Plant Growth Promoting Rhizobacteria (PGPR) have different mechanisms of action in the development of plants, such as growth promotion, production of phytohormones and antibiotic substances and changes in root exudates. These help to control plant diseases. In order to evaluate the potential of microorganisms in the control of Meloidogyne javanica and Ditylenchus spp., five rhizobacteria isolated from rhizosphere of garlic cultivated in the Curitibanos (SC) region were tested. Hatching chambers were set on Petri dishes, in which were added 10mL of bacterial suspension and 1mL of M. javanica eggs suspension, at the rate of 4500, on the filter paper of each chamber. The same procedure was performed with 300 juvenile Ditylenchus spp. The experimental design was completely randomized, with four replications. The evaluations were performed every 72h for nine days. The antagonized population of nematodes was determined in Peters counting chamber, determining the percentage hatching (for M. javanica) and motility (for Ditylenchus spp). Isolates CBSAL02 and CBSAL05 significantly reduced the hatching of M. javanica eggs (74% and 54.77%, respectively) and the motility of Ditylenchus spp. (55.19% and 53.53%, respectively) in vitro. Isolates were identified as belonging to the genera Pseudomonas (CBSAL05) and Bacillus (CBSAL02).
Some of the plant crops commodity cultivated in Curitibanos region (SC) are garlic (Allium sativum) and soybean (Glycine max (L) Merril.). Those two crops are used in the main rotation system adopted by local farmers. In the case of garlic production, Curitibanos ranks as the leading producer in the state of Santa Catarina. Soybean production in the region is low; however, the genetic potential of the crop generates a secure market and serves as an alternative crop to increase the income of the producer.
As with all crops, garlic and soybeans are also susceptible to a variety of pests and diseases. Among biotic factors, nematodes cause significant drop in production. These organisms parasitize both the roots and the aerial parts of the plants.
The main nematode parasite in garlic is Ditylenchus dipsaci, which is distributed in all production regions of Brazil.1 One of the factors hindering its control is the anhydrobiosis ability of the fourth instar juvenile.2 In addition, few chemicals are released by the Ministry of Agriculture Livestock and Food Supply to control this agricultural pest.3
On the other hand, the nematodes of the genus Meloidogyne cause the most damage to soybean.4 Due to monoculture or rotation with plant species that are also the host, coupled with low ground cover, the genus finds a favorable environment for its development.
Currently, control of nematodes is accomplished through the use of nematicides that are high-priced products and have residual action, as well as high toxicity to the environment and soil microorganisms.5 Because of the possible negative impacts that it can cause6 and restrictions in the use of these products, biological control can become a great ally in the control of these plant parasites.7
An alternative to chemical control is the use of antagonist microorganisms. The Plant Growth Promoting Rhizobacteria (PGPR) can be powerful agents for biological control of plant parasitic nematodes. Bacillus and fluorescent Pseudomonas group are among the most studied and with the highest correlation to soil suppressiveness.8 Rhizobacteria have several mechanisms of action for the plant disease control.9 The main mode of action of this group of bacteria is through the production of enzymes, antibiotics, siderophores, changes in root exudates, and induced resistance, among others.10 According to Becker et al.,11 changes in root exudates can inhibit the hatching of eggs in nematodes, or even reduce the attractiveness of these to the roots of plants. Freitas et al.12 confirmed the potential of Pseudomonas fluorescens isolates to control nematodes by achieving a 75% control of Heterodera schachtii. The same isolates also controlled Meloidogyne spp. and Radopholus similis in maize, banana and tomato. Isolates of the same bacterial species have been reported as effective in the inhibition of M. incognita on tobacco, when inoculated into the soil with Trichoderma harzianum, and the action resembles the nematicides Furadan and phorate.13
In this paper, the objective was to evaluate the effect of rhizobacteria, belonging to Rhizobacteria collection of Laboratório de Microbiologia da UFSC Centro de Curitibanos, in the control of Meloidogyne javanica and Ditylenchus spp.
Materials and methodsEffect of rhizobacteria isolates in the hatching of eggs of M. javanica in vitroFive isolates of rhizobacteria (CBSAL02, CBSAL05, CBSAL14, CBSAL18 and CBSAL21) from Rhizobacteria Collection of the Laboratório de Microbiologia from Universidade Federal de Santa Catarina Centro Curitibanos were selected. The rhizobacteria were grown in Petri dishes containing solid King B medium and subsequently incubated for 24h at 28°C. After growth, the suspension of these isolates was held in sterile distilled water with the aid of Drigalski strap, under aseptic conditions. Each suspension was placed into sterile glass bottles. The optical density of the bacterial suspensions was adjusted to 0.2 optical density (wavelength 625nm).
