Applied Research and Testing (SICART), Vallabh Vidyanagar, Gujarat, India. Samples were analyzed in KBr cell. Data was collected between 400 and 4000 wave numbers/cm, and processed with IR analytical software.
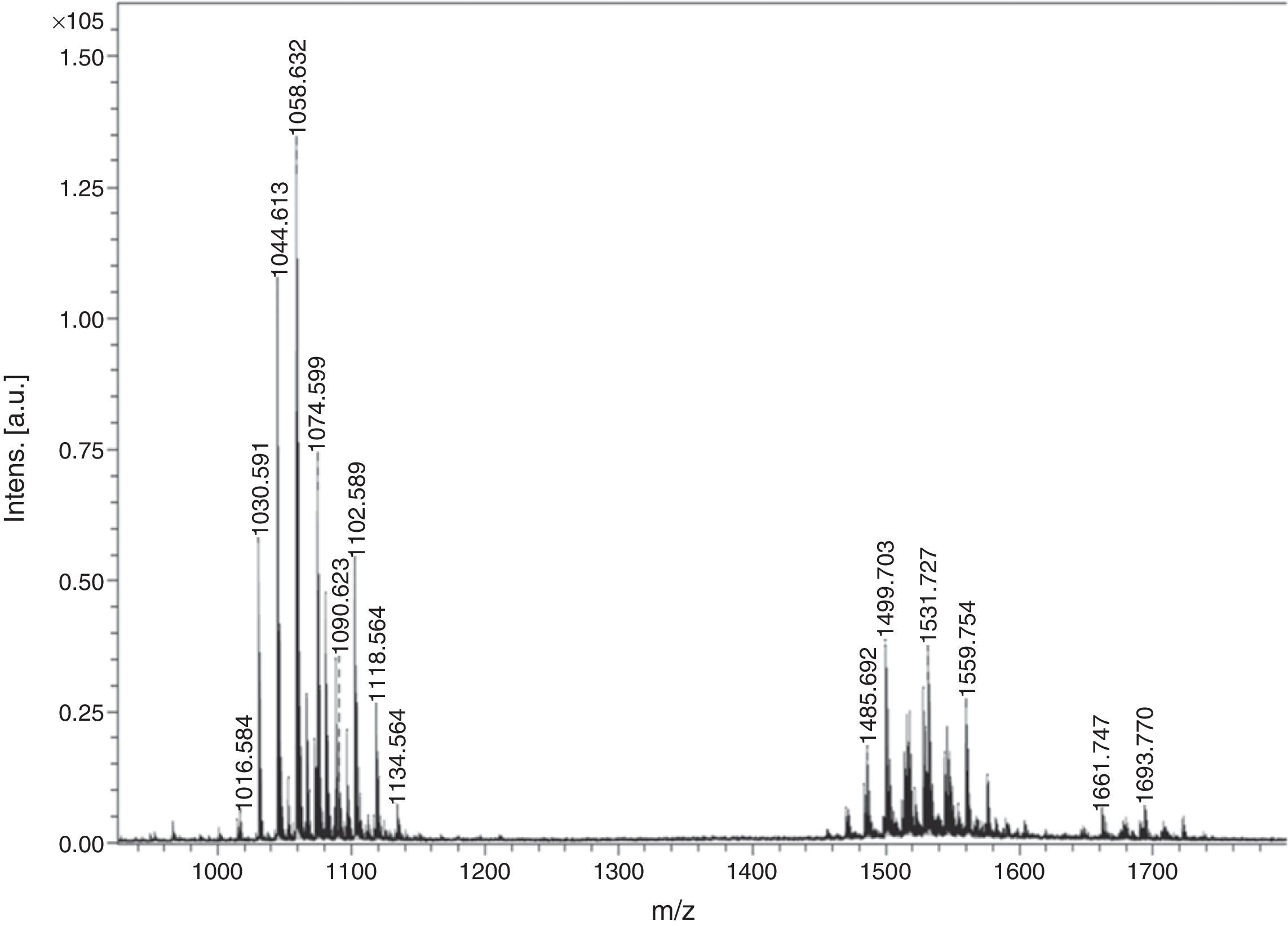
Matrix assisted laser desorption ionization time-of-flight mass spectroscopy (MALDI-TOF MS)The biosurfactant was characterized by ULTRFLEX-TOF/TOF (Bruker Daltonics) in reflector mode using a matrix of 2, 5–Dihydroxybenzoic acid (DHB). The reflector voltage was 25kVA and nitrogen laser was used with 150 laser shots (337nm, 50Hz).
