Dermatophytes are classified in three genera, Epidermophyton, Microsporum and Trichophyton. They have the capacity to invade keratinized tissue to produce a cutaneous infection known as dermatophytoses. This investigation was performed to study the effect of gaseous ozone and ozonized oil on three specific properties of six different dermatophytes. These properties included sporulation, mycelia leakage of sugar and nutrients and the activity of their hydrolytic enzymes. Generally, ozonized oil was found to be more efficacious than gaseous ozone. Microsporum gypseum and Microsporum canis were the most susceptible, while Trichophyton interdigitale and T. mentagrophytes were relatively resistant. The study revealed a steady decline in spore production of M. gypseum and M. canis on application of ozonated oil. An increase in leakage of electrolytes and sugar was noticed after treatment with ozonized oil in the case of M. gypseum, M. canis, T. interdigitale, T. mentagrophytes and T. rubrum. The results also revealed loss in urease, amylase, alkaline phosphatase, lipase and keratinase enzyme producing capacity of the investigated fungi.
Dermatophytosis constitutes an important public health problem, not only in underdeveloped countries but also in elderly and immuno-compromised patients worldwide.1,2
The treatment with systemic antifungal chemical agents such as ketoconazole, fluonazole and itraconazole derivatives have side effects, in particular, when these chemicals are used for longterm. Therefore, the search for suitable alternatives to these drugs has been going on. One possible approach is to use ozone therapy. The ozone gas molecule has powerful anti-microbial, germicide properties against viruses, bacteria, parasites and fungi. The interaction of ozone molecule with the oxidizable molecules of cellular components particularly those containing double bonds, sulfhydryl groups, and phenolic rings leads to an oxidation reaction that stunts their growth. Hence, membrane phospholipids, intracellular enzymes, and genomic materials are targeted by ozone. These reactions result in cell damage and death of microorganisms.3,4
The cell wall of fungi is multilayered and composed of approximately 80% carbohydrates and 20% of proteins and glycoproteins. The presence of many disulfide bonds making this a possible site for oxidative inactivation by ozone. Ozone has the capacity to diffuse through the fungal wall, enters into its cytoplasm and disrupting vital cellular functions. The inhibitory effect of ozone on spore germination, spore production and biomass production in two Aspergillus species was examined by Antony-Babu and Singleton.5
The reaction of ozone with olive oil occurs almost exclusively with the carbon–carbon double bonds present in unsaturated fatty acids producing different toxic products such as several oxygenated compounds, ozonides, aldehydes and peroxides. These compounds could be also responsible for the wide antimicrobial activity of ozonized olive oil. The safety of oleozone was reported by Gundarova et al. and Alvarez et al.6–9
The aim of this investigation was to study the effect of ozone on the spore germination of various dermatophytes. Since their pathogenecity depends on the activity of keratinolytic and other hydrolysing enzymes, it was important to test the effect of ozone on the production and activity of keratinase, phosphatase, urease, amylase and lipase.
Materials and methodsTest organismsFive dermatophyte species (Microsporum canis, M. gypseum, Trichophyton rubrum, T. mentagrophytes, and T. interdigitales) used in this study were obtained from medical laboratory of microbiology at Kasr elainy hospital and identified by routine mycological procedures. The fungi were separately inoculated into fresh plates of Sabouraud dextrose agar (SDA) “for ozone gas exposure” and into fresh slants of SDA “for ozonized oil treatment”, then incubated for 3 weeks at 28°C. From the culture slants, the spore suspensions were prepared to be a working suspension of (8×104conidia/ml). This suspension was used for ozonized oil treatment.
Test procedure3.2, 2.0, 1.6, 0.8, 0.4, 0.2 and 0.1μg/ml concentrations of ozonized olive oil were prepared in DMSO and added to spore suspensions of each fungus for 2min. The control remained without treatment. In a parallel experiment, different concentrations of gaseous ozone (20, 16, 12, 8, 4, 2 and 0.5μg/ml) were passed through the culture plates of each tested fungi for 2h. The control remained without exposure. After exposure, the microconidia were harvested and adjusted to 8×104conidia/ml.
Minimum inhibitory concentration (MIC)MIC endpoints for growth were performed by plating 0.01ml of a 1:10 dilution of each adjusted inoculum on SDA plates. The plates were incubated and then examined for the presence of fungal colonies. For MIC endpoints for spore germination, a drop of a 1:400 dilution of each adjusted inoculum was transferred to a glass slide. The MICs were determined as 80% growth and germination inhibition was compared with the control.
Effect of the MICs of ozonized oil on sporulation of fungiFour spore suspension tubes of each tested fungi were prepared. Two of these tubes were treated for 2min with the MIC of ozonized oil (specific for each fungus) and the other two tubes remained without treatment and were considered as control. Conidial germination was counted as log cfu/ml.
Effect of the MICs of ozonized oil on mycelium permeabilityThe mycelium of tested dermatophytes “previously treated with MIC (for growth) of ozonized oil” was filtered off and washed thoroughly with sterile distilled water.