The eggs of M. javanica used in the experiment were provided by Dr. Bruno Barbosa Unesp Jaboticabal (SP). Hatching chambers were assembled according to the methodology proposed by Alves et al.,14 in which were added 10mL of each bacterial suspension. Then, onto the filter paper was added 1mL of the egg suspension of M. javanica, containing approximately 4500 eggs. As a control treatment, only sterile water with M. javanica eggs was used. The chambers were conditioned at a temperature of 29°C for a period of nine days. The statistical design was completely randomized, with six treatments and four replications, each of which has a hatching chamber. For every 72h (three days), aliquots were removed from the suspensions of the chambers for evaluation of the degree of inhibition of egg hatching by each bacterial suspension.
The population of nematodes hatched, which was retained on the filter paper, was determined with the aid of an optical microscope and Peters counting chamber. Nematodes recovered below the paper were recorded as not antagonized. For counting was determined the hatched individuals in the control (100% hatching) and then, in the other treatments. The percentage of hatching was calculated by comparison with the number in the control. The volume of bacterial suspension removed was replaced individually in each chamber, after the withdrawal according to each treatment.
Effects of rhizobacteria isolates on the motility of Ditylenchus spp.The preparation of the inoculum followed the same methodology described above.
The population of nematodes was extracted from garlic plants contaminated by the nematode samples. The methodology used was flotation–centrifugation in sucrose solution with kaolin.15 After extraction, individual nematodes were placed in Petri dishes containing 20% formaldehyde. Later, the nematodes were identified with the help of the identification key proposed by Mekete et al.16 After being identified as belonging to Ditylenchus genus, the population was established as 300nematodes/mL in the extracted suspension, regardless of the stage of development of the individual.
Hatching chambers were prepared and to it 10mL of each bacterial suspension was added. Then, to the filter paper, 1mL of Ditylenchus spp. suspension containing approximately 250 individuals, at different stages of development, was added. As a control treatment, only sterile water added to the filter paper with nematodes was used. The chambers were placed in 29°C, for a period of nine days. The statistical design was completely randomized, with six treatments and four replications, with each plot having a hatching chamber.
At intervals of 72h, samples were taken from the suspensions in the chambers to evaluate the degree of antagonism of each isolate.
The population of antagonized nematodes retained on the filter paper was determined with the aid of an optical microscope and Peters counting chamber. Nematodes recovered below the paper were recorded as not antagonized. To calculate the degree of antagonism, the number of individuals in the control (considered 100% not antagonized) and then the number in the other treatments were determined. The percentage of antagonism was calculated by comparing with the number in the control. The volume of bacterial suspension removed was replaced individually in each chamber after the withdrawal according to each treatment.
Statistical analysisThe data relating to M. javanica hatching test and motility of Ditylenchus spp. were subjected to analysis of variance and the means were compared by Tukey test at 1% and 5% probability to the statistical program Assistat version 7.7.
Amplification and sequencing of 16S rRNA geneThe bacteria were initially grown in King B medium for 24h and then subjected to DNA extraction using the Wizard Clean up extraction kit following the manufacturer's recommendations. Amplification was performed in a final volume of 50μL, containing approximately 50ng of template DNA, 1× buffer, 1.75mM Mg Cl2, 0.25mM dNTP each, primers 27F and 1492R and 0.20mM each Taq polymerase 1.5U. Sequencing was performed using sense and antisense primers in equipment of Applied Biosystems 3500. The assembly of the contigs was done using BioNumerics 7.0 program.
The sequences obtained were subjected to a similarity analysis in the NCBI database (National Center for Biotechnology Information) using the BLAST tool (Basic Local Alignment Search Tool).17
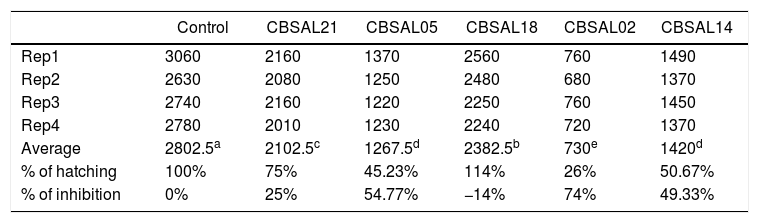
ResultsEffect of isolates of rhizobacteria in the hatching of eggs of M. javanica and the motility of Ditylenchus spp. in vitroTo calculate the percentage of control, comparison index was used. The control treatment that received no bacteria was considered as 100% hatching of M. javanica eggs or 0% of control. The isolates CBSAL02, CBSAL05, CBSAL14 and CBSAL21 presented at the end of the evaluation averages significantly higher than other treatments, taking into account the overall average of the treatments. These showed 74% control rates, 54.77%, 49.33% and 25%, respectively (Table 1). Isolated CBSAL18 showed an inhibition percent significantly lower than the control, suggesting no antagonistic effect.
