The use of dark septate fungi (DSE) to promote plant growth can be beneficial to agriculture, and these organisms are important allies in the search for sustainable agriculture practices. This study investigates the contribution of dark septate fungi to the absorption of nutrients by rice plants and their ensuing growth. Four dark septate fungi isolates that were identified by Internal transcribed spacer phylogeny were inoculated in rice seeds (Cv. Piauí). The resulting root colonization was estimated and the kinetic parameters Vmax and Km were calculated from the nitrate contents of the nutrient solution. The macronutrient levels in the shoots, and the NO3−-N, NH4+-N, free amino-N and soluble sugars in the roots, sheathes and leaves were measured. The rice roots were significantly colonized by all of the fungi, but in particular, isolate A103 increased the fresh and dry biomass of the shoots and the number of tillers per plant, amino-N, and soluble sugars as well as the N, P, K, Mg and S contents in comparison with the control treatment. When inoculated with isolates A103 and A101, the plants presented lower Km values, indicating affinity increases for NO3−-N absorption. Therefore, the A103 Pleosporales fungus presented the highest potential for the promotion of rice plant growth, increasing the tillering and nutrients uptake, especially N (due to an enhanced affinity for N uptake) and P.
Despite the increasing yields caused by genetic progress over the last 30 years, improvements in crop yields have not been very evident, especially for rice,1,2 which is the most frequently cultivated and consumed cereal in the world.3 That said, farmers have to face the increased costs of seeds, chemicals, and fertilizers.2 In this scenario, increasing crop yields at lower costs could be achieved by increasing the root surface provided by associations with fungi, culminating in better nutrient uptake and plant health.4,5
Dark septate fungi (DSE) are ascomycetes, and they are characterized by having dark pigmentation, microsclerotia and melanized septate hyphae that colonize the root epidermis and cortex inter- and intracellulary in the host roots.6–11 Many of these fungi are able to colonize the root cells of plants, promoting growth without causing pathologies.12,13
These fungi often inhabit oligotrophic soils that are associated with the roots of hundreds of plant species in all climate regions and major biome types.12,14–20 Although DSE research is increasing, our knowledge of the diversity of these fungi remains restricted.21 DSE classification is gradually being improved and new species are being described, but many isolates do not yet have adequate taxonomic positioning.21,22 For example, the three novel genera Darksidea, Aquilomyces and Flavomyces belonging to Lentitheciaceae, Morosphaeriaceae and, Massarinaceae family were recently documented.21 To date, approximately 40 DSE species have been described.6,21,23–26
The ability of these fungi to promote plant growth has attracted attention because of their potential use in different species, such as conifers, grasses, and cabbages, among others.6,22,27–29 Furthermore, because they readily grow in culture media and are not biotrophic, DSEs have advantages over other fungi because their inoculant production can be easier.6,30 Newsham13 performed a meta-analysis of 18 independent studies to assess the inoculation of DSE in different crops, concluding that this practice raised the nitrogen (N) and phosphorus (P) contents as well as the plant biomass by 26–103%. Conversely, another meta-analysis suggested negative to neutral effects from inoculating with non-clavicipitaceous root fungal endophytes (including DSE) on the plant biomass and N content.31 Although the influence of DSE on its host is still under debate, the identification of DSE with biotechnological potential expands the horizons for its use in agriculture, as is also the case for nitrogen-fixing bacteria and other beneficial microorganisms.
In Brazil, recent studies have shown the presence of DSE in the roots of healthy Oryza glumaepatula plants in the natural environment.32 In this study, some isolates were able to colonize and contribute to the development of Oryza sativa L. without causing disease symptoms.32–34 In the same way, based on intergenic spaces, Bonfim et al.35 identified 35 DSE groups representing 27 species that were isolated from 7 native trees, indicating the high diversity of these fungi. Likewise, a new dark septate fungus species from China (Harpophora oryzae) was recently identified in the healthy roots of Oryza granulata, which was able to increase the biomass of O. sativa, also without triggering disease symptoms.22
The way in which DSE establish their association with the host is not fully understood, but studies have indicated that growth promotion can be performed directly, by enhancing the nutrient uptake; or indirectly, by protecting the plant against abiotic stresses (drought, salinity, and high concentrations of heavy metals) and the production of phytohormones or analogous substances.27,36–38 In Brassica campestris L., a mutualistic association was found with the fungus Heteroconium chaetospira, in which the fungus supplies N to the plant and the plant provides carbon to the fungus, resulting in significant increases in plant biomass.27
The best growth promotion responses from DSE use have been observed when organic sources of N are used.12,13,27,28 However, because of the generalized occurrence of dark septate fungi in a wide range of environments,20,39 there are likely DSE species that are also able to assist in nutrient uptake from inorganic sources.27
DSEs have been shown to form intraradical structures resembling those formed during mycorrhizal symbioses. For example, Acephala macrosclerotiorum formed ectomycorrhizae in pine and spruce29 and H. chaetospira formed loose intracellular hyphal loops that morphologically resembled the ericoid mycorrhizae in Rhododendron obtusum var. kaempferi.40 However, the roles of these intraradical hyphal structures are still at the hypothesis level.
In a previous study, the compositional diversity of the DSE that colonized native rice (O. glumaepatula) in the Brazilian Amazon was investigated.33 Based on in vitro tests, 32 nonpathogenic isolates were considered as DSE fungi.33 This study was performed to address the phylogenetic position and to assess the contribution of dark septate fungal isolates (A101, A103, A104 and A105) obtained from O. glumaepatula33 to the absorption of nutrients and the growth of rice plants (O. sativa) under controlled conditions.
Materials and methodsFungal isolates, DNA extraction, and phylogenetic analysesAll of the isolates investigated here are deposited in the CRB-JD (Centro de Recursos Biológicos Joanna Döbereiner at Embrapa Agrobiologia – www.embrapa.br/agrobiologia/crb-jd) (A101, A103, A104, and A105). For the phylogenetic analyses, genomic DNA was extracted from eight-day-old cultures grown in PDA medium. Five discs of 6mm each were transferred from the plate to 2mL sterile microtubes, to which 0.2g of glass beads (106μm; Sigma) and 1mL of sterilized ultrapure water were added. The suspension was vigorously agitated by vortex and centrifuged (1.5min; 13000rpm). The supernatant was transferred to clean microtubes, and 600μL of the cell lysis solution from the kit Wizard® Genomic DNA Purification (Promega) was added. The samples were vigorously agitated by vortex (10min) and subjected to three cycles of 95°C/ice, with 10min for each step. These cycles were followed by a Wizard® Genomic DNA Purification protocol. The ITS1-5.8S-ITS2 region of the rDNA fragment was amplified using primers ITS1 and ITS4.41 PCR was performed with 25μL reactions consisting of 14.63μL of water, 1× buffer, 2.5mM MgCl2, 0.25μM dNTP, 0.2μM of each primer (ITS1 and ITS4), 1.25U of Taq DNA polymerase (5UμL−1), and 1.0μL of DNA template (approx. 50ng). The amplification conditions from Maharachchikumbura et al.42 were used. DNA sequencing was performed for both DNA strands, and the sequences were assembled with Bionumerics software v. 7.1.