Antifungal activityA bacterial culture grown in LB to a concentration of 108cfu/mL was spotted on the Petri dish containing potato dextrose agar (PDA). Simultaneously a 1cm2 (diameter) agar plug containing 48h grown fungal mycelium in PDA was placed in the center of the plate. The inhibition effect on fungal growth was evaluated after 4–5 days incubation at 28°C. All in vitro antagonism assays were performed in triplicates, as described previously.13
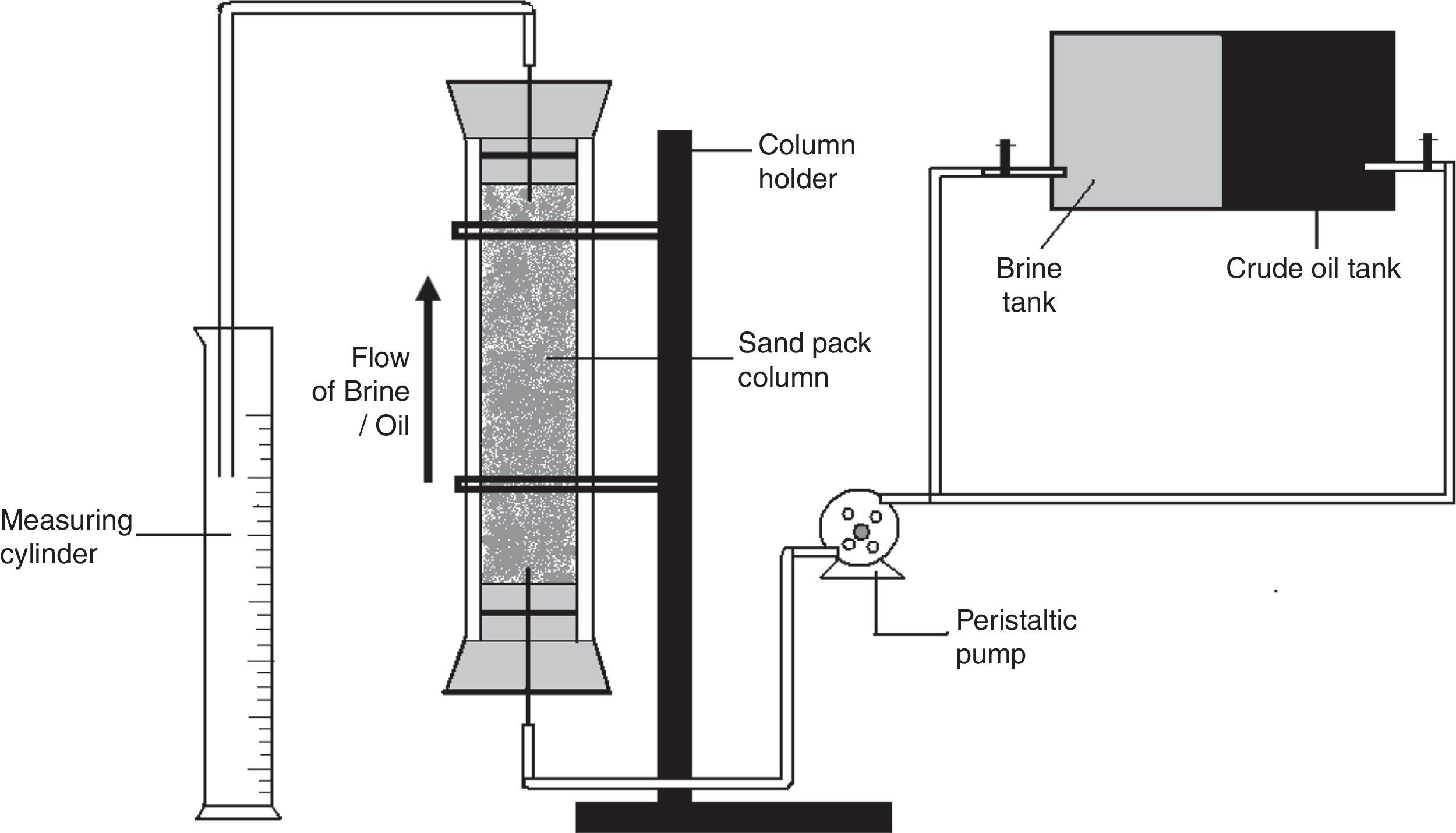
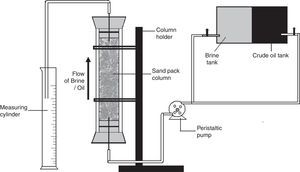
Oil displacement studies using glass sand pack columnsThe application of the biosurfactant in MEOR was evaluated using the glass sand pack columns as described previously.14 Where briefly, glass columns (45.7cm×2.5cm) were packed with 100g of acid washed sand (100 mesh size), and saturated with brine (5.0% NaCl). Each column was then vertically flooded upwards with light crude oil to irreducible water saturation, the amount of oil in place was calculated and then the column was flooded with brine until no further oil appeared in the effluent. The amount of oil recovered was measured and then cell free Broth was pumped upwards through the sand pack column. The rate of movement of the broth was about 5in. per hour. The amount of oil released with biosurfactant flooding was measured, and the additional oil recovery percentage was calculated.
Statistical analysisAll of the experimental data are expressed in terms of arithmetic averages of at least three independent replicates, with standard deviation (±), and the mutants were analyzed for any significant increase in efficiency using SigmaPlot 12.5 (Systat Software, Inc., USA).
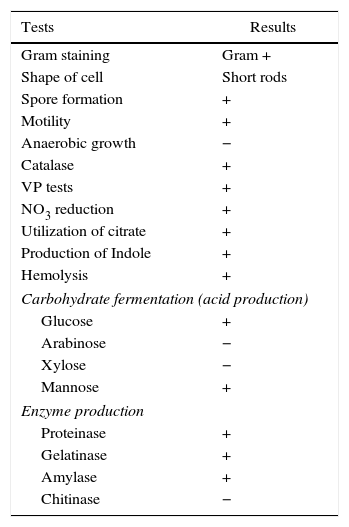
Results and discussionDifferent isolates were screened for biosurfactant production on blood agar plates and one isolate R1 was selected for further screening. It was studied for morphological and biochemical tests, and was found to be Gram positive, motile short rods, with spores. The details are as presented in Table 1. It was identified as B. subtilis by 16S rRNA gene sequencing and the nucleotide sequence was deposited in NCBI GenBank (GenBank accession no: DQ922949). Previously we’ve reported biosurfactant production by B. subtilis R1 in molasses media.2,14 Molasses is a cheaper agro-industrial byproduct. The production of molasses is related to the production of sugarcane crop. It was reported that the cane molasses production has fluctuated significantly in the last one decade in India (India's Sugar Industry: Analysing Domestic Demand and Recent Trends, accessed on 13th September 2015). Molasses contains substantial amount of sugar, that is not extractable and is widely utilized for alcohol production by fermentation. It has been estimated that almost all of the molasses (∼95%) produced in India is used for the production of Alcohol, and it is the raw material for potable alcohol, industrial alcohol and bioethanol. Thus almost all available molasses is expended by ethanol industry and is tightly regulated in the Indian market. Most of the states do not allow free interstate movements and selling of any molasses requires a permit NOC from the state excise departments (http://www.indiansugar.com/NewsDetails.aspx?nid=1774; and India's Sugar Industry: Analysing Domestic Demand and Recent Trends, accessed on 13th September 2015). Due to all those uncertainties in availability and fluctuating prices of cane molasses, we tested glucose based minimal medium for biosurfactant production.