Inhibition of egg hatching in Meloidogyne javanica by the PGPR isolates.
| Control | CBSAL21 | CBSAL05 | CBSAL18 | CBSAL02 | CBSAL14 | |
|---|---|---|---|---|---|---|
| Rep1 | 3060 | 2160 | 1370 | 2560 | 760 | 1490 |
| Rep2 | 2630 | 2080 | 1250 | 2480 | 680 | 1370 |
| Rep3 | 2740 | 2160 | 1220 | 2250 | 760 | 1450 |
| Rep4 | 2780 | 2010 | 1230 | 2240 | 720 | 1370 |
| Average | 2802.5a | 2102.5c | 1267.5d | 2382.5b | 730e | 1420d |
| % of hatching | 100% | 75% | 45.23% | 114% | 26% | 50.67% |
| % of inhibition | 0% | 25% | 54.77% | −14% | 74% | 49.33% |
Means followed by the same letter do not differ. Tukey test at 5% probability.
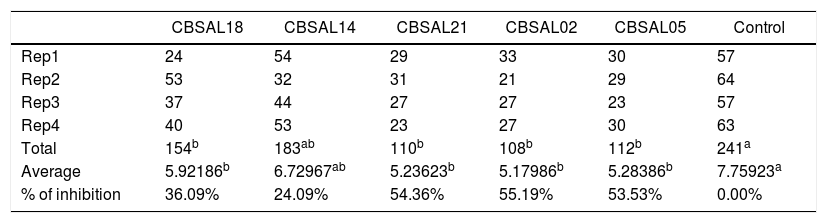
Regarding the results of the tests inhibiting the motility of Ditylenchus spp., isolates CBSAL02, CBSAL05, CBSAL18, and CBSAL21 statistically differed from the control, while the isolate CBSAL14 statistically matched other treatments and the control (Table 2). The isolates CBSAL02, CBSAL21, CBSAL05 and CBSAL18 exhibited control of 55.19%, 54.36%, 53.53% and 36.09%, respectively. CBSAL14 presented a percentage of 24.09% (Table 2); however, compared to the control, the difference was not considered significant.
Inhibition of the motility of different juvenile stages of Ditylenchus spp. by the PGPR isolates.
| CBSAL18 | CBSAL14 | CBSAL21 | CBSAL02 | CBSAL05 | Control | |
|---|---|---|---|---|---|---|
| Rep1 | 24 | 54 | 29 | 33 | 30 | 57 |
| Rep2 | 53 | 32 | 31 | 21 | 29 | 64 |
| Rep3 | 37 | 44 | 27 | 27 | 23 | 57 |
| Rep4 | 40 | 53 | 23 | 27 | 30 | 63 |
| Total | 154b | 183ab | 110b | 108b | 112b | 241a |
| Average | 5.92186b | 6.72967ab | 5.23623b | 5.17986b | 5.28386b | 7.75923a |
| % of inhibition | 36.09% | 24.09% | 54.36% | 55.19% | 53.53% | 0.00% |
Means followed by the same letter do not differ. Tukey test at 5% probability.
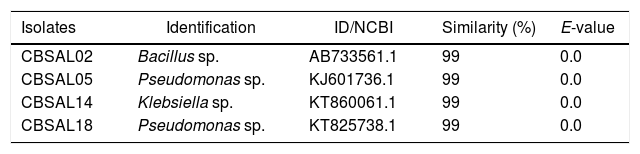
Comparison of 16S rRNA sequences of five isolates (CBSAL02, CBSAL05, CBSAL14, CBSAL18 and CBSAL21) showed that three isolates (CBSAL05, CBSAL18 and CBSAL21) belonged to the genus Pseudomonas, with 99% similarity (Table 3). Other isolates were identified as Bacillus (CBSAL02) and Klebsiella (CBSAL14); both also showed 99% similarity (Table 3).
DiscussionFour of the isolates tested belonged to Pseudomonas and Bacillus genera, which are commonly described as inhibitors of various pathogens.9,18,19 The Klebsiella genus is described endophytic, diazotrophic and a plant growth promoter as well.20 Information regarding its effect on plant pathogens was found in the literature.