The dataset used for the phylogenetic analysis contained taxon belonging to dothideomycete orders and only Pleosporales was represented by their known families. The order-level dataset was used to gain information about the phylogenetic position of A101, A104 and A105 isolates in Pleosporales (Fig. 2). Alignments of our sequences together with sequences from GenBank were performed using MUSCLE in MEGA v. 7.43 The sequences were edited using MEGA v. 7. Aligned dataset of ITS was analyzed using Maximum likelihood (ML). The best model for ML was selected based on the Akaike Information Criterion (AIC), which was calculated in MEGA v. 7. ML analysis was done by calculating an initial tree using BioNJ and the subsequent Heuristic search done with the Nearest-Neighbour-Interchange (NNI) option. Distance matrices were calculated using the Kimura three-parameter substitution model43 and the robustness of the tree nodes was evaluated by bootstrap analysis44 using 1000 replicates. The software default parameters were considered in all analyses. The ITS region sequences were deposited in GenBank (KR817246=A101, KR817248=A103, KR817249=A104, and KR817250=A105).
Experimental design, treatments, and conditions of the incubator roomThis experiment was performed in an incubator room at Embrapa Agrobiologia, in Seropédica, Rio de Janeiro state. The experimental design was completely randomized, with rice plant treatments grown without (control) and with the inoculation of dark septate endophytic fungi (DSE). Each treatment including the control had 4 replications, and each replication had four plants. The Piauí rice variety, a genotype adapted to the natural low-fertility of Brazilian soil was used, along with the fungal isolates A101, A103, A104 and A105. The plants were grown with a 13h/11h (light/dark) photoperiod, a luminosity of 384μmolm−2s−1 (photosynthetically active photon flux), a relative humidity of 50–60% and a temperature of 28°C/24°C (day/night).
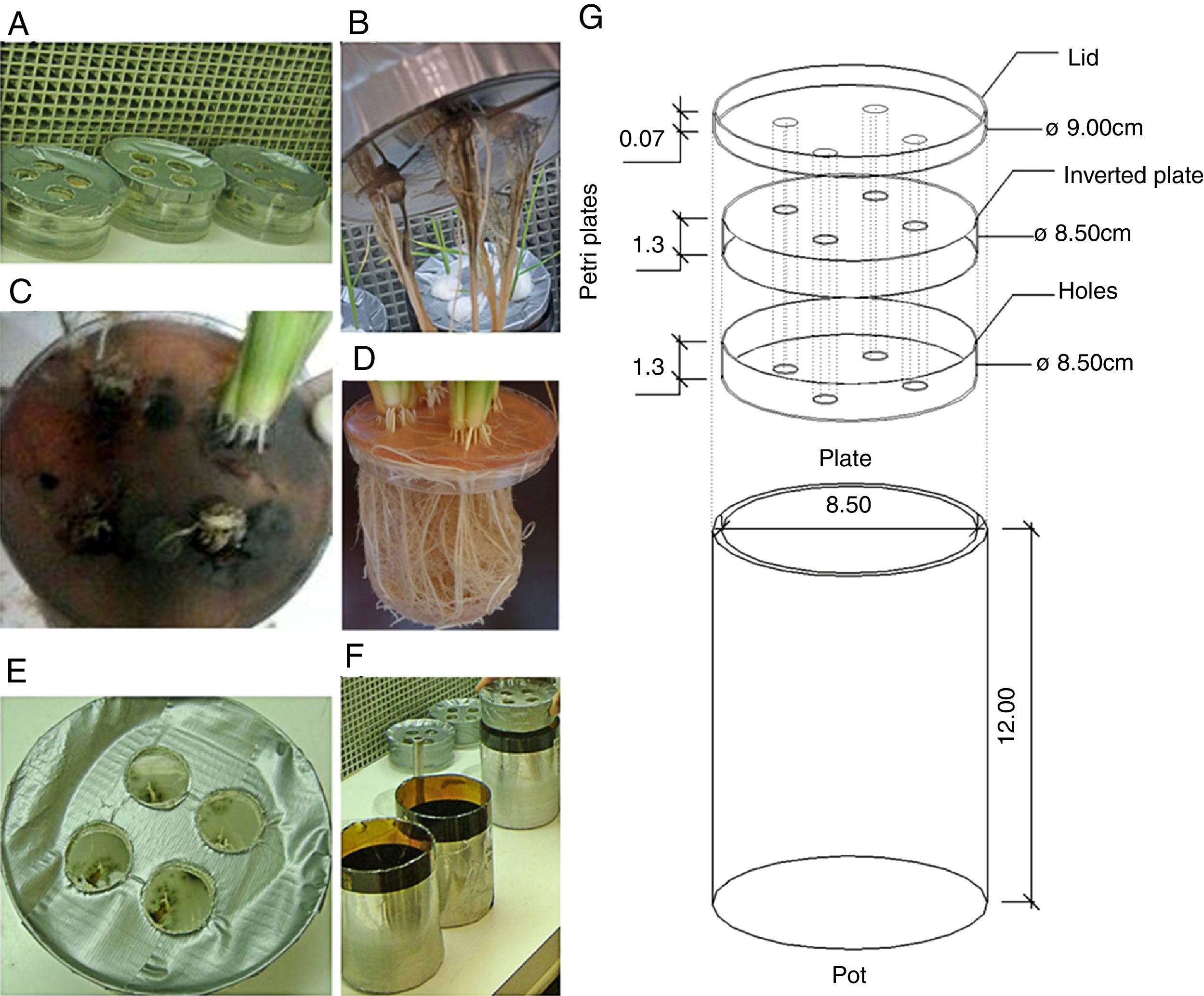
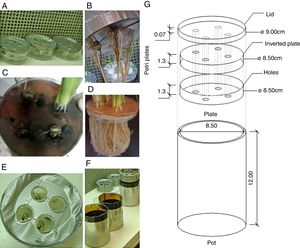
Inoculation and growth conditionsThe rice plants were grown in disposable Petri plates containing sterilized agar-water (1% concentration), and they were adapted to allow for the mycelial growth. Two plates (one inverted) and one lid contained four equidistant holes (approximately 8mm in diameter), and the holes on the bottom were sealed with adhesive tape to hold the previously autoclaved agar water (Fig. 1). The set of plates was sealed with high-resistance adhesive tape (Fig. 1) and then exposed to ultraviolet light for 20min in a laminar flow hood to prevent contamination.