Morphological and biochemical characteristics of B. subtilis R1.
| Tests | Results |
|---|---|
| Gram staining | Gram + |
| Shape of cell | Short rods |
| Spore formation | + |
| Motility | + |
| Anaerobic growth | − |
| Catalase | + |
| VP tests | + |
| NO3 reduction | + |
| Utilization of citrate | + |
| Production of Indole | + |
| Hemolysis | + |
| Carbohydrate fermentation (acid production) | |
| Glucose | + |
| Arabinose | − |
| Xylose | − |
| Mannose | + |
| Enzyme production | |
| Proteinase | + |
| Gelatinase | + |
| Amylase | + |
| Chitinase | − |
‘+’, positive reaction; ‘−’, negative reaction.
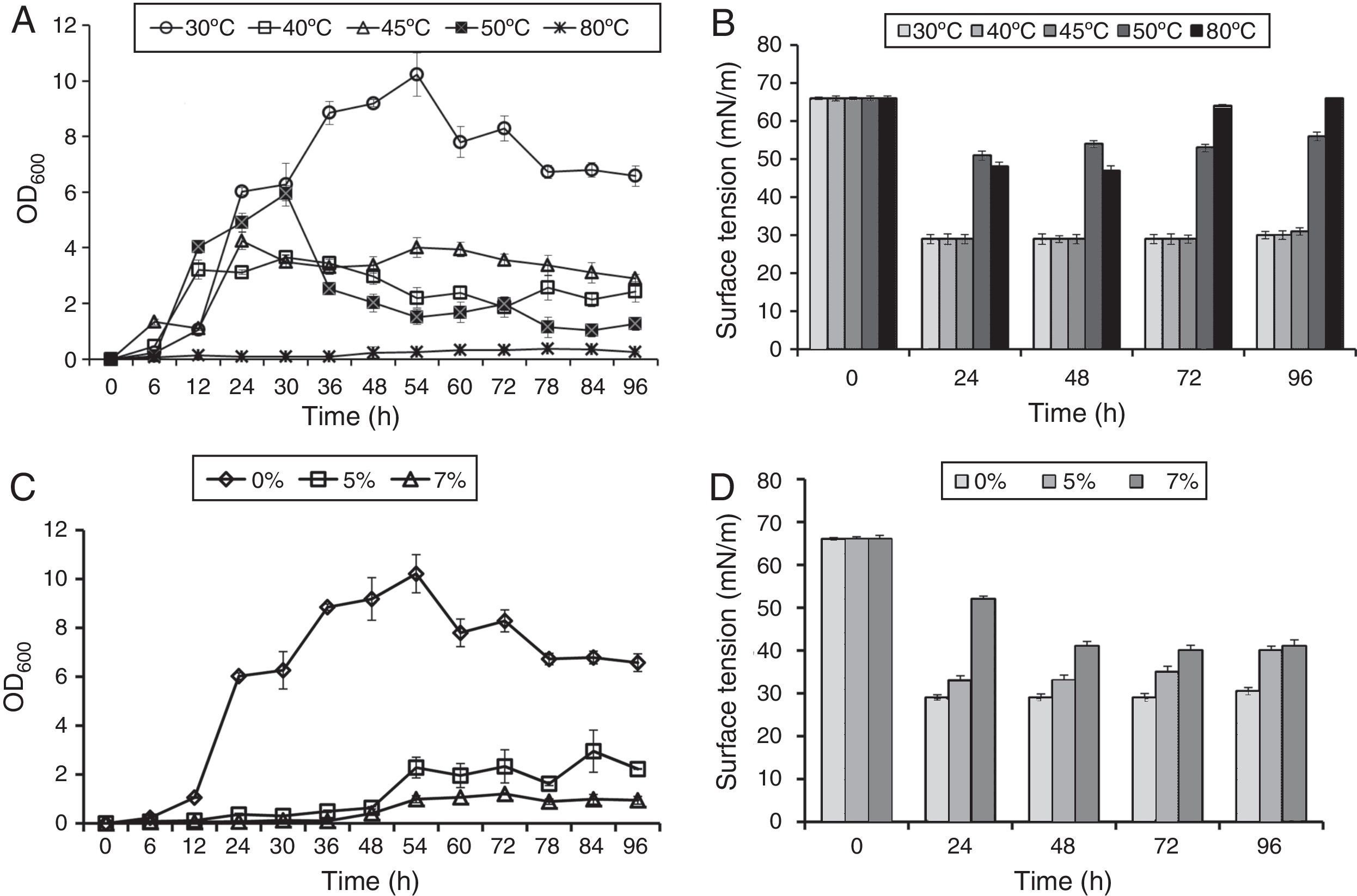
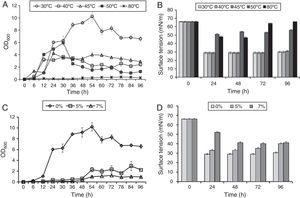
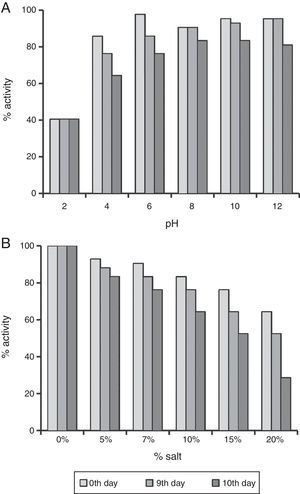
In order to study effect of various environmental factors on growth and biosurfactant production B. subtilis R1 was grown at different temperatures (30, 40, 45, 50 and 80°C) and different concentrations of NaCl (0%, 5%, 7%), in minimal medium with 2% (w/v) glucose as a carbon source. The growth was monitored by measuring OD600 and ST was measured after every 24h till 96h. B. subtilis R1 showed growth at temperature range of 30–50°C, but not at 80°C (Fig. 1A). Where highest growth was observed at 30°C, followed by 40–50°C. B. subtilis R1 also produced biosurfactant from 30 to 45°C, which substantially reduced the ST of the culture medium from 66±1.25mN/m to 29±0.85mN/m within 24h (Fig. 1B). Production of biosurfactant at thermophilic condition has apparent advantages while large scale production or scale-up, such as if organism can grow at high temperature it would reduce cost of cooling and also there are less chances of contamination. B. subtilis R1 also showed growth in presence of up to 7% salt, where in absence of salt better growth was observed (Fig. 1C). B. subtilis R1 produced biosurfactant thus reducing the ST in presence of up to 5% salt, where ST was reduced from 66±1.25mN/m to 29±0.85mN/m within 