Despite recent advances in food production technology, food-borne diseases (FBD) remain a challenging public health concern. In several countries, including Brazil, Clostridium perfringens is among the five main causative agents of food-borne diseases. The present study determines antimicrobial activities of essential oils of six condiments commonly used in Brazil, viz., Ocimum basilicum L. (basil), Rosmarinus officinalis L. (rosemary), Origanum majorana L. (marjoram), Mentha × piperita L. var. Piperita (peppermint), Thymus vulgaris L. (thyme) and Pimpinella anisum L. (anise) against C. perfringens strain A. Chemical compositions of the oils were determined by GC–MS (gas chromatography–mass spectrometry). The identities of the isolated compounds were established from the respective Kováts indices, and a comparison of mass spectral data was made with those reported earlier. The antibacterial activity was assessed from minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using the microdilution method. Minimum inhibitory concentration values were 1.25mgmL−1 for thyme, 5.0mgmL−1 for basil and marjoram, and 10mgmL−1 for rosemary, peppermint and anise. All oils showed bactericidal activity at their minimum inhibitory concentration, except anise oil, which was only bacteriostatic. The use of essential oils from these common spices might serve as an alternative to the use of chemical preservatives in the control and inactivation of pathogens in commercially produced food systems.
Food-borne disease (FBD) is characterized by diverse symptoms that arise from consumption of contaminated foods or beverages. Despite the recent advances in food production technology and processing, FBD remains a major cause of morbidity and mortality, constituting both an important public health concern and a significant economic problem at a global level.1–4
One of the most common causes of FBD is the Gram-positive, anaerobic, spore-forming bacterium Clostridium perfringens (family Clostridiaceae), which is widely distributed in the environment and in foodstuffs. The spores of this bacterium, compared to the vegetative cells, are very robust and resistant to heating, drying, pH and certain toxic compounds. This allows the microorganism to persist until conditions become favorable for germination and growth. There are five strain-types of C. perfringens, designated as A–E. Each of them produces a unique spectrum of exotoxins. Type A strains of the bacterium cause food poisoning in the classical form, while the strains of type C cause necrotic enteritis – a disorder that can be severe and is often fatal but, fortunately, its occurrence is rare. The microorganism prefers substrates, such as meat products, poultry, and sauces, which contain high level of moisture and rich in protein. These are the main classes of food materials involved in occurrence of this disease. Specific factors that favor the spread of this agent are prolonged cooling and non-refrigerated storage, wherein sausage, canned fish, pate, cheese and fermented oyster provide ideal conditions for the development of the bacterium.1,2,5
Many of the spices commonly used in food not only improve palatability and flavor but also assist in the preservation of the food itself. The antimicrobial activity of such spices is bestowed mainly by the essential oils that contain terpenoids hydrocarbons, alcohols, aldehydes, ketones, phenolics and their derivatives. The quantity, quality, and chemical profiles of the essential oils derived from a single plant species can vary considerably according to the geographic origin, climatic conditions, soil composition, the part of the plant used, and the age and season when the material is collected. Besides, drying process and storage time can alter, quantitatively, and qualitatively, the essential oil composition.6 While the antimicrobial activities of essential oils are well reported, the mechanisms of their action are not yet fully understood, although there might be several different microbial target sites.7,8
In the present study, the essential oils of some of the spices most commonly used in Brazil, namely, Ocimum basilicum L. (basil), Rosmarinus officinalis L. (rosemary), Origanum majorana L. (marjoram), Mentha × piperita L. var. Piperita (peppermint), Thymus vulgaris L. (thyme) and Pimpinella anisum L. (anise), were assessed with regard to their antimicrobial activities against C. perfringens strain A. The criteria for selection of plants were their popular use as spices, availability to common people, and ensuring that organic production methods have been used in accordance with the Law: 10,831.9 The chemical compositions of the essential oils were determined by GC–MS (gas chromatography–mass spectrometry), and minimum inhibitory and bactericidal concentrations were determined using the microdilution method.
Materials and methodsPlant materialsDried, fragmented leaves of basil, rosemary, marjoram, peppermint, and thyme and dried fruits of anise were purchased commercially in São Paulo, Brazil, in October 2013. All samples were acquired in accordance with the terms of expiry mentioned on the labels.