Among the three isolates identified as Pseudomonas, one inhibited over 50% the two nematodes, while the other two showed inhibition percentage at around 50% to only one of the parasitic nematodes. The results indicated an antagonistic action of the isolates to the nematodes, suggesting potential control. It was noted that two of the five isolates (CBSAL02 and CBSAL05) had percentages of control higher than 50% to the two nematodes examined. The control ability of CBSAL21 to Ditylenchus spp. was more than double of that observed for M. javanica (54, 36% and 25% respectively). A reverse condition was observed for CBSAL14, wherein the percentage for M. javanica was 49.33% and 24.09% for Ditylenchus spp. For CBSAL18, there was inhibition of 36% of Ditylenchus spp., while for M. javanica, there was no effect on hatching. These results suggest specific action of some rhizobacteria to taxonomic groups of nematodes.
The results are consistent with many previous studies to assess the effect of rhizobacteria on the control nematodes. Alves et al.14 demonstrated through in vitro experiments the potential of different isolates of rhizobacteria, with regard to the motility and ovicidal action of M. javanica, M. incognita and P. zeae. Naves et al.21 observed that endophytic bacteria filtered suspensions were able to significantly reduce the mobility, and consequently increase mortality, as well as the hatching of the young second stage of M. javanica.
Some authors have reported rhizobacterial antagonistic action to be dependent on the production ability of secondary metabolites, which could explain the effect variance observed in some isolates by the production or not of specific compounds. Studies by Stirling22 confirm that some rhizobacterial isolates are able to synthesize toxic metabolites, affecting the movement and the emergence of the young in different kinds of nematodes.
Extracellular enzymes are the most studied rhizobacterial metabolites with inhibitory action against pathogens. This action has also been studied in other microorganisms. Khan et al.23 reported the production of proteases and chitinases by an isolated fungus Paecilomyces lilacinus. Those substances were observed to operate together, destroying the lipid layer that is essential for the development and maintenance of nematode in the egg; in addition, they caused the hydrolysis of egg chitin layer and affected the integrity of the vitelline layer, thus having an impact on both development and hatching.
It is suggested that the inhibitory action of rhizobacterial isolates may be linked to the production of enzymes, such as chitinase and other cell wall degrading substances. These would assist in the degradation of the wall of M. javanica eggs and, subsequently, impair the development of that nematode, as well as Ditylenchus spp. Some PGPR produce these lytic enzymes that break down the walls of the eggs of species of Meloidogyne, delay the onset of second stage juveniles (J2), cause the death of adult nematodes or interfere with their host plant recognition process.24
The recognition of the plant host is the primary factor in the infection by nematode. The plant identification is performed through the root exudates, where nematodes will migrate to the infection site. Rhizobacteria as those of the genus Pseudomonas possess the ability to alter root exudates, thus hampering the recognition of the feeding site by the nematode present in the soil.25 Siddiqui and Mahmood26 and Tian et al.27 observed that the interference in the recognition process by exudate changes was caused by endophytic bacteria as a control mechanism.
The action of rhizobacterial isolates in plants has been reported in several studies. Araújo et al.28 demonstrated the efficacy of Bacillus spp. and Pseudomonas spp. in the control of R. similis, which causes root necrosis in banana plants. They also pointed out the prevalence of these bacterial genera in the rhizosphere of different plants. De Souza Júnior et al.29 evaluated the effect caused by the mixing of rhizobacterial isolates for the control of Meloidogyne graminicola in rice seedlings grown from microbiolized seeds. In this study, the authors observed a reduction in the number of galls, number of eggs and reproduction of M. graminicola.
Five bacterial isolates were inoculated in lettuce pre-inoculated seedlings grown in substrate (vermiculite:sand – 2:1) with M. javanica. However, due to unfavorable environmental factors in the development of nematode, there was no gall formation.
Tested rhizobacteria also exhibited plant growth promoting activity in vitro and in vivo, indicating its potential use in production and plant protection.30
The bacterial isolates CBSAL02, CBSAL05, CBSAL14 and CBSAL21 provided ovicidal action M. javanica. Aside from CBSAL14, all the isolates showed potential control to Ditylenchus spp. when compared to the control treatment. The results indicate that two isolates (CBSAL02 and CBSAL05) exhibited strong potential for use as biological control agents for both the nematodes. Detailed studies of the action of these isolates on nematodes should be performed, as well as evaluations on their potential for plant protection.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Professor Jaime Maia dos Santos and Dr. Bruno F. F. Barbosa, Unesp Jaboticabal, for the assignment regarding the nematodes and technical support.