24h (Fig. 1D). In presence of 7% salt the ST was reduced to ∼41mN/m. Elzzazy et al.15 reported similar observation for the halophilic strain V. salarius (KSA-T) where highest biosurfactant was produced in presence of 4% (w/v) salt (NaCl), however, in presence of higher salt (10% w/v) it lost 25% of its activity.
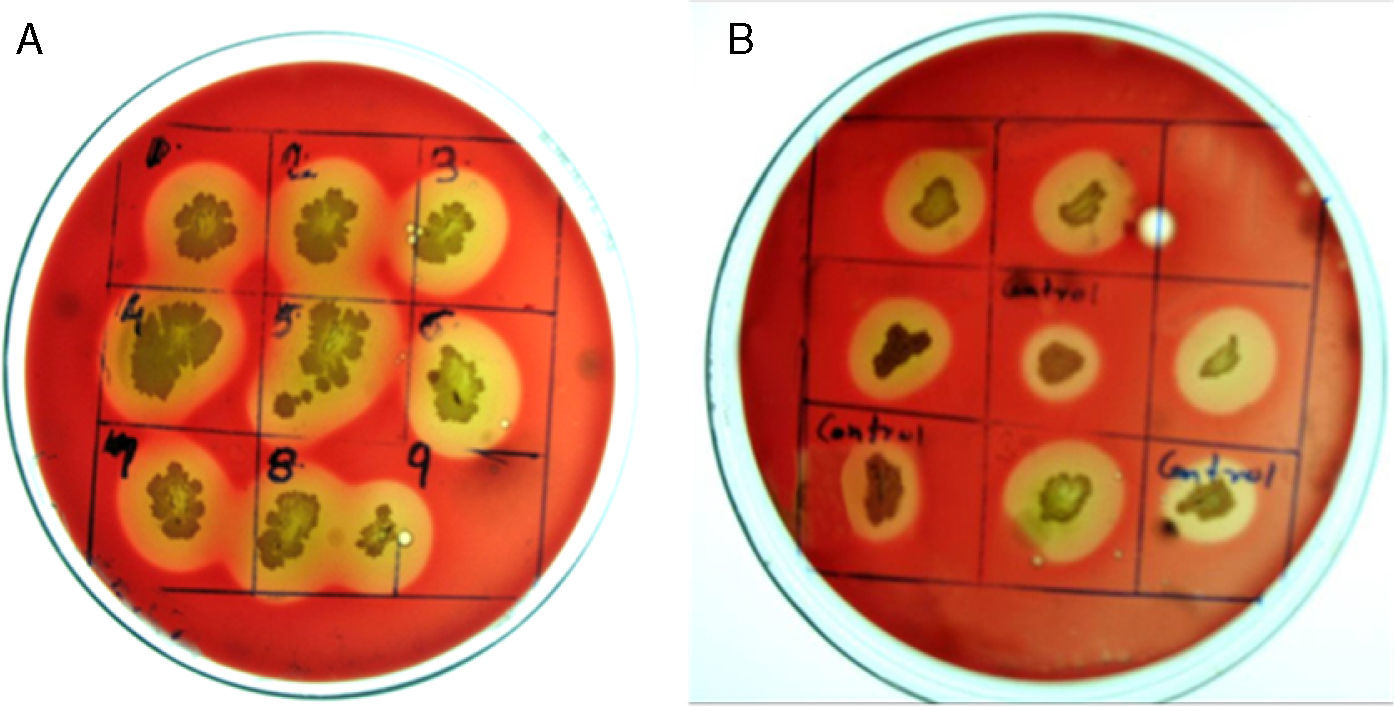
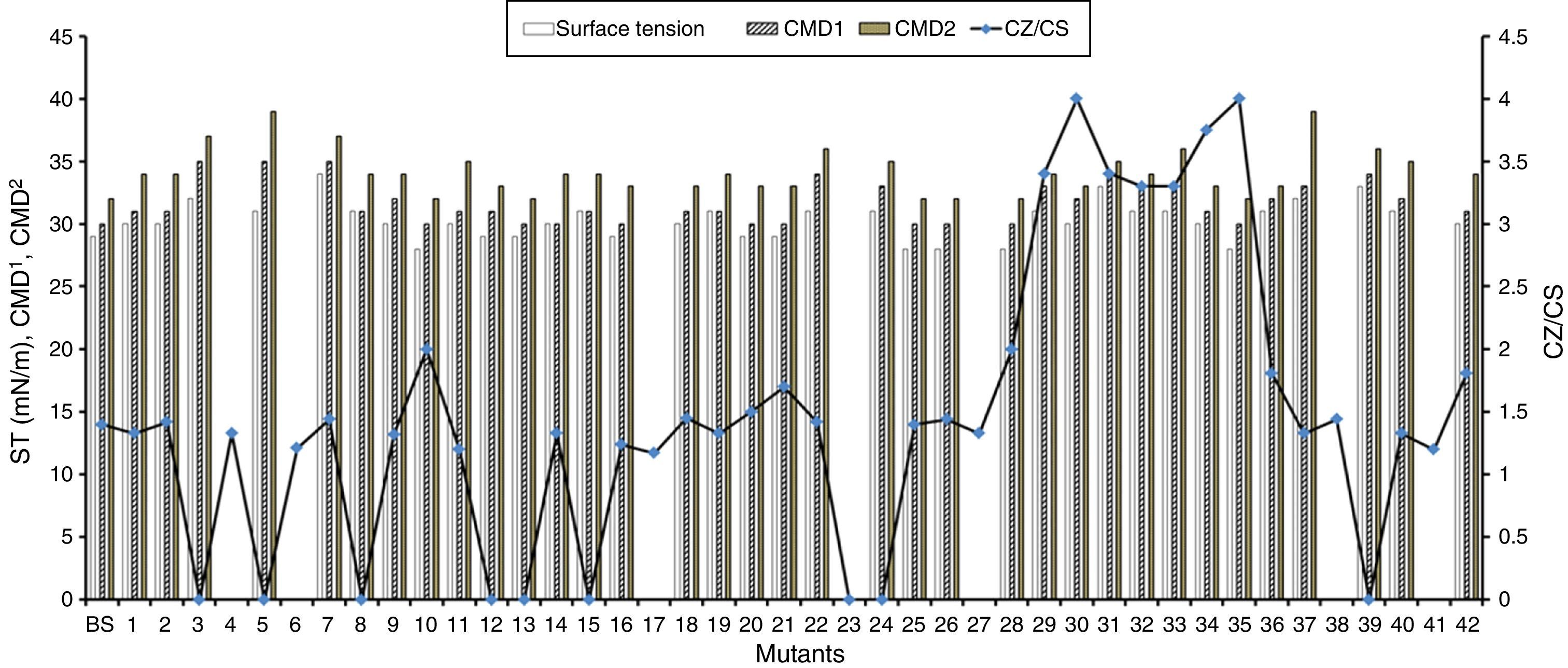
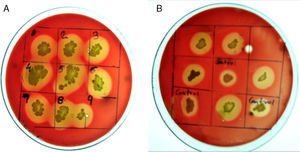
Random mutagenesis using chemical mutagen for enhanced production of biosurfactantProduction economy is the limiting factor of any biotechnological process, even in case of biosurfactant production. The success of biosurfactant production depends on development of process which reduces the overall cost either by use of cheaper raw material or by enhancing the biosurfactant production capacity of the bacteria by altering the genetic makeup by either random or site-directed mutagenesis. We reported use of cheaper raw materials like molasses and cheese whey for biosurfactant production by B. subtilis R1, where it produced biosurfactant thus reducing the ST to 29–34mN/m.2 In present paper we describe the random mutagenesis of B. subtilis R1 using alkylating agent EMS to enhance the biosurfactant production. EMS is an organic mutagenic compound (C3H8SO3), which induces random mutations in genetic material by nucleotide substitution; particularly by guanine alkylation.16 Random mutagenesis by using physical (U.V. light) or chemical mutagens (like EMS, NTG etc.) are reported to be time consuming but successful in finding out improved mutants. Tahzibi et al.17 reported biosurfactant production by Pseudomonas aeruginosa mutants derived by random mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG). The mutant designated P. aeruginosa PTCC1637 produced rhamnolipid at concentration 10 times higher than parent strain. Lin et al.18 reported a B. licheniformis mutant derived by random mutagenesis with