Extraction of essential oilsDried leaves of basil, marjoram, peppermint, rosemary and thyme, and the dried fruits of anise were powdered (10–20 mesh) and samples were extracted by hydro-distillation (plant:water ratio 1:10, w/v) for 3.5h in a modified Clevenger apparatus. The oily phase was removed, dried over anhydrous sodium sulfate, and stored in a freezer at –20°C. Microbiological analyses were performed seven days after the extraction.
Chemical compositions of essential oilsEssential oil samples were submitted for GC–MS analysis to the Laboratory of Chromatography, Energy Research Center, Federal University of Roraima, Boa Vista, RR, Brazil. The analyses were performed using Shimadzu GC2010 system with an autoinjector AOC-20i and Plus mass detector QP2110, and equipped with an HP5-MS fused silica capillary column (30m×0.25mm×0.25μm). The chromatographic conditions were as follows: carrier gas, helium at a flow rate of 1.02mLmin–1; oven temperature programmed initially at 60°C and increased to 310°C at a ramp of 3°Cmin–1; injector temperature, 220°C; injector mode in split ratio of 1:20 with 2mLmin–1 purge; MS interface temperature, 280°C; ion source temperature, 260°C; and ionization energy, 70eV. The oil samples (15mg) were dissolved in 1.5mL of purified ethyl acetate and 1μL volume of that was injected for analysis. The isolated compounds were identified by their respective Kováts retention indices determined in reference to a series of n-alkanes, and verified by a comparison of mass spectral data with those obtained using pure standards and with those reported in the literature,10 and eventually by comparing their mass spectra with the GC–MS spectral library (Wiley 8 and FFNSC 1.2 libraries).
For GC-FID, HP-5 MS column (30m×0.25×0.25μm) at same temperature as that of GC–MS, using hydrogen and nitrogen carrier gas, was used. The FID temperature was 260°C. The relative compositions of the oils were calculated from the peak areas (uncorrected for specific response factors) of the isolated compounds.
Antimicrobial activities of essential oilsThe minimum inhibitory concentration (MIC) and the MBC of the oil samples were assessed against type A strain of C. perfringens ATCC 13124 (a gas gangrene isolate) using the microdilution method.
Standardization of inoculumC. perfringens (ATCC 13124) was revived according to standard procedures in tryptose sulfite-cycloserine agar (Oxoid) with d-cycloserine (Sigma) under anaerobic conditions at 36±1°C for 24h.11 The bacterial concentration in the inoculum was standardized at 0.5 on the McFarland turbidity scale, equivalent to 108CFUmL–1. An aliquot (1mL) of this suspension was transferred to a sterile tube and the volume was adjusted to 10mL with sodium chloride solution (0.8%, w/v) to obtain a concentration of 107CFUmL–1. Working inoculums were prepared by transferring 200μL aliquots of this suspension to three test tubes and adjusting the volumes to 10mL with reinforced clostridial medium (RCM; Oxoid) to give final concentrations of 2.0×105CFUmL–1.12
Minimal inhibitory concentrationsAll microbiological assays were performed under anaerobic conditions. MIC determinations were performed in 96-well microplates according to procedures described by the Clinical and Laboratory Standards Institute.13 Each essential oil (200mg) was dissolved in dimethyl sulfoxide (40μL) and the volume was made to 5mL with sterile RCM containing 1% Tween 80 to provide a stock solution containing 40mgmL–1 of oil. Serial twofold dilutions of each essential oil stock were made with RCM to yield final concentrations ranging from 20 to 0.625mgmL–1. The diluted samples (100μL) were transferred to microplate wells and mixed well with the micropipette. Chloramphenicol was employed as a positive control in the concentration range 0.1–0.003125mgmL–1, while the negative controls comprised sterile RCM either alone or with dimethyl sulfoxide (at concentrations used in the dilutions). In order to ascertain aseptic conditions, the control wells contained sterile RCM but without inoculum. The inoculated microplates were incubated at 36±1°C for 48h under anaerobic conditions; and the bacterial growth was confirmed by adding 10μL of a sterile 0.5% aqueous solution of triphenyltetrazolium chloride (TTC, Sigma–Aldrich) and incubating at 36°C for 30min.14 The viable bacterial cells reduced the yellow TTC to pink/red 1,3,5-triphenylformazan (TPF). All assays were performed in triplicate.