Vessel used to study the NO3−-N uptake kinetics in rice plants (var. Piauí) that were inoculated with dark septate fungi and subjected to 0.5mM of NO3−-N after 72h of N nutrient deprivation. (A) Petri dishes containing agar-water with seeds and mycelium discs; (B) fungal biofilm of A103 (10 DAG); (C) fungal characteristics at the collection time (35 DAG); (D) non-inoculated plant at 41 DAG; (E) the start of A103 establishment (3 DAG); (F) Pot used; (G) overview of vessel.
The fungal isolates were previously grown on PDA medium for seven days at 28°C. The rice seeds were washed in 70% alcohol for 5min and disinfested with 2.5% sodium hypochlorite for 10min, followed by successive washing in autoclaved distilled water. The seeds were placed on the agar-water medium at the spots where each plate was perforated. Additionally, one disk of PDA medium (approximately 8mm) in diameter containing mycelia was placed alongside of each seed. The Petri plates for the control plants were inoculated with PDA plugs without fungal mycelium. After their sowing, the plates were incubated for three days at 28°C to favor fungal germination and establishment.
After the seeds had germinated, four plates with uniformly sized seedlings were selected for transfer to glass pots containing only autoclaved, distilled water (120°C for 1h). After five days, the water contained in the pots was replaced by nutrient solution that was formulated according to45 at 1/4 of the ionic strength, which was modified with 1.5mmolL−1 of NO3−-N (KNO3 as source). After three days, this solution was replaced by another solution at 1/2 ionic strength, with 2.0mmolL−1 of NO3−-N and 0.5mmolL−1 of NH4+-N, with Ca(NO3)2 and (NH4)2SO4, respectively. This strategy was adopted to avoid causing salt stress in the plantlets.46 Thereafter, the 1/2 ionic strength solution was replaced every three days.
Thirty-two days after germination (DAG), the plants were deprived of nutrient solution NO3−-N for a period of 72h to increase the roots’ capacity to absorb N47 after which a nutrient solution containing 0.5mmolL−1 of NO3−, again by using KNO3 as the source, was supplied. During 11h, samples of the solution were then collected every 30min by removing 0.5mL aliquots from each pot to plot the depletion curves and determine the kinetic parameters Vmax and Km.48,49 The samples were placed in microtubes and the NO3− concentration was measured according to50 as adjusted following Alves et al.51 The Vmax and Km values were determined by using the mathematical graphing method proposed by Cometti et al.52 with the Cineticawin 1.0 program (Universidade Federal de Viçosa, Minas Gerais, Brazil).
Soluble fraction and colonization measurementFollowing the collection of the last nutrient solution sample, the plants were cut and the heights and numbers of tillers from each plant were determined. The fresh and dry masses of the roots and shoots were measured, in the second case after being dried in a forced-air chamber at 65°C until reaching a constant weight. The fresh samples (one gram of root, sheath and leaf tissue) were homogenized in 80% ethanol, and after partitioning the samples with chloroform,53 the levels of NO3−-N,50 free amino-N,54 NH4+-N,55 and soluble sugars56 in the soluble fraction were measured. The remaining shoot material was used to determine the macronutrient concentrations.57 After removing the shoots, a fresh root was removed from each pot and kept on alcohol 50% to observe the colonized roots. The diafanization and staining of the roots were performed according to.58 The root sections (approx. 