MNNG, thus producing 12 times more biosurfactant than the parent strain. In present study total 42 mutant colonies obtained after mutagenesis were screened for enhanced biosurfactant production using BA plates (Fig. 2). The isolated mutants (Fig. 2A) and parent strain R1 (Fig. 2B) were compared for zone of hemolysis on BA plates. The hemolysis zone surrounding the parent strain B. subtilis R1 and mutant colonies on BA plate (Cz), and the size of colonies (Cs) were measured (mm) and the efficiency was calculated as a ratio of Cz/Cs. Total 11 mutants showed bigger hemolysis zone (1.7–4mm) than parent strain R1 (1.4±0.2mm), and 9 mutants showed no zone of hemolysis (Fig. 3). All the mutants were screened for biosurfactant production in minimal media, and ST was measured (undiluted, 10× diluted – CMD−1, and 100× diluted – CMD−2), where 7 mutants showed no growth in minimal medium. The mutant number 10, 13, 25, 26, 28, and 35 showed similar or lower ST than parent strain R1 (28–29mN/m), but showed similar CMD−1 and CMD−2 values after 10× and 100× dilutions (Fig. 3). When the data of Cz/Cs, ST, CMD−1 and CMD−2 of all 42 mutants and parent strain R1 were statistically analyzed using ‘One-Sample Signed Rank Test’, it was observed to be statistical significant with p value <0.001. Hence, the six selected mutants and parent strain R1 were further analyzed by ‘Kruskal–Wallis One Way Analysis of Variance (ANOVA) on Ranks’, where the difference in the median values among the treatment were statistical insignificant (p=0.423 for ST and p=1.000 for CMD−1 and CMD−2). Hence as observed, based on zone of hemolysis, mutants showed better biosurfactant production, but when the data for ST, CMD−1 and CMD−2 were analyzed, all the mutants were either similar or poor biosurfactant producers, as compared to parent strain B. subtilis R1 (Fig. 3). Therefore none of the mutants were selected and only parent strain R1 was used for all further experiments.