Minimum bactericidal concentrationsMBCs were determined by inoculating the assay mixtures from the wells showing no microbial growth onto the surface of sterile Shahidi-Ferguson Perfringens agar medium as recommended by the Ministério da Agricultura, Pecuária e Abastecimento.15 The plates were incubated under anaerobic conditions for 24h in an oven maintained at 36±1°C and subjected to visual inspection. The presence of microbial growth on the medium indicated that the essential oil possessed bacteriostatic activity, while the absence of the growth implied bactericidal activity of the oil sample.
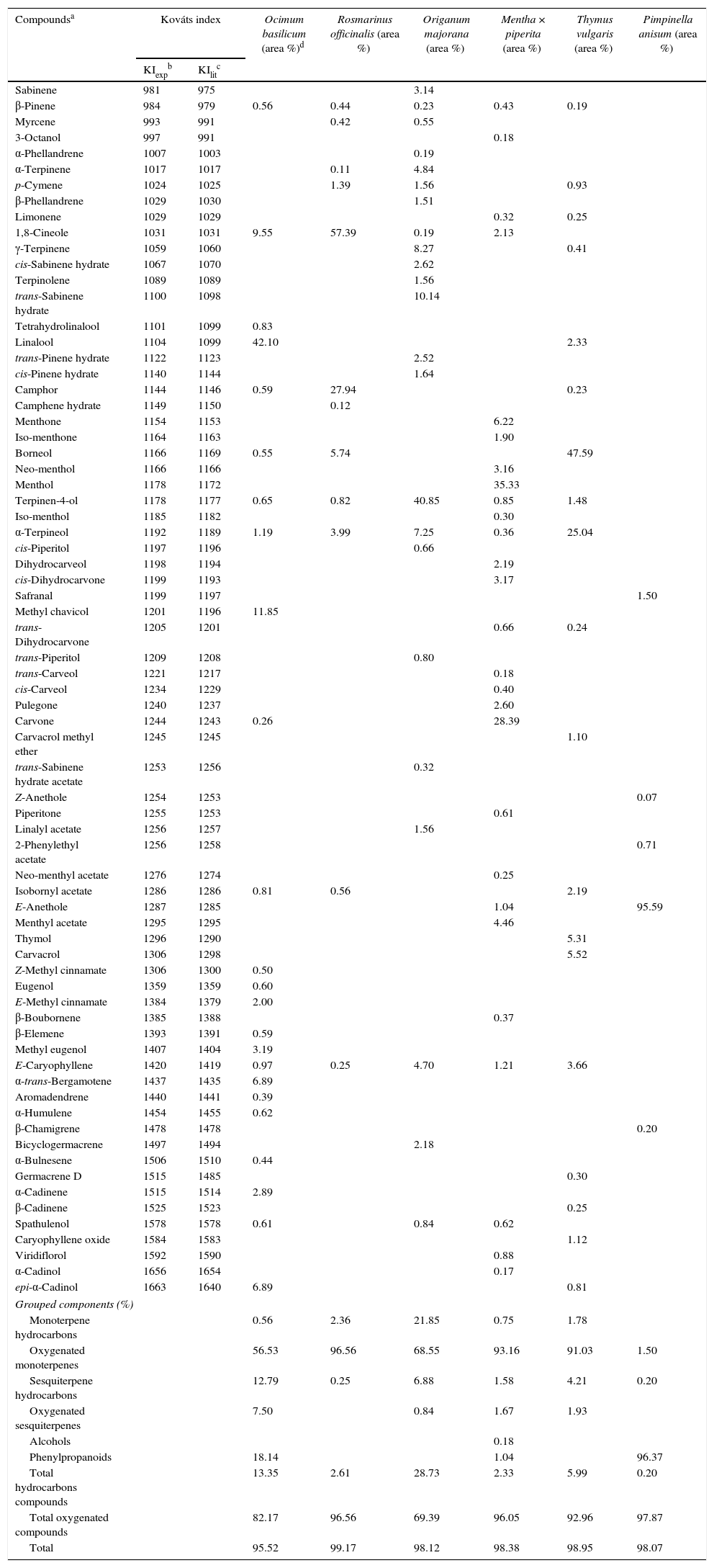
Results and discussionYields and chemical profiles of the essential oilsThe yields of the essential oils were 0.24% for basil, 1.57% for rosemary, 0.47% for marjoram oil, 0.49% for peppermint, 0.97% for thyme and 1.29% for anise.