1cm) were placed in slides with glycerin and screened at high magnifications (400× and 1000×) using an Axioplan light microscope (Carl Zeiss, Jena, Germany) equipped with an Axiocam MRC5 digital camera (Carl Zeiss). The microsclerotia and intraradical hyphae were counted in 100 microscopic fields per root system under 400× magnification using point-intercept method.29,59,60 To prepare cross-sections of colonized roots for light microscopy, the samples were dehydrated twice in an ethanol series of 70, 90 and 100% for 1h each. After dehydration, the colonized roots were infiltrated with historesin (Leica, Wetzlar, Germany) and 100% ethanol (1:1, v/v) for 12h and then with 100% historesin for 24h before being embedded in historesin. Sections (that were approximately 5μm thick) were sliced using a rotary microtome (Leica). The samples were observed and the images were analyzed as described above.
Statistical analysesAn analysis of variance and a t-test for the comparison of means were applied to the data (p<0.05) with Assistat61 and Sisvar software.62
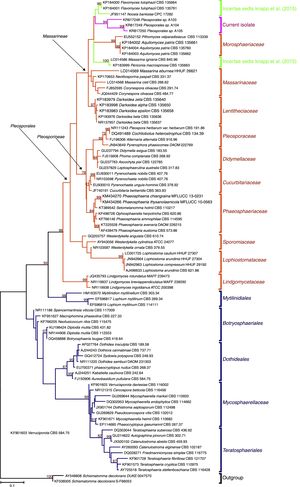
Results and discussionPhylogenetics of the isolates based on ITSThe sequences obtained from the isolated fungi were first subjected to a BLAST search (http://www.ncbi.nlm.nih.gov) and to pairwise sequence alignment (http://www.fungalbarcoding.org). For the A101 fungus, only matches with less than 88% similarity were found, indicating that it represented an unknown taxon. For the other three isolates, a similarity to a fungus belonging to the Pleosporales order was found; this is the largest order in the Dothideomycetes class and encompasses some recognized dark septate fungal families.21
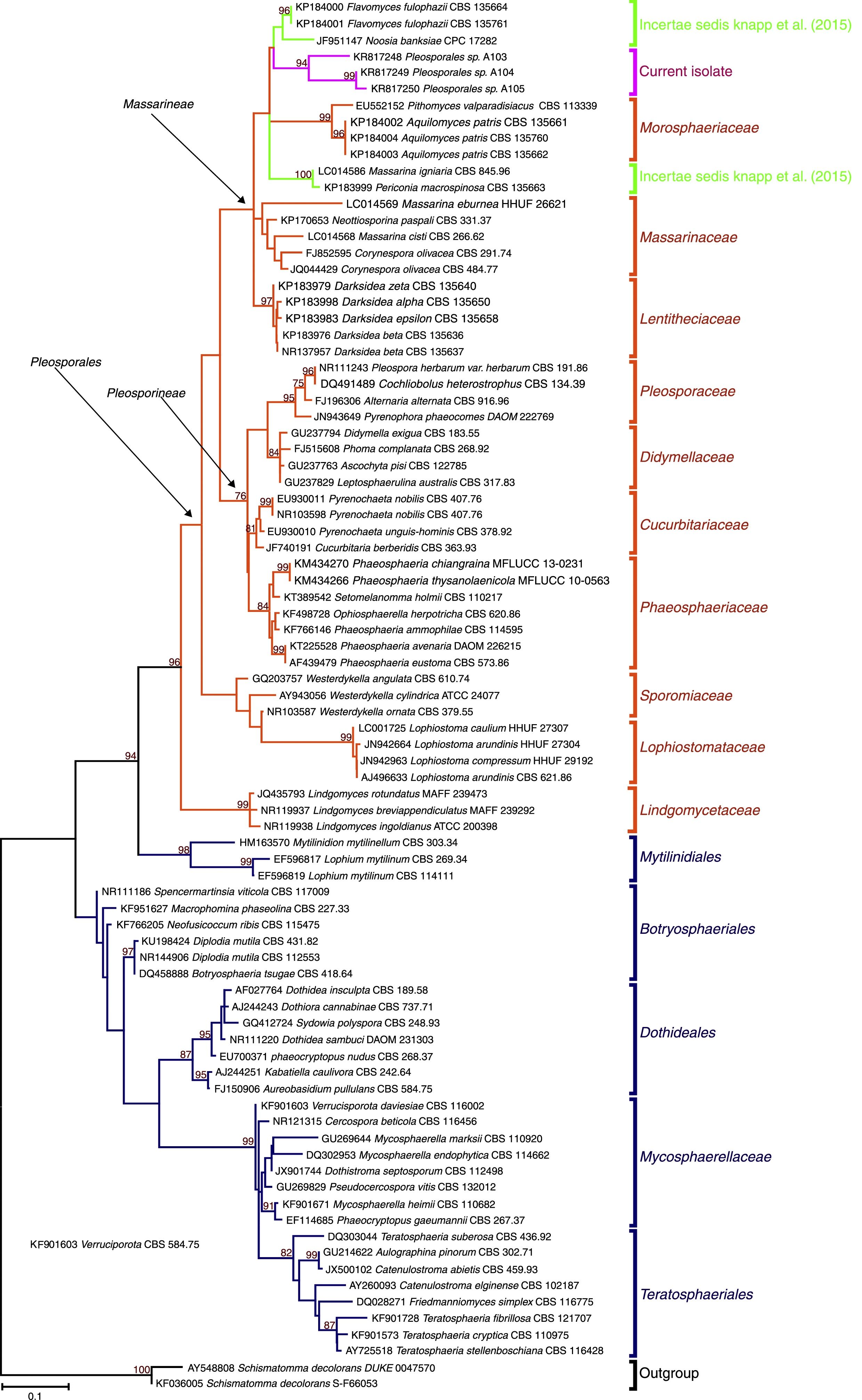
According to the results of the phylogenetic analyses, the three isolates (A103, A104 and A105) belonged to the Pleosporales order and are nested in families of the suborder Massarineae, near the Morosphaeriaceae (Fig. 2). Additionally, the three new isolates are closely related to some genera that were treated as incertae sedis (a genus not yet placed within a specific family/genus), such as Massarina igniaria (CBS 845.96), Periconia macrospinosa (CBS 135663), Noosia bankssiae (CPC 17282), Flavomyces fulophazii (CBS 135761) and Flavomyces fulophazii (CBS 135664) (Fig. 2).21 Therefore, the ITS sequence analysis indicated that our isolates represent new taxa that will require a proper polyphasic analysis in the future, but at least three isolates belong to the classical dark septate fungal group, i.e., the Pleosporales order.
ML phylogenies of ITS sequences from Dothydeomycetes showing the phylogenetic placement of the dark septate endophytes A103, A104 and A105 (labeled in pink). Schismatomma decolorans (DUKE 0047570 and S-F66053) was chosen as the out-group. The numbers at the branching nodes represent bootstrap values (1000 replicates) of 70% and higher. Ex-type and ex-epitype sequences are in bold. Families highlighted in orange sections and blue are orders. Selected model: Tamura 3-parameter (T92)+K2+G.
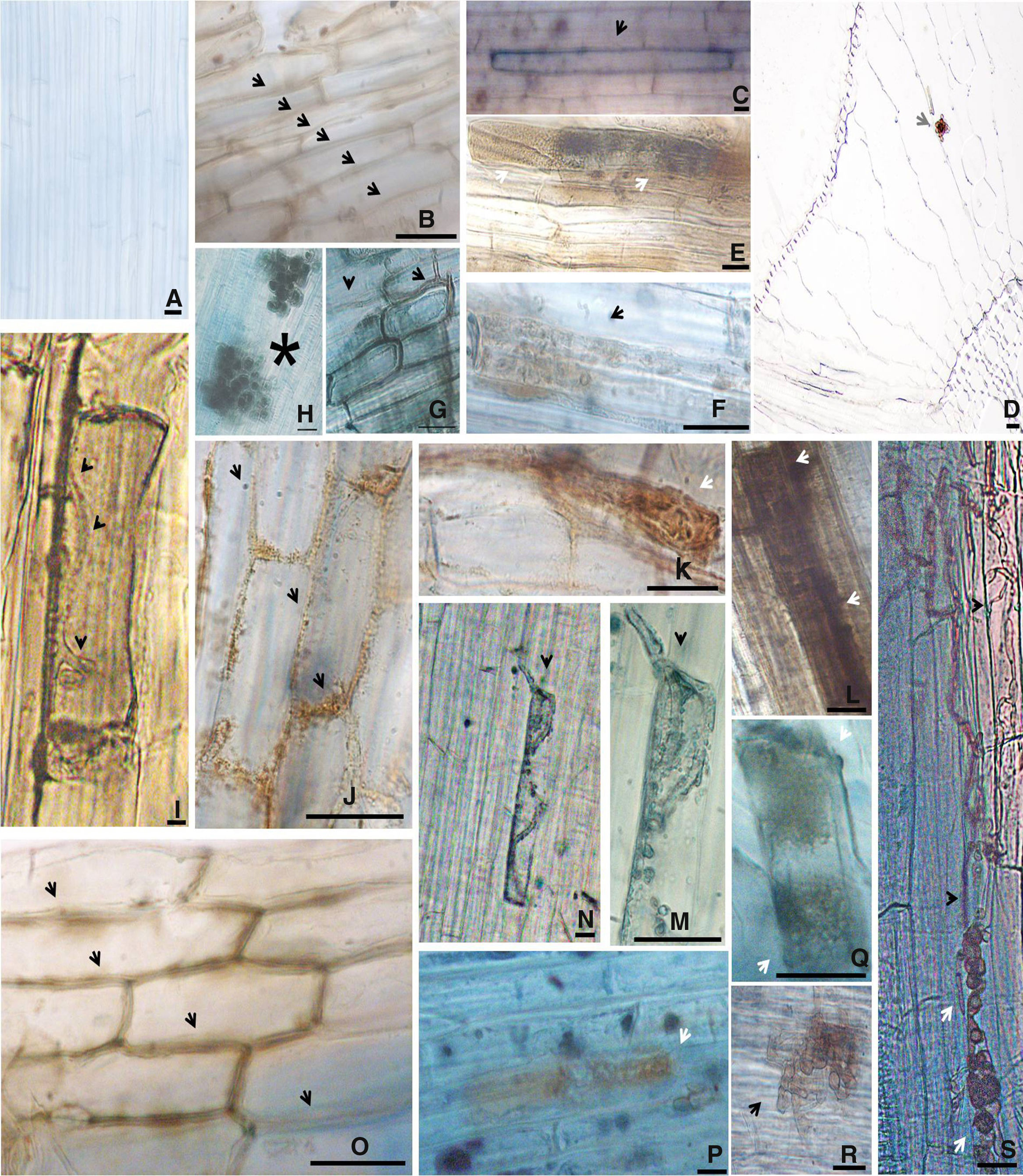
The inoculation of the rice plants with four different DSE isolates did not cause any disease symptoms, indicating the compatibility of the association. Other studies also indicated that DSE can colonize the root cortex of plants without causing any pathologies.13 In the same way, the roots of all of the plants that were inoculated with A103, A104 and A105 fungi had intercellular melanized hyphae that formed structures resembling anastomoses (Fig. 3B, J, and O) and intracellular microsclerotia in the cortex root (Fig. 3E, D, F, K, L, P, Q and R). A104 and A105 formed intracellular hyphae that were stained with methyl blue in the roots of all of the inoculated rice plants (Fig. 3I, M, and N). A103 and A101 