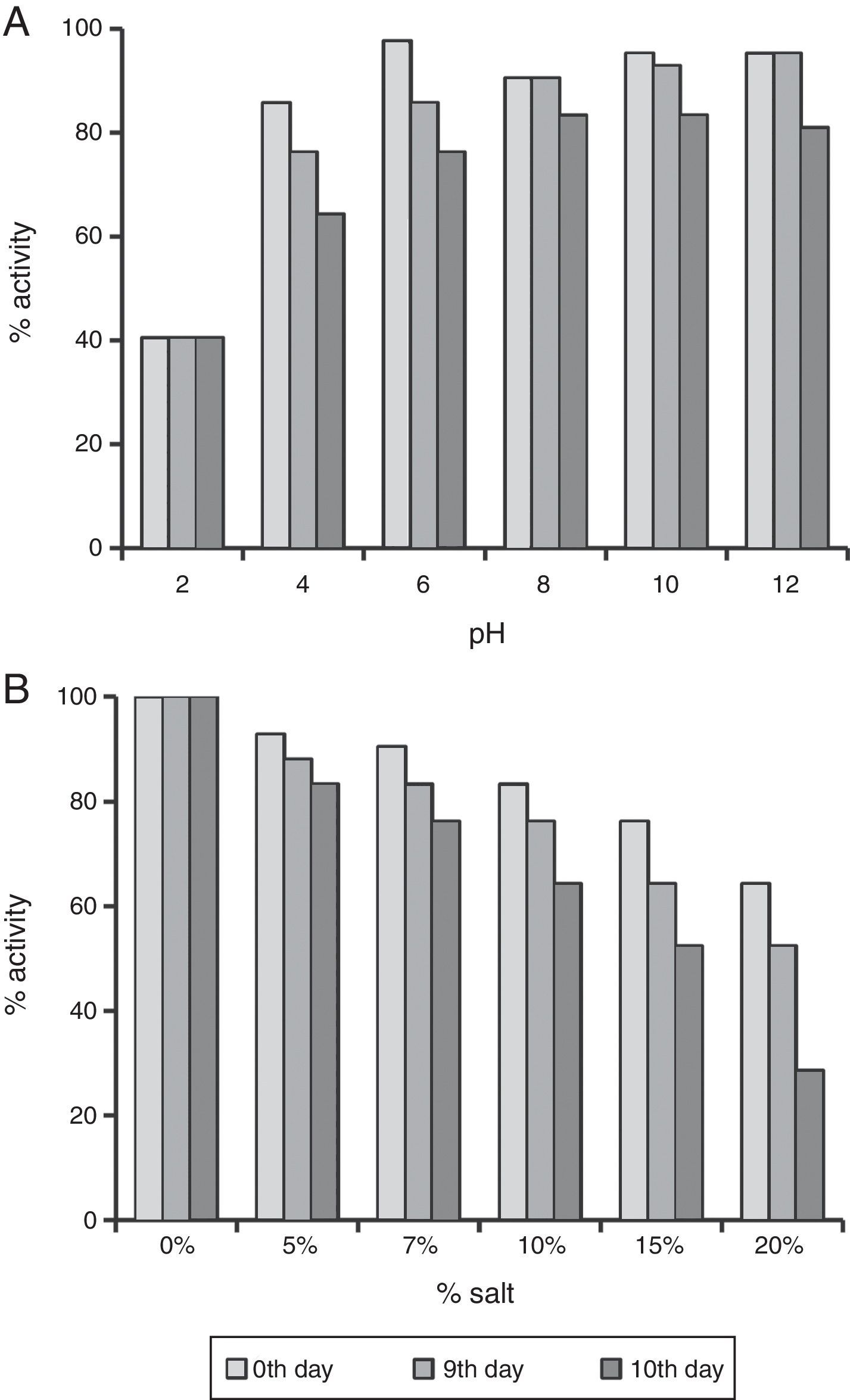
The enhanced stability under harsh reservoir conditions is one of the important criterions for selecting any compound for EOR applications. Thus we studied the produced biosurfactant under different temperatures, salt concentrations and pH values. It was completely stable for 10 days at 30–80°C, and no change was observed in ST. It also retained 64.28–80.96% surface activity till 10th day at pH 4.0–12.0 (Fig. 4A). The surface activity was reduced to 40% at pH 2.0, and it started precipitating. Whereas, it retained ≥64% surface activity even at 10% salt (NaCl), after 10 days (Fig. 4B), and at 15–20% salt, the biosurfactant activity was reduced to 64–52%, after 5th day itself (Fig. 4B). Thus the biosurfactant produced by B. subtilis R1 was found to be stable up to 80°C, pH range of 4.0–12.0, and 10% salt till 10 days. It is similar to other repots for stability of biosurfactant produced by different microorganisms.4,11,15,19–22 The use of such stable biosurfactant is advantageous in any future commercial application in oil industry for cleaning oil-sludge in storage tanks, oil immobilization and MEOR.