As shown in Table 1, the most abundant compounds in all of the essential oils were oxygenated compounds, especially oxygenated monoterpenes and phenylpropanoids. The combination of oxygenated monoterpenes and sesquiterpenes, phenylpropanoids and alcohols accounted 82.17% of the oxygenated compounds for O. basilicum, 96.56% for R. officinalis, 69.39% for O. majorana, 96.05% for Menta × piperita, 92.96% for T. vulgaris and 97.87% for P. anisum.
Chemical composition of the essential oils obtained from the spices.
| Compoundsa | Kováts index | Ocimum basilicum (area %)d | Rosmarinus officinalis (area %) | Origanum majorana (area %) | Mentha × piperita (area %) | Thymus vulgaris (area %) | Pimpinella anisum (area %) | |
|---|---|---|---|---|---|---|---|---|
| KIexpb | KIlitc | |||||||
| Sabinene | 981 | 975 | 3.14 | |||||
| β-Pinene | 984 | 979 | 0.56 | 0.44 | 0.23 | 0.43 | 0.19 | |
| Myrcene | 993 | 991 | 0.42 | 0.55 | ||||
| 3-Octanol | 997 | 991 | 0.18 | |||||
| α-Phellandrene | 1007 | 1003 | 0.19 | |||||
| α-Terpinene | 1017 | 1017 | 0.11 | 4.84 | ||||
| p-Cymene | 1024 | 1025 | 1.39 | 1.56 | 0.93 | |||
| β-Phellandrene | 1029 | 1030 | 1.51 | |||||
| Limonene | 1029 | 1029 | 0.32 | 0.25 | ||||
| 1,8-Cineole | 1031 | 1031 | 9.55 | 57.39 | 0.19 | 2.13 | ||
| γ-Terpinene | 1059 | 1060 | 8.27 | 0.41 | ||||
| cis-Sabinene hydrate | 1067 | 1070 | 2.62 | |||||
| Terpinolene | 1089 | 1089 | 1.56 | |||||
| trans-Sabinene hydrate | 1100 | 1098 | 10.14 | |||||
| Tetrahydrolinalool | 1101 | 1099 | 0.83 | |||||
| Linalool | 1104 | 1099 | 42.10 | 2.33 | ||||
| trans-Pinene hydrate | 1122 | 1123 | 2.52 | |||||
| cis-Pinene hydrate | 1140 | 1144 | 1.64 | |||||
| Camphor | 1144 | 1146 | 0.59 | 27.94 | 0.23 | |||
| Camphene hydrate | 1149 | 1150 | 0.12 | |||||
| Menthone | 1154 | 1153 | 6.22 | |||||
| Iso-menthone | 1164 | 1163 | 1.90 | |||||
| Borneol | 1166 | 1169 | 0.55 | 5.74 | 47.59 | |||
| Neo-menthol | 1166 | 1166 | 3.16 | |||||
| Menthol | 1178 | 1172 | 35.33 | |||||
| Terpinen-4-ol | 1178 | 1177 | 0.65 | 0.82 | 40.85 | 0.85 | 1.48 | |
| Iso-menthol | 1185 | 1182 | 0.30 | |||||
| α-Terpineol | 1192 | 1189 | 1.19 | 3.99 | 7.25 | 0.36 | 25.04 | |
| cis-Piperitol | 1197 | 1196 | 0.66 | |||||
| Dihydrocarveol | 1198 | 1194 | 2.19 | |||||
| cis-Dihydrocarvone | 1199 | 1193 | 3.17 | |||||
| Safranal | 1199 | 1197 | 1.50 | |||||
| Methyl chavicol | 1201 | 1196 | 11.85 | |||||
| trans-Dihydrocarvone | 1205 | 1201 | 0.66 | 0.24 | ||||
| trans-Piperitol | 1209 | 1208 | 0.80 | |||||
| trans-Carveol | 1221 | 1217 | 0.18 | |||||
| cis-Carveol | 1234 | 1229 | 0.40 | |||||
| Pulegone | 1240 | 1237 | 2.60 | |||||
| Carvone | 1244 | 1243 | 0.26 | 28.39 | ||||
| Carvacrol methyl ether | 1245 | 1245 | 1.10 | |||||
| trans-Sabinene hydrate acetate | 1253 | 1256 | 0.32 | |||||
| Z-Anethole | 1254 | 1253 | 0.07 | |||||
| Piperitone | 1255 | 1253 | 0.61 | |||||
| Linalyl acetate | 1256 | 1257 | 1.56 | |||||
| 2-Phenylethyl acetate | 1256 | 1258 | 0.71 | |||||
| Neo-menthyl acetate | 1276 | 1274 | 0.25 | |||||
| Isobornyl acetate | 1286 | 1286 | 0.81 | 0.56 | 2.19 | |||
| E-Anethole | 1287 | 1285 | 1.04 | 95.59 | ||||
| Menthyl acetate | 1295 | 1295 | 4.46 | |||||
| Thymol | 1296 | 1290 | 5.31 | |||||
| Carvacrol | 1306 | 1298 | 5.52 | |||||
| Z-Methyl cinnamate | 1306 | 1300 | 0.50 | |||||
| Eugenol | 1359 | 1359 | 0.60 | |||||
| E-Methyl cinnamate | 1384 | 1379 | 2.00 | |||||
| β-Boubornene | 1385 | 1388 | 0.37 | |||||
| β-Elemene | 1393 | 1391 | 0.59 | |||||
| Methyl eugenol | 1407 | 1404 | 3.19 | |||||