formed intracellular melanized septate hyphae (Fig. 3G and S), and the A103 fungus also formed chlamydospore-like structures that surrounded the vascular bundle region (Fig. 3H) as well as a loose intracellular hyphal loop (Fig. 3C) and A101 formed microsclerotia (Fig. 3S) in the roots of all of the inoculated rice plants. In addition, during the experiment, the fungus was found to be growing abundantly all over the water-agar in the Petri dish that had been tossed into the pot planted with rice (Fig. 1C). Moreover, abundant fungal growth was directly observed over the root surface, even when the roots were submerged in the nutrient solution (Fig. 1B). The control plants did not display any fungal colonization (Figs. 1D and 3A). However, in all of the treatments with dark septate inoculation, abundant colonizations were observed, varying from 33 to 77.2% (Table 1). The percentage of colonized roots in the plants inoculated with A103, A104, A105 was significantly higher than that of A101 fungus (Table 1). These results are similar to results obtained recently by Lukešová et al.,29 in which the authors observed a loose intracellular hyphal loop formed by A. macrosclerotiorum in birch roots, with colonization varying from 51–61% when the birches were inoculated with Acephala applanata and A. macrosclerotiorum and 50–80% when blueberries were inoculated with Phialocephala fortiniis s.l. – A. applanata species complex (PAC).
Morphological aspects of rice (var. Piauí) roots at 35 DAG on control (non-inoculated) and colonized by A103, A105, A104 and A101 fungi, unstained (d) or stained with methyl blue (others). (A) Non-inoculated control; (B–H) intercellular melanized hyphae forming structures resembling anastomoses (arrows) (B), a loose intracellular hyphal loop (arrow) (C), melanized intracellular microsclerotia (arrows) (E and F), a cross-section showing melanized intracellular microsclerotium (arrow) in the cortex region tissue of root (D), intracellular melanized septate hyphae (arrowhead) and a cell surrounded by melanized septate hyphae (arrow) (G), an intraradical chlamydospore-like structure (asterisk) in the vascular bundle region (H) formed by A103 fungus in the rice roots; (I–L) intracellular hyphae (arrowheads) (I), melanized intracellular microsclerotia (arrows) (K and L) formed by A105 fungus in rice roots; (M–R) intracellular hyphae (arrowhead) stained with methyl blue (M and N), intercellular melanized hyphae forming structures resembling anastomoses (arrows) (O), an early developmental stage of an intracellular microsclerotia (arrows) (P and Q), melanized microsclerotia (R) formed by A104 fungus in the rice roots; (S) intracellular melanized septate hyphae (arrowhead) and melanized microsclerotia (white arrows) formed by A101 fungus (R). DAG (days after germination). Bar=20μm.
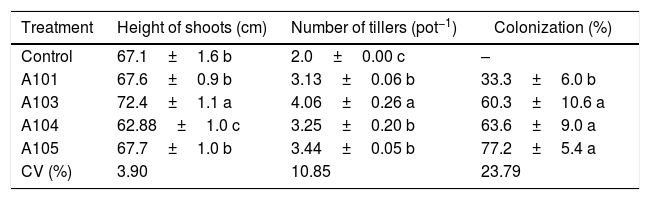
Heights of the shoots, number of rice plant tillers (Piauí variety) at 35 DAG, with and without inoculation with different dark septate fungal isolates and the percentage fungal colonization of rice roots.
| Treatment | Height of shoots (cm) | Number of tillers (pot−1) | Colonization (%) |
|---|---|---|---|
| Control | 67.1±1.6 b | 2.0±0.00 c | – |
| A101 | 67.6±0.9 b | 3.13±0.06 b | 33.3±6.0 b |
| A103 | 72.4±1.1 a | 4.06±0.26 a | 60.3±10.6 a |
| A104 | 62.88±1.0 c | 3.25±0.20 b | 63.6±9.0 a |
| A105 | 67.7±1.0 b | 3.44±0.05 b | 77.2±5.4 a |
| CV (%) | 3.90 | 10.85 | 23.79 |
Means±SE (n=4) followed by the same lower-case letters in the column do not differ significantly (t-test (LSD), p<0.05). DAG, days after germination. The means of four composed repetitions (each repetition had four plants per pot). SE, standard error.