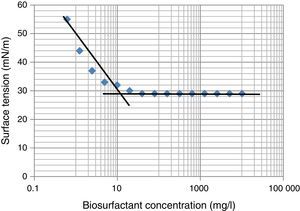
Biosurfactant yield and CMC determinationThe biosurfactant was extracted and partially purified using acid precipitation method followed by lyophilization to collect crude off-white biosurfactant powder. The observed biosurfactant yield was 0.7±0.23g/L. The CMC value characterizes the efficiency of biosurfactant, where lower the CMC – better the biosurfactant. For measurement of CMC, biosurfactant obtained after lyophilization was dissolved in distilled water and ST was measured. We observed maximum reduction in ST (∼30 to 29mN/m) at 19–20mg/L biosurfactant, beyond which concentration ST remained unchanged (Fig. 5), thus it was considered as CMC for current biosurfactant. Previously in molasses media we observed biosurfactant production by B. subtilis R1 with CMC value of 39.5±0.66mg/L,14 whereas in current glucose based minimal media the CMC observed was 20±0.56mg/L (Fig. 5). Thus we observed biosurfactant with better CMC value in glucose containing minimal medium, as compared to previously reported molasses medium.
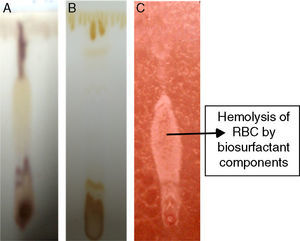
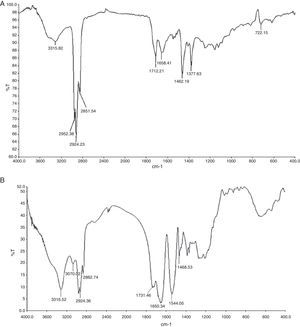
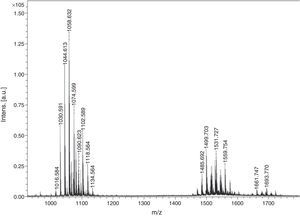
Biosurfactant characterizationPreliminary characterization of Biosurfactant was done by TLC. The separated biosurfactant components were detected by three different methods – saturating plates with Iodine vapor, spraying with methanol:sulphuric acid (95:5) reagent followed by charring plates at 100°C for 30min, and also by spraying the plates with blood reagent. Brown colored spots were detected after saturation with Iodine vapors and the spots were charred and observed as dark black spots with methanol–sulfuric acid reagent (Fig. 6), which can be correlated to lipidic-organic compounds. When the plates were sprayed with 20% blood reagent, hemolysis was observed surrounding the separated biosurfactant after 20min, which showed the presence of hemolytic biosurfactant fractions. The FTIR spectrum of partially purified biosurfactant (Fig. 7A) showed bands characteristic of peptides at 3315cm−1 (NH stretching mode) and at 1658cm−1 (stretching mode of the CON bond). The bands at 2952–2924cm−1, 2851cm−1 and at 1462cm−1, 1377cm−1 reflect aliphatic chains (CH3, CH2). The FTIR spectrum of biosurfactant showed similarity to cyclic lipopeptides like surfactin (Fig. 7B) and lichenysin produced by bacilli, as reported by other researchers.8,13 The biosurfactant was further analyzed by MALDI-TOF-MS, in the positive mode, where it primarily showed the sodium adducts for the compounds. MALDI showed three main ions, being the sodium adducts at m/z 1030, 1044 and 1058 (Fig. 8). The MALDI analysis showed that the sample also contained a small amount of other lipopeptides at m/z 1485, 1499, 1531, 1559, 1661 and 1639 (Fig. 8). However those lipopeptides are present in minor quantities, and may be similar to lipopeptide–fengycin.23,24 From the analysis it was observed that biosurfactant contained lipid and peptide moiety, and major fraction was similar to a mixture of C13–15 fatty acid-surfactin homologues.25–27
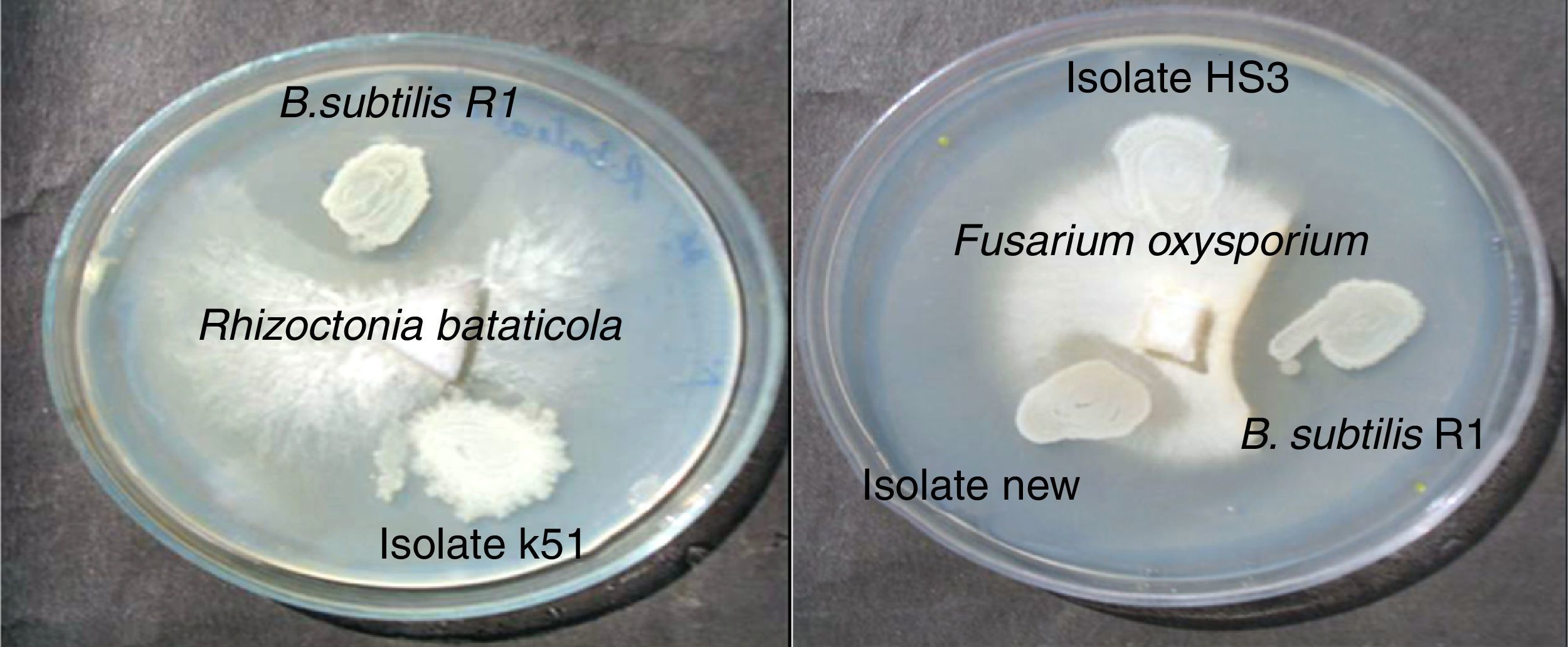
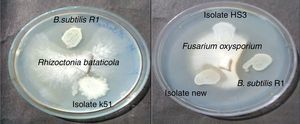
Bacteria of the genus Bacillus are known producers of a number of lipopeptides with antimicrobial activity like iturin, surfactin and fengycin. The fengycin is reported to inhibit the growth of filamentous fungi, and surfactin is reported for inhibiting the growth of bacteria and viruses, but has little or no inhibition effects on filamentous fungi.28–30 Asaka and Shoda,31 reported a B. subtilis strain producing iturin A and surfactin, to be effective for the control of damping-off caused by R. solani in tomato plants. Joshi et al.13 also reported the biosurfactant production and inhibition