| E-Caryophyllene | 1420 | 1419 | 0.97 | 0.25 | 4.70 | 1.21 | 3.66 | |
| α-trans-Bergamotene | 1437 | 1435 | 6.89 | |||||
| Aromadendrene | 1440 | 1441 | 0.39 | |||||
| α-Humulene | 1454 | 1455 | 0.62 | |||||
| β-Chamigrene | 1478 | 1478 | 0.20 | |||||
| Bicyclogermacrene | 1497 | 1494 | 2.18 | |||||
| α-Bulnesene | 1506 | 1510 | 0.44 | |||||
| Germacrene D | 1515 | 1485 | 0.30 | |||||
| α-Cadinene | 1515 | 1514 | 2.89 | |||||
| β-Cadinene | 1525 | 1523 | 0.25 | |||||
| Spathulenol | 1578 | 1578 | 0.61 | 0.84 | 0.62 | |||
| Caryophyllene oxide | 1584 | 1583 | 1.12 | |||||
| Viridiflorol | 1592 | 1590 | 0.88 | |||||
| α-Cadinol | 1656 | 1654 | 0.17 | |||||
| epi-α-Cadinol | 1663 | 1640 | 6.89 | 0.81 | ||||
| Grouped components (%) | ||||||||
| Monoterpene hydrocarbons | 0.56 | 2.36 | 21.85 | 0.75 | 1.78 | |||
| Oxygenated monoterpenes | 56.53 | 96.56 | 68.55 | 93.16 | 91.03 | 1.50 | ||
| Sesquiterpene hydrocarbons | 12.79 | 0.25 | 6.88 | 1.58 | 4.21 | 0.20 | ||
| Oxygenated sesquiterpenes | 7.50 | 0.84 | 1.67 | 1.93 | ||||
| Alcohols | 0.18 | |||||||
| Phenylpropanoids | 18.14 | 1.04 | 96.37 | |||||
| Total hydrocarbons compounds | 13.35 | 2.61 | 28.73 | 2.33 | 5.99 | 0.20 | ||
| Total oxygenated compounds | 82.17 | 96.56 | 69.39 | 96.05 | 92.96 | 97.87 | ||
| Total | 95.52 | 99.17 | 98.12 | 98.38 | 98.95 | 98.07 | ||
In the analyzed oil samples, most of the compounds were identified unambiguously from the Kováts index and mass spectral data (Table 1). For basil oil, the major compounds were linalool, methyl chavicol and 1,8-cineole, with trace amounts of α-trans-bergamotene and epi-α-cadinol. Hussain, Anawar, Sherazi, and Przybylski analyzed the essential oils from the aerial parts of O. basilicum harvested in different seasons and found significant variations in the major compounds including linalool (56.70–60.60%), α-bergamotene (7.60–9.20%), γ-cadinene (3.20–5.40%) and epi-α-cadinol (8.60–12.40%).16 Linalool was reported as the major component (66.40%) of the oil extracted from one of the three botanical varieties and cultivars of O. basilicum, while the other two contained α-trans-bergamotene (6.84–7.96%).17
Rosemary oil contains two major compounds, 1,8-cineole and camphor, together with borneol and α-terpineol in much smaller amounts. In a recent evaluation of the antimicrobial activity of R. officinalis, Jiang et al. identified 1,8-cineole (26.54%), α-pinene (20.14%), camphor (12.88%), camphene (11.38%) and β-pinene (6.95%) as the main constituents of the essential oil. In marjoram oil, the major components were terpinen-4-ol, trans-sabinene hydrate, γ-terpinene and α-terpineol.18 Busatta et al. evaluated the antimicrobial activity of the essential oil from dried leaves of O. majorana used in a processed food, and found main constituents as terpinen-4-ol (30.41%), γ-terpinene (13.94%), cis-sabinene hydrate (9.64%), and α-terpineol (4.47%).19
In peppermint oil, the major constituents are menthol and carvone with minute amounts of menthone and menthyl acetate. Tyagi and Malik reported the main constituents of M. piperita oil as menthol (19.10%), iso-menthone (14.80%), menthone (14.80%), limonene (10.60%), iso-menthol (8.80%), menthyl acetate (6.60%), β-pinene (5.60%) and α-pinene (4.80%).20 The essential oil of thyme analyzed herein contained borneol and α-terpineol as the major compounds, together with trace amounts of thymol and carvacrol. Rota, Herrera, Martinez, Sotomayor, and Jordan determined the chemical composition and antimicrobial activity of the essential oil of T. vulgaris (thymol chemotype) and identified the main constituents as thymol (57.70%), p-cymene (18.70%) and carvacrol (2.80%).21