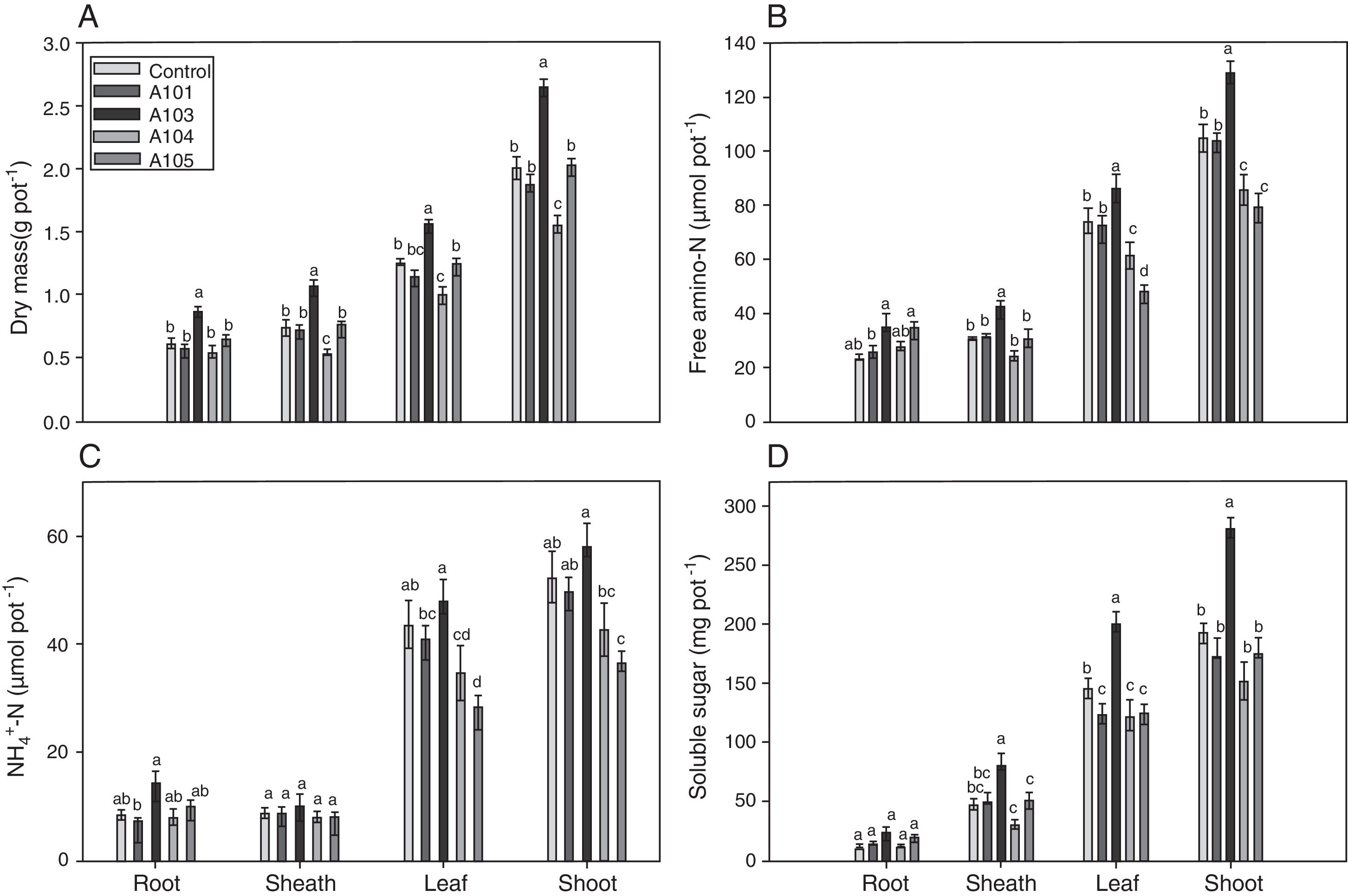
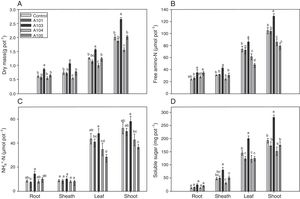
An evaluation of the dry matter showed that the plants that were inoculated with isolate A103 had higher values for the roots, sheathes, leaves and shoots, with increases of 44%, 43%, 25% and 32%, respectively, in comparison with the control, indicating that this fungus contributes to rice plant growth (Fig. 4A). However, the inoculation with A104 caused reductions of 24%, 11% and 22% in the dry matter of the sheaths, leaves and shoots, respectively (Fig. 4A), and isolates A101 and A105 did not influence the plant dry matter (Fig. 4A). Likewise, the heights of the plants were influenced positively by A103 inoculation. However, A104 caused a negative effect, and there was no effect from A101 and A105 in comparison with the control (Table 1). The higher root mass probably indicates the greater presence of lateral and secondary roots and root hairs, which is a characteristic of roots that are morphologically adapted for a more efficient uptake of nutrients. This trait is desirable, primarily because it is an important attribute for the absorption of nutrients such as N and P under low soil fertility conditions.48,63,64
Dry mass (A), the contents of free amino-N (B), free NH4+-N (C) and soluble sugars (D) as determined at 35 DAG in rice plants (Piauí variety) without inoculation (white bar) and those inoculated with the following different dark septate fungal isolates: A101 (checkered bars), A103 (closed bars), A104 (grey bars) and A105 (striped bars). Different letters within a given plant tissue (root or sheath or leaf or shoot) indicate significant differences among the treatment at p<0.05 (t-test (LSD)). The error bars are standard error (n=4). The means of four composed repetitions (each repetition had four plants per pot). The shoot biomass is the sum of the sheathes and leaves.
The results of the dry matter and plant height evaluations were widely variable among the fungal isolates. The A103 isolate promoted the greatest accumulation of biomass and the greatest elongation of the shoot, which was previously observed. For example, in Dendrobium nobile65 and Saussurea involucrata,66 DSE inoculation also promoted greater plant heights and biomasses in the shoots and roots in comparison with the control plants. Moreover, the inoculation of the host plants (blueberries) and birch seedlings with A. applanata and A. macrosclerotiorum showed increasing dry shoot weights and fresh root weights.29 However, the latter author observed negative effects when the blueberries were inoculated with PAC, which is in accordance with.31
For the dry matter values and heights of the plants treated with A103, there was a larger number of tillers in the plants inoculated with the fungal isolates, with the standout again being the plants that were inoculated with A103, with an increase of 100% compared with the control (Table 1). This more intense tillering likely results from the greater plant vigor that occurs when inoculating with this isolate because other studies have shown a high correlation between the nutritional state and supplementation of carbohydrates.67,68 Newsham69 found that the DSE fungus P. graminicola, which is currently called Harpophora radicicola, was beneficial to the grass Vulpia ciliata because inoculated grasses had increased tiller numbers, root lengths, and dry biomasses for the shoots and roots, when compared with non-inoculated seedlings in a growth room and a glasshouse experiment.
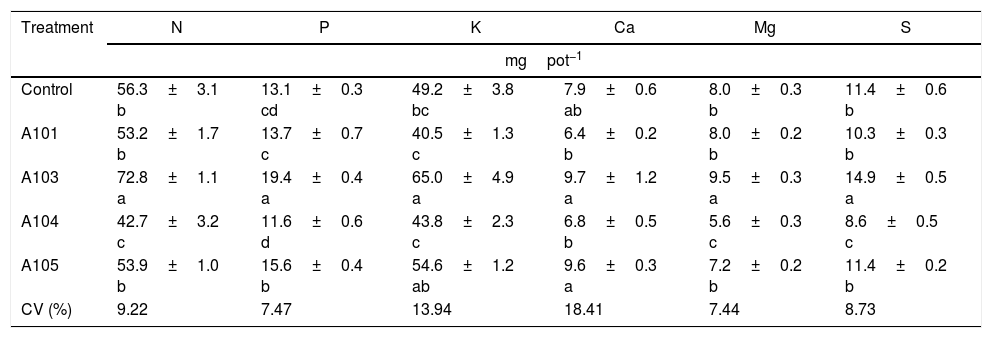
An assessment of the macronutrient contents in the shoots showed that the A103 treatment increased the N, Mg and S contents in relation to the control (by 29, 19 and 30%, respectively), and those levels were lower in the inoculation with A104; there were no differences with A101 and A105 (Table 2). Therefore, A103 inoculation promoted a greater accumulation of these nutrients while the other fungal isolates tended not to produce effects.