of various plant pathogenic fungi like Chrysosporium indicum, Alternaria burnsii, Fusarium oxysporium, and Rhizoctonia bataticola by the growth of B. subtilis 20B. In current paper also we checked antifungal activity of the produced biosurfactant against some fungal plant pathogens on PDA plates. B. subtilis R1 showed production of antifungal compound on PDA plates, co-inoculated with various fungi. Among the fungi tested, growth of Rhizoctonia bataticola and Fusarium oxysporium (Fig. 9) was inhibited by B. subtilis R1, whereas, the growth of Aspergillus niger and Penicillium chrysogenium were not inhibited. B. subtilis R1 showed no chitinase production but antifungal activity against mycelial growth of plant pathogenic fungi, so it can be hypothesized that it produces antifungal compound similar to fengycin, as also evident by MALDI-TOF results. Kim et al.23 reported co-production of biosurfactant lipopeptides – Iturin A, Fengycin, and Surfactin A, from B. subtilis CMB32, and its inhibitory effect on fungal pathogen Colletotrichum gloeosporioides. They observed that among the three lipopeptides produced, iturin A and fengycin showed antifungal activity, whereas surfactin A only acted as a synergistic factor for the antifungal effect of iturin. The co-production of surfactants and fungicide is an interesting characteristic with potential practical applications in petroleum, environmental and agricultural industries. The observed results showed that the co-production of biosurfactant and antifungal compounds have potential applications to reduce the usage of synthetic surfactants and chemical fungicides, thus contributing to the environmental protection.
Oil displacement studies using sand pack columnPreviously we observed 32.18% additional oil recovery (AOR) using biosurfactant produced by different isolates including B. subtilis R1, using molasses as a carbon source.14 In the present paper we describe the production of biosurfactant using glucose based medium and its application in MEOR. In sand pack experiments (Fig. 10) residual oil in columns was mobilized during passage of the biosurfactant broth and nearly 33±1.25% of residual oil was recovered using biosurfactant broth. The results indicated that biosurfactant mobilized oil in the sand column model and aided in biosurfactant based MEOR. It is similar to other reports of AOR using biosurfactants, where they have observed 20–37% AOR in sand-pack columns or core-flood experiments.11,22,32,33
ConclusionIt was concluded that isolate B. subtilis R1 could grow and produce biosurfactant at up to 45°C and in presence of 7% salt, in glucose based minimal medium. It was subjected to random mutagenesis using EMS, where 42 mutants were isolated and screened for biosurfactant production. None of the mutants showed statistically significant difference in biosurfactant production as compared to parent strain R1. Thus parent strain R1 was selected for further experiments. It produced 0.7±0.23g/L biosurfactant with CMC of 20±0.56mg/L. It was also quite stable at high temperature, salinity and pH values for 10 days. It was structurally similar to lipopeptides – surfactin and fengycin. Sand pack column studies showed that biosurfactant can recover 33±1.25% additional crude oil and it also inhibited some of the plant pathogenic fungi. The strain B. subtilis R1 can be potentially explored for biosurfactant based MEOR applications and as a biocontrol agent in agriculture field.
Conflicts of interestThe authors declare no conflicts of interest.
All authors kindly acknowledge the guidance provided by Prof. Anjana J. Desai, and Department of Microbiology and Biotechnology Centre, The M.S. University of Baroda for providing the research facility and consumables needed for the research project.