Nocardia is an opportunistic pathogen that causes respiratory infections in immunocompromised patients. The aim of this study was to analyze the epidemiology, clinical significance and antimicrobial susceptibility of Nocardia species isolated from eight children with cystic fibrosis. The isolated species were identified as Nocardia farcinica, Nocardia transvalensis, Nocardia pneumoniae, Nocardia veterana and Nocardia wallacei. N. farcinica was isolated in three patients and all of them presented lung affectation with a chronic colonization and pneumonia. N. farcinica showed resistance against gentamicin, tobramycin, cefotaxime, but was susceptible to trimethoprim-sulfamethoxazole and amikacin. N. transvalensis, which was isolated from two patients, showed an association with chronic colonization. N. transvalensis was resistant to tobramycin and amikacin, but susceptible to ciprofloxacin, trimethoprim-sulfamethoxazole and cefotaxime. N. veterana, N. pneumoniae and N. wallacei were isolated from three different patients and appeared in transitory lung colonization. N. veterana and N. pneumoniae were susceptible to imipenem, trimethoprim-sulfamethoxazole, amikacin, tobramycin, and cefotaxime. N. wallacei was resistant to amikacin, tobramycin, imipenem, and trimethoprim-sulfamethoxazole and susceptible to ciprofloxacin and cefotaxime. All the isolates were identified up to species level by 16S rRNA gene sequencing. The presence of Nocardia in the sputum of patients with cystic fibrosis is not always an indication of an active infection; therefore, the need for a treatment should be evaluated on an individual basis. The detection of multidrug-resistant species needs molecular identification and susceptibility testing, and should be performed for all Nocardia infections.
Cystic fibrosis (CF) is a common autosomal recessive disorder caused by mutation in the CF transmembrane conductance regulator gene.1 The most important complication of CF is a chronic bacterial infection and a concomitant airway inflammation, mostly caused by Staphylococcus aureus and Pseudomonas aeruginosa.2 In purview of this information, an isolation of Nocardia from the respiratory tract of patients with CF is an uncommon finding; and there are very few reports in the literature about this. Thorn et al.,3 for example, have recently reported in the United States that Nocardia asteroides was the most incident species found in 13 out of 17 studied patients, while other isolated species were Nocardia farcinica, Nocardia transvalensis, Nocardia cyriacigeorgica and Nocardia otitidiscaviarum. Despite their presence in the sputum, the clinical significance of these microorganisms in CF patients is considered uncertain,4–6 as their isolation does not necessarily imply an infection and should be considered individually in the clinical context of an individual patient.7
Nocardiae are aerobic actinomycetes ubiquitous in soil and aquatic habitats. Nocardia are beaded, branching Gram-positive rods that are partially acid-fast and generally slow growing. The genus Nocardia contains more than 100 described species8; and about 30 of them are known to cause diseases in human.9 The microorganism was first described by Nocard in 1888 as a fungus but was later classified as an aerobic bacterium under the genus Nocardia, and order Actinomycetales.10Nocardia spp. are able to produce infections such as mycetomas, nodular lymphangitic syndrome, and pneumonia in immunocompromised patients, where pneumonia is most frequently observed. There are possibilities of the dissemination of a primary pulmonary infection to almost every organ.11
The main aim of this study was to identify Nocardia isolates obtained from Spanish children with cystic fibrosis at a species level, test their antimicrobial susceptibility, and assess the clinical significance.
Material and methodsNocardia spp. were isolated in eight out of 37 patients being followed between 1995 and 2008 in the CF unit of Miguel Servet Hospital. Zaragoza. Spain. Sputum specimens were collected from patients coming for a routine microbiological investigation, and the samples were grown on common culture media (sheep blood agar, chocolate agar, MacConkey agar and Sabouraud chloramphenicol agar plates all from Oxoid (UK).12 All spontaneous sputa were subjected to Gram staining.13 In the case, when Gram-positive branching filamentous organisms were observed, the sample was inoculated onto an additional plate of buffered charcoal yeast extract agar. All plates were incubated for 14 days before being discarded as negative.
The evaluated variables were age, sex, clinical manifestations, underlying treatment, clinical course and additional organisms isolated from the patients infected or colonized by Nocardia spp.