Macronutrient contents in the rice plant shoots (Piauí variety), at 35 DAG, with and without inoculation with different dark septate fungal isolates.
| Treatment | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| mgpot−1 | ||||||
| Control | 56.3±3.1 b | 13.1±0.3 cd | 49.2±3.8 bc | 7.9±0.6 ab | 8.0±0.3 b | 11.4±0.6 b |
| A101 | 53.2±1.7 b | 13.7±0.7 c | 40.5±1.3 c | 6.4±0.2 b | 8.0±0.2 b | 10.3±0.3 b |
| A103 | 72.8±1.1 a | 19.4±0.4 a | 65.0±4.9 a | 9.7±1.2 a | 9.5±0.3 a | 14.9±0.5 a |
| A104 | 42.7±3.2 c | 11.6±0.6 d | 43.8±2.3 c | 6.8±0.5 b | 5.6±0.3 c | 8.6±0.5 c |
| A105 | 53.9±1.0 b | 15.6±0.4 b | 54.6±1.2 ab | 9.6±0.3 a | 7.2±0.2 b | 11.4±0.2 b |
| CV (%) | 9.22 | 7.47 | 13.94 | 18.41 | 7.44 | 8.73 |
Means±SE (n=4) followed by the same lower-case letters in the column do not differ significantly (t-test (LSD), p<0.05). DAG, days after germination. Means of four composed repetitions (each repetition had four plants per pot). SE, standard error; N, nitrogen; P, phosphorus; K, potassium; Ca, calcium; Mg, magnesium; S, sulphur.
The 30% higher accumulation of N found in this study in the A103-inoculated plants when ammonium nitrate was applied as the N source appears to indicate that greater N accumulation does not necessarily occur when the N source is organic. This finding might result from the low availability of this type of nitrogen source or the specialization of this fungus for the absorption of mineral N in tropical soils, as previously observed for mycorrhizal fungi.70–73
The P and K contents in the shoots of the plants that were inoculated with A103 and A105 were higher than those of the control plants (for increases of 48 and 32% with A103 and 18 and 10% with A105), and the levels in the plants that were inoculated with A101 and A104 were equal to that of the control. Increases in the P contents in the shoots of DSE-inoculated plants were observed when they were supplemented with an inorganic source of P.12,13 The Ca level was equal for all of the treatments in comparison with the control, even though there was a tendency for higher accumulation in the plants that were inoculated with A103 and A105 (Table 2).
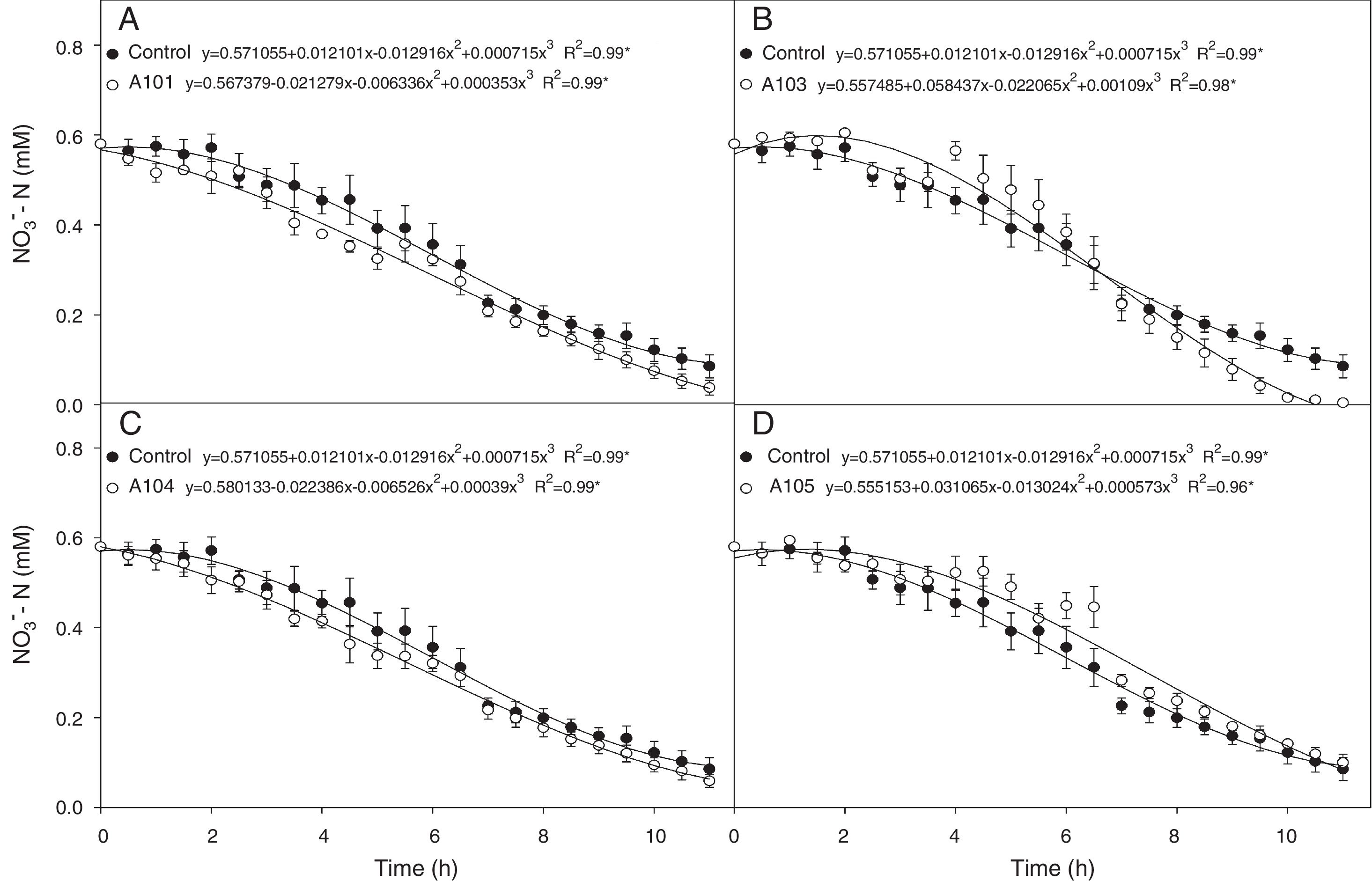
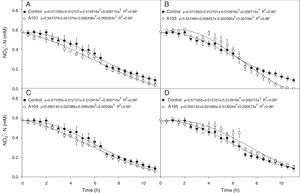
Nitrogen absorption kineticsIn evaluating the NO3− exhaustion rate obtained from the nutrient solution, inoculating with A101 and A103 increased NO3− consumption in comparison with the control treatment (Fig. 5A and B). In general, the absorption speed was higher during the first hours with A101, but in the plants inoculated with A103, the highest absorption speed was observed approximately 7h after inoculation, and the NO3− content of the nutrient solution was depleted 11h after the start of the experiment (Fig. 5B). However, inoculating with A104 and A105 did not cause any change in relation to the control.