The oil derived from the dried fruits of anise contained almost entirely (95.59%) E-anethole. Tepe et al. studied the antioxidative and antimicrobial activities of P. anisetum and found E-anethole as the main constituent, accounting for 82.80% of the total oil.22
The differences between the essential oil profiles reported herein and those in other studies can be attributed to the use of commercially available dried plant materials rather than the fresh plant parts.
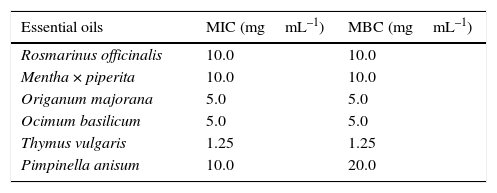
Antimicrobial activities of essential oilsIncubation of the assay plates for 48h was sufficient for all negative controls to show significant microbial growth (in the form of well-dispersed colonies), while the wells with positive control (chloramphenicol) and the system control (RCM without inoculum) remained clear with no observable colony formation. The presence of viable microorganisms in microplate wells via indication of colony formation was verified by reduction of yellow TTC to pink TPF; this color change was not observed in the wells without any microbial growth. In this way, the exact concentration of each of the essential oils able to inhibit the growth of C. perfringens was determined. The values obtained for MIC and MBC are shown in Table 2.
Minimal inhibitory concentration (MIC) and minimal bactericide concentration (MBC) of essential oils against Clostridium perfringens.a
| Essential oils | MIC (mgmL–1) | MBC (mgmL–1) |
|---|---|---|
| Rosmarinus officinalis | 10.0 | 10.0 |
| Mentha × piperita | 10.0 | 10.0 |
| Origanum majorana | 5.0 | 5.0 |
| Ocimum basilicum | 5.0 | 5.0 |
| Thymus vulgaris | 1.25 | 1.25 |
| Pimpinella anisum | 10.0 | 20.0 |
The essential oil from leaves of T. vulgaris showed the lowest MIC and MBC values (1.25mgmL–1) against C. perfringens, since both values are similar, the oil must be considered to possess strong bactericidal activity. Al-Bayati reported an MIC value of 0.5mgmL–1 for thyme oil against Klebsiella pneumoniae, 0.25mgmL–1 against Salmonella typhi, 0.125mgmL–1 against S. typhimurium, 0.625mgmL–1 against Escherichia coli and Proteus mirabilis, and 0.312mgmL–1 against Staphylococcus aureus and P. vulgaris.23
The essential oils from leaves of O. basilicum and O. majorana showed MIC and MBC values of 5.0mgmL−1 against C. perfringens, indicating that these oils possess bactericidal properties. Lv, Liang, Yuan, and Li determined MIC values of 1.25mgmL–1 for basil oil against E. coli and S. aureus, and 0.625mgmL–1 against Bacillus subtilis.24 In a study on marjoram oil, Busatta et al. determined MIC values of 2.3mgmL–1 against Enterococcus faecalis, Serratia sp. and Streptococcus mutans, 0.92mgmL–1 against Aeromonas sp., E. coli, K. pneumoniae and Salmonella choleraesuis, 0.782mgmL–1 against Shigella flexneri and Staphylococcus aureus, and 0.069mgmL–1 against B. subtilis.19
The MIC and MBC values for R. officinalis and M. piperita oils against C. perfringens were considerably higher (10mgmL–1) than the other samples tested suggesting that these oils possess much weaker bactericidal activities. In the case of rosemary oil extracted from fresh plant material, Okoh, Sadimenko, and Afolayan reported an MIC of 3.75mgmL–1 and an MBC of 7.5mgmL–1 against S. aureus, an MIC of 7.5mgmL–1 and an MBC>7.5mgmL–1 against E. coli, and an MIC of 1.88mgmL–1 and an MBC of 7.5mgmL–1 against B. subtilis.25 For peppermint oil, Tyagi and Malik found an MIC of 1.13mgmL–1 and an MBC of 4.5mgmL−1 against E. coli, an MIC value of 2.25mgmL–1 and an MBC value of 9.0mgmL–1 against Pseudomonas aeruginosa and P. fluorescens, and an MIC value of 1.13mgmL–1 and an MBC value of 2.25mgmL–1 against B. subtilis and S. aureus.20