The identification of a microorganism as member of the genus Nocardia was based on macroscopic, microscopic, and biochemical characteristics (physical features of colonies, microscopic morphology and Gram and modified acid-fast staining, casein, xanthine and tyrosine hydrolysis, opacification of Middlebrook 7H10 agar, arylsulfatase production after 14 days of incubation) as described by Boiron et al.14
The susceptibility of the isolates to different antimicrobials was determined by broth microdilution method, as recommended by the CLSI for antimicrobial susceptibility testing of Nocardiae.15 Appropriate dilutions for MIC determinations were obtained by employing EMIZA 9EF Sensititre® plates (Izasa). The recommended control reference strains (Escherichia coli ATCC® 35218 and S. aureus ATCC 29213®) were also used as positive controls. Susceptibility testing was done with the recommended primary antimicrobials (amikacin, amoxicillin-clavulanic acid, ciprofloxacin, imipenem, trimethoprim-sulfamethoxazole, and tobramycin) and two secondary antimicrobials (cefotaxime and gentamicin). The plates were incubated at 37°C for 72h and read manually with a mirrored box. The MICs were interpreted according to CLSI.15
The species level identification was done by sequencing a 606bp long fragment of the 16S rRNA gene. DNA extraction, PCR amplification, and sequencing were carried out by using primers Noc1 (5′-GCT TAA ACC ATG CAA GTCG-3′) and Noc2 (5′-GAA TTC CAG TCT CCC CTG-3′) as described previously.16 Each strain was sequenced in both the senses in order to determine a consensus sequence. To determine the percentage of similarity to the closest type strain, each sequence was used as a query using BLAST search against the GenBank database.
ResultsEight cases (2 male, 6 female; median age 13 years, range 10–17 years) of nocardial infections or colonization in CF patients were diagnosed between 1995 and 2008.
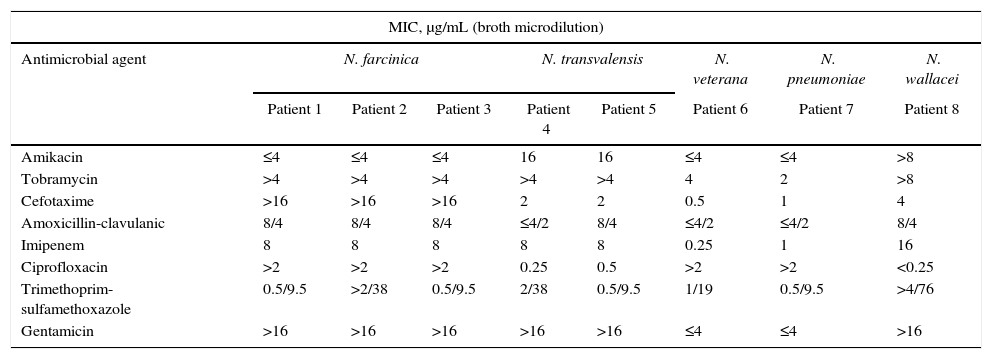
The isolated Nocardia species were identified as N. farcinica (3 cases, 100% of sequence similarity), N. transvalensis (2 cases, 100% of sequence similarity), Nocardia pneumoniae (1 case, 99% of sequence similarity), Nocardia veterana (1 case, 99% of sequence similarity) and Nocardia wallacei (1 case, 100% of sequence similarity). These isolates were tested for antimicrobial susceptibility; and their in vitro patterns of antibiotic susceptibility are shown in Table 1.
In vitro antimicrobial susceptibility patterns of Nocardia in CF patients.
| MIC, μg/mL (broth microdilution) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antimicrobial agent | N. farcinica | N. transvalensis | N. veterana | N. pneumoniae | N. wallacei | |||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
| Amikacin | ≤4 | ≤4 | ≤4 | 16 | 16 | ≤4 | ≤4 | >8 |
| Tobramycin | >4 | >4 | >4 | >4 | >4 | 4 | 2 | >8 |
| Cefotaxime | >16 | >16 | >16 | 2 | 2 | 0.5 | 1 | 4 |
| Amoxicillin-clavulanic | 8/4 | 8/4 | 8/4 | ≤4/2 | 8/4 | ≤4/2 | ≤4/2 | 8/4 |
| Imipenem | 8 | 8 | 8 | 8 | 8 | 0.25 | 1 | 16 |
| Ciprofloxacin | >2 | >2 | >2 | 0.25 | 0.5 | >2 | >2 | <0.25 |
| Trimethoprim-sulfamethoxazole | 0.5/9.5 | >2/38 | 0.5/9.5 | 2/38 | 0.5/9.5 | 1/19 | 0.5/9.5 | >4/76 |
| Gentamicin | >16 | >16 | >16 | >16 | >16 | ≤4 | ≤4 | >16 |
N. farcinica could be isolated from three patients and showed a rather typical multidrug resistance pattern, characterized by resistance to cefotaxime, gentamicin, tobramycin; susceptibility to amikacin, amoxicillin-clavulanic acid and trimethoprim-sulfamethoxazole; and intermediate susceptibility to imipenem; this was similar to previous reports for the same species.17,18 The three patients having N. farcinica infection were treated with trimethoprim-sulfamethoxazole, although one of the isolates became resistant during the treatment. The patients with N. farcinica presented lung affectation with chronic colonization and pneumonia. Two of them showed bronchiectasis in the course of disease evolution and the other one showed a good clinical evolution.