Depletion of N-NO3− in the nutrient solution for each rice plant (Piauí variety) inoculated with dark septate fungi A101 (A), A103 (B), A104 (C) and A105 (D), then subjected to 0.5mM of N-NO3− after 72h of N deprivation. * Significant according to the F-test at 5% probability. The error bars are standard error (n=4).
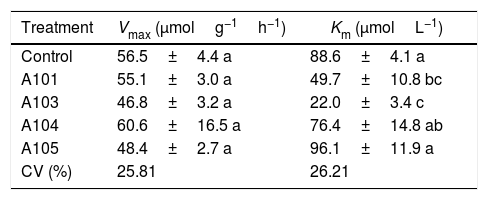
Among the kinetic parameters of NO3− absorption, there was only a significant effect for Km (Table 3). The A101 and A103 inoculation led to lower Km values than the control.
Kinetic parameters Km and Vmax for NO3− absorption as determined at 35 DAG in rice plants (Piauí variety) with and without inoculation with different dark septate fungal isolates.
| Treatment | Vmax (μmolg−1h−1) | Km (μmolL−1) |
|---|---|---|
| Control | 56.5±4.4 a | 88.6±4.1 a |
| A101 | 55.1±3.0 a | 49.7±10.8 bc |
| A103 | 46.8±3.2 a | 22.0±3.4 c |
| A104 | 60.6±16.5 a | 76.4±14.8 ab |
| A105 | 48.4±2.7 a | 96.1±11.9 a |
| CV (%) | 25.81 | 26.21 |
Means±SE (n=4) followed by the same lower-case letters in the column do not differ significantly (t-test (LSD), p<0.05). The means of four composed repetitions (each repetition had four plants per pot). SE, standard error.
Lower Km values were estimated for the A101 and A103 treatments (44 and 75% lower than the control, respectively), indicating greater affinity for transporting the NO3− of the plants that were colonized by these two isolates in comparison with the other treatments. In the same way, the better nitrate uptake efficiency of the colonized tomato root by arbuscular mycorrhizal fungi (AMF) compared with a non-inoculated control was preferentially mediated by a higher expression of NRT 2.3, a member of the high-affinity transport system.74 Another study showed that soil inoculation with AMF increased the plant growth and N uptake of wheat when compared with a non-inoculated control, and it was attributed to upregulation by the AMF of nitrate transporter genes.75
Morphology, physiology and root system development, among other aspects, determine the variations in the absorption kinetics factors (Km, Vmax and Cmin) of plant species. This variation is associated in turn with the adaptation of plants to different ecosystems.48 These morphophysiological traits of the root system are influenced by the microorganisms that are present in the rhizosphere.75,76 Therefore, it can be assumed that the greater NO3− absorption efficiency observed in the A103-colonized plants is explained by the lower Km values caused by the presence of this fungus. This greater efficiency in acquiring NO3− when there are low concentrations in solution could be an indication of plant adaptations to nutritional stress conditions in tropical regions, which are caused by the nitrogen dynamic of the environment.49
Plant soluble fractionThe nitrate contents of the roots, sheathes, leaves and shoots of the rice plants were not influenced by the treatments, confirming the ability of the Piauí variety to accumulate nitrate, along with amino-N in the sheathes, as already observed in previous studies.49,64,77–79 However, the free amino-N content varied depending on the given treatment (Fig. 4B). A103 stood out from the others because the sheathes, leaves and shoots that were inoculated with this isolate presented a 20% increase in the amino-N content in relation to the control (Fig. 4B). This higher accumulation of amino-N indicates a greater influx of N and a better potential to make proteins in the plants that were inoculated with A103 compared with the other treatments. For the A104 and A105-inoculated plants, the leaves and shoots presented lower contents of amino-N than the control plants, with respective reductions of 18% and 24%. The amino-N decrease in the shoots could be related to the transport of amino acids from this part to the root, to supply the metabolic demands of the A104 and A105 fungi because there are reports that some dark septate fungi use free amino acids as their sole source of carbon and nitrogen.27 Other studies have shown that the metabolic activity of mycorrhizal fungi can cause considerable changes in the N metabolism, with a reduced concentration of free amino-N.80–82
The NH4+-N content was generally higher in the leaves than in the roots and sheathes (Fig. 4C). Similar results have been observed in this variety by other authors.64,78,79,83 The inoculation did not influence the NH4+-N contents in the roots and sheathes (Fig. 4C). In contrast, there was lower NH4+-N accumulation in the leaves for A104 and A105 treatments compared with the control (Fig. 4C). In the shoots, with the exception of the A105 treatment, which presented a reduction of 30% in the NH4+-N content, all of the treatments presented NH4+-N contents equal to that of the control. Another study found an NH4+-N reduction in transgenic carrot roots that were inoculated with AMF.82
In general, the soluble sugar contents of the roots were lower than those of the sheathes, which in turn contained a lower content than the leaves (Fig. 4D). Similar results were obtained by49 for the same rice variety. The soluble sugar content of the roots was not influenced by the presence of the fungi (Fig. 4D). By contrast, there was a difference between the treatments in the sheathes, leaves and shoots, with the A103 inoculation causing the highest soluble sugar contents, at an increase of 46% compared with the control. This result indicates that inoculating rice plants (Piauí variety) with A103 can enhance maintenance respiration and plant growth. The higher observed sugar content is an indication of more efficient photosynthesis in the A103-inoculated plants. This finding can be explained by the fact that plants associated with DSE can present higher levels of chlorophyll and better efficiency of photosystem II photochemistry.84 In this case, the establishment of the association between the host plant and DSE should be similar to the effect of mycorrhizae formation, which results in changes in the host plant's physiology, with increases in the chlorophyll levels in the leaves and a consequently higher production of photoassimilates and carbohydrates.85,86
The increases in the root, sheath, leaf and shoot masses along with the greater height and number of tillers in the plants treated with the A103 fungal isolate is an indication of the better nutritional state of these plants as promoted by an association with this fungus. This trend was also evidenced by the higher accumulations of soluble sugars and the more efficient uptake of nutrients, especially nitrogen (as indicated by the lower value of Km).
Concluding remarksThere are important differences in rice plant benefits, depending on the associated fungi and ranging from the promotion of plant growth to no effect. The Pleosporales fungus A103 presented high potential for use in the promotion of rice plant growth, increasing the tillering and absorption of nutrients, especially N (with an enhanced affinity for absorbing this element) and P.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank the Universidade Federal Rural do Rio de Janeiro (UFRRJ), particularly for the Laboratory Nutrition of Plants and Biochemical Plant, the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), especially for LBCT, the Empresa Brasileira de Pesquisa Agropecuária (Embrapa), the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support. We are grateful to Marcus Sperandio, Marcela Jacques, Orlando Huertas, Peter Soares de Medeiros and Guilherme Gomes for their support.