For the essential oil from fruits of Pimpinella anisum, the MBC value (20mgmL–1) was twofold higher than the MIC value indicating only bacteriostatic activity of the oil at a concentration of 10mgmL–1. Al-Bayati reported MIC values of 0.125mgmL–1 for anise oil against S. aureus and Proteus mirabilis, 0.25mgmL–1 against Salmonella typhimurium, 0.5mgmL–1 against S. typhi and values>0.5mgmL–1 against K. pneumoniae, Pseudomonas aeruginosa and E. coli.23
Although the mechanisms associated with the antimicrobial activities of essential oils are not fully understood; numerous modes of action have been proposed involving, for example, degradation of the bacterial cell wall, modification of proteins of the cytoplasmic membrane, alteration of membrane permeability, inactivation of extracellular enzymes, reduction of intracellular ATP, leakage of cellular contents, coagulation of cytoplasm, and interruption of electron flow and active transport.7,8,26 However, some studies have reported the specific mechanism of action for some oil constituents. Thymol and carvacrol are believed to act by increasing the permeability of cell membranes.27 In this context, Ultee, Bennik, and Moezelaar showed that carvacrol accumulates in the lipid phase of the membrane by changing the conformation of the phospholipid bilayer, which causes expansion of the membrane and leakage of ions, thereby increasing membrane fluidity and permeability.28p-Cymene also accumulates in large amounts and acts by causing expansion of the membrane phospholipids by increasing spaces through which ion leakage might occur.28 In the case of carvone, the compound is believed to be partitioned in the lipid membrane, thereby disturbing selective barrier function and the conservation of metabolic energy.29 Terpinen-4-ol, on the other hand, has been shown to inhibit cellular respiration and to damage the structure of the cell membrane attenuating its role as a permeable barrier.30 However, in general, it is believed that the antimicrobial efficacy of an essential oil is not associated exclusively with a specific constituent but rather a synergistic effect of all of the constituents contained.
ConclusionsThe results obtained in this study demonstrate that the essential oils of basil, rosemary, marjoram, peppermint, thyme and anise exhibit in vitro antimicrobial activities against C. perfringens. The essential oil from T. vulgaris showed the lowest MIC value among all of the oils tested and was the most effective bactericide against one of the main causes of food poisoning in Brazil. The use of essential oils from commonly employed spices clearly offers an alternative to the chemical preservatives in the control and inactivation of pathogens in food, but further studies are needed in order to verify direct application in commercially produced food systems. The results suggest that the oxygenated compound, especially oxygenated monoterpenes and phenylpropanoids, might be responsible for the antimicrobial activity against C. perfringens, but the synergistic effects of these chemicals with other minor constituents of the essential oil should also be considered.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Microbiology Laboratory at Univates for the C. perfringens strain and technical assistance with microbiological assays.