The susceptibility pattern of N. transvalensis was similar to the previously published data17 (Table 1). The two patients with N. transvalensis presented an identical susceptibility pattern and the same clinical significance (chronic colonization) (Table 2). They were treated with trimethoprim-sulfamethoxazole, and one died because of massive hemoptysis.
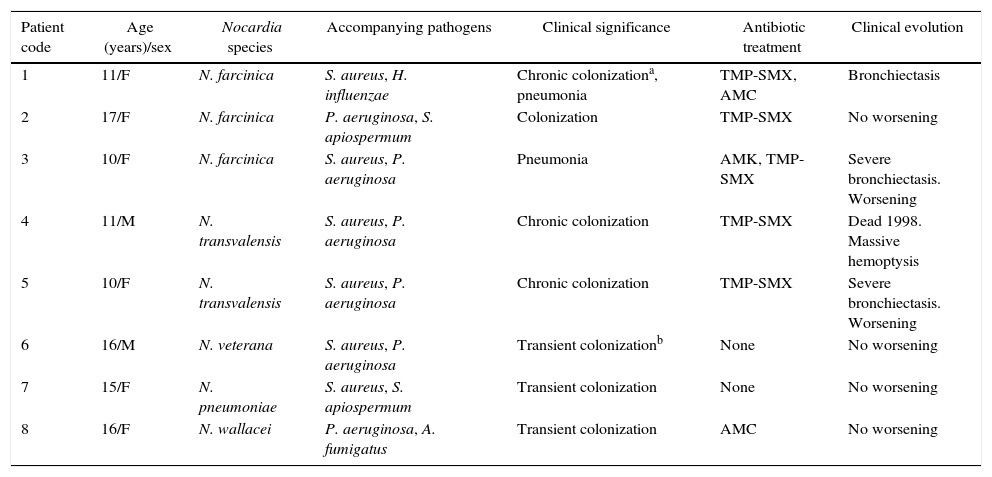
Characteristics of CF patients.
| Patient code | Age (years)/sex | Nocardia species | Accompanying pathogens | Clinical significance | Antibiotic treatment | Clinical evolution |
|---|---|---|---|---|---|---|
| 1 | 11/F | N. farcinica | S. aureus, H. influenzae | Chronic colonizationa, pneumonia | TMP-SMX, AMC | Bronchiectasis |
| 2 | 17/F | N. farcinica | P. aeruginosa, S. apiospermum | Colonization | TMP-SMX | No worsening |
| 3 | 10/F | N. farcinica | S. aureus, P. aeruginosa | Pneumonia | AMK, TMP-SMX | Severe bronchiectasis. Worsening |
| 4 | 11/M | N. transvalensis | S. aureus, P. aeruginosa | Chronic colonization | TMP-SMX | Dead 1998. Massive hemoptysis |
| 5 | 10/F | N. transvalensis | S. aureus, P. aeruginosa | Chronic colonization | TMP-SMX | Severe bronchiectasis. Worsening |
| 6 | 16/M | N. veterana | S. aureus, P. aeruginosa | Transient colonizationb | None | No worsening |
| 7 | 15/F | N. pneumoniae | S. aureus, S. apiospermum | Transient colonization | None | No worsening |
| 8 | 16/F | N. wallacei | P. aeruginosa, A. fumigatus | Transient colonization | AMC | No worsening |
F, female; M, male; TMP-SMX, trimethoprim-sulfamethoxazole; AMC, amoxicillin-clavulanic acid; AMK, amikacin.
N. veterana, N. pneumoniae, and N. wallacei were isolated from transitory lung colonization of three different patients. These patients showed improvement and only one child required antibiotic treatment (Table 2).
The demographic and clinical data of the patients are summarized in Table 2. Among the eight patients with isolation of Nocardia species, two (25%) had a clinical manifestations (pneumonia), and the other six (75%) only presented lung colonization. Other microorganisms isolated from the patients were: S. aureus, P. aeruginosa, Aspergillus fumigatus, Scedosporium apiospermum and Haemophilus influenzae (Table 2).
DiscussionNocardia can cause lung disease, skin disease, systemic disorders involving the central nervous system, and colonize the airways asymptomatically.19 Although the presence of Nocardia has been detected in healthy individuals, the risk is higher in patients with depressed cell-mediated immunity, like patients with neoplastic disease and congenital or acquired immunodeficiency (HIV infection).20 This bacterial group has also been isolated in patients receiving treatment with corticosteroids or immunosuppressive drugs, or having lung disease, such as bronchiectasis, chronic obstructive pulmonary disease, asthma, mycobacterial infection, tuberculosis, chronic granulomatosis, alveolar proteinosis or CF.21 The lung disease altering local airway defense mechanisms is a known risk factor for nocardiosis. However, nocardiosis has only been described sporadically in bronchiectasis and CF.7
In CF patients, the presence of microorganisms in sputum cultures does not always imply a disease. There are few reports in the literature that categorize Nocardia as colonizing bacteria even in the absence of clinical findings.22 A significant level of nocardiosis is usually associated with clinical findings and with a positive Gram-stain of respiratory specimens containing partially branching, acid-fast and Gram-positive bacteria. Therefore, it would be convenient to include Gram staining in the respiratory samples of CF patients, and if any organism resembling Nocardia is observed, selective media culture plates should be included followed by a longer incubation period.
In our group of CF patients, the isolation of N. farcinica or N. transvalensis was associated with pneumonia and a chronic lung colonization, as previously reported,9,23,24 while the isolation of N. veterana, N. pneumoniae and N. wallacei was related with a transient colonization. N. pneumoniae and N. wallacei did not show any clinical relevance in our patients. This fact, in addition with limited published data, does not allow a definite evaluation of their pathogenicity. This study shows that Nocardiae can cause lung colonization in cystic fibrosis patients, similar to other more casual bacterial pathogens (e.g., P. aeruginosa or S. aureus).
The species-specific drug susceptibility pattern of Nocardia and the multidrug resistant species isolated in our patients, as N. farcinica, suggest that new molecular methods like 16S rRNA gene sequencing for Nocardia identification are crucial in determining the need of a specific and individualized antibiotic treatment. On the other hand, identification by standard methods is a long process25 that can delay a timely antibiotic therapy; therefore, the development of molecular methods will facilitate a rapid diagnosis,26,27 epidemiological studies, and the prompt initiation of the appropriate antibiotic therapy in CF patients with clinical symptoms.
Nocardiosis is often difficult to treat. The choice of therapy should therefore be guided by in vitro susceptibility testing. The situation can be further complicated as different strains of the same species may display different antibiotic sensitivities.
However, a sulfonamide containing regimen is still considered as a treatment of choice, though some species show in vitro resistance. Whether this is an indication of the treatment failure needs further investigation.28
There are currently no guidelines as to when (immediately or not) and how Nocardia infections should be treated. However, in the literature, treatments from 6 to 12 months are recommended for serious lung infections or in patients with strong immunosuppression. If symptoms are few, treatment could be reduced to 1–3 months.6 When cystic fibrosis is involved, the duration of treatment is less defined. But still in such a case, for serious infections, treatment should also last from 6 to 12 months; and if symptoms are few, it may be spanned from 2 to 3 weeks, as in cases of exacerbations by other microorganisms.4
Molecular techniques have made identification of many species of Nocardia more rapid and precise providing data for a better management of antibiotic treatment. Further studies involving a larger number of samples using molecular methods27 are needed to determine the most common Nocardia species and their role in lung infection or colonization in CF patients. A high level of variations in the species and susceptibility patterns observed in such a small number of cases require further attention. Usually, in Nocardia infection the first line of treatment is trimethoprim-sulfamethoxazole, but other antibiotics, such as imipenem and amikacin, could be administered in severe infections in children with CF, as reported in the literature.4
Conflicts of interestThe authors declare no conflicts of interest.