Measurement of leakage of electrolytesThe method adopted by Emam was used and the result was expressed as μmohs/g fresh weight.10
Measurement of sugar leakageLeakage from mycelium was determined using the anthrone sulfuric acid method described by Fales and modified by Badour. Sugar amount was expressed as μg/ml and the result was tabulated as % increase in sugar permeability.11,12
Effect of the MICs of ozonized oil on the activity of some enzymes secreted by tested fungiAn inoculum from each organism “treated with its specific MIC of ozonized oil or control” was inoculated into enzyme induction medium. At the end of the growth period, the fungal mycelium and the residual hair were removed and the culture filtrates were tested for enzyme assay.13
Keratinolytic activity was measured by the method of Yu et al., urease activity was measured using the method of Weatherburn with some modifications. Alkaline phosphatase activity was measured by the method of Harsanyit and Dorn. Amylase activity by the method of Kaufman and Tietz, lipase enzyme activity by the method of Lott et al. The results of all were tabulated as % reduction of its activity.14–19
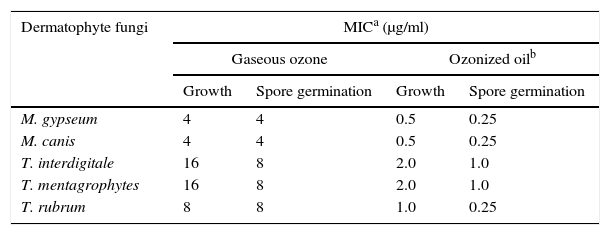
ResultsMinimum inhibitory concentration (MIC) for growth and spore germinationIn Table 1, the MICs for fungal growth and spore germination are shown in presence of gaseous ozone and ozonized oil. The MICs were as high as 16g/ml and 8g/ml for both T. interdigitale and T. mentagrophytes, respectively when treated with gaseous ozone, as compared to 2.0μg/ml and 1.0μg/ml, respectively for the same species treated with ozonized oil. For M. gypseum and M. canis however, the MICs for growth and spore germination were the same at 4μg/ml in the case of ozone applied as gas and was 0.5 and 0.25μg/ml for growth and spore germination, respectively for the same fungi in the case of ozonized oil.
Minimum inhibitory concentration (MIC) of ozone applied as a gas for 2h or as ozonized oil for 2min against growth and spore germination of the tested dermatophyte fungi.
Since the ozonized oil was found to be more efficacious than ozone gas, so we conducted all further experiments focused only on the effect of ozonized oil. Table 2 shows the effect of ozonized oil on sporulation (log CFU/ml) of the tested dermatophytes. The previously determined MIC values (shown in Table 1) of ozonized oil were used. There was a steady reduction in sporulation of M. gypseum and M. canis reaching 98.71 and 97.05%, respectively as compared to the control on application of 0.5μg/ml of ozonized oil. The least reduction in sporulation, under the same conditions, was recorded for T. rubrum (72.6%).
Effect of the minimum inhibitory concentration (MIC)a of ozonized oil (for growth) on sporulation (log CFU/ml) of the tested dermatophyte fungi.
Change in conductance of bathing solutions containing mycelia of the tested dermatophytes previously treated with MIC of ozonized oil is shown in Table 3. There was an increase in percent conductivity and leakage of electrolytes reaching 40.51, 34.87, 20.62, 21.17 and 11.52% of the total conductance in the case of M. gypseum, M. canis, T. interdigitale, T. mentagrophytes and T. rubrum, respectively. Sugar amount also increased reaching 102.6% and 82.7% for M. gypseum and M. canis, respectively (Table 4). Moderate sugar leakage was observed in the case of T. interdigitale and T. mentagrophytes, while the lowest sugar leakage was noticed for T. rubrum.
Change in conductance of the bathing solutions containing mycelia of dermatophyte fungi previously treated with the minimum inhibitory concentration (MIC)a of ozonized oil.
| Dermatophyte fungi | Conductivity after 8h (μmohs/g fresh weight) | Leakage as% of total conductance | ||
|---|---|---|---|---|
| Control | Ozonized oilb | Total conductancec | ||
| Microsporum gypseum | 24.00±2.41 | 44.03±3.82 | 49.44±2.98 | 40.51 |
| M. canis | 24.64±3.98 | 41.90±4.87 | 49.50±6.32 | 34.87 |
| Trichophyton interdigitale | 22.98±3.02 | 32.51±4.01 | 46.22±4.93 | 20.62 |
| T. mentagrophytes | 21.51±2.21 | 31.03±3.18 | 44.97±5.32 | 21.17 |
| T. rubrum | 22.92±3.88 | 28.02±2.92 | 44.29±3.98 | 11.52 |
Sugar amount in bathing solutions after 8h incubation of mycelia of dermatophyte fungi previously treated with the minimum inhibitory concentration (MIC) of ozonized oil.
| Dermatophyte fungi | Sugar amount in bathing solution (μg/ml) | % increase in sugar permeability | |
|---|---|---|---|
| Control | Ozonized oil | ||
| Microsporum gypseum | 154±18 | 312±14 | 102.6 |
| M. canis | 133±09 | 243±15 | 82.7 |
| Trichophyton interdigitale | 108±11 | 159±11 | 47.2 |
| T. mentagrophytes | 110±10 | 171±10 | 55.5 |
| T. rubrum | 95±08 | 131±10 | 37.9 |
Data in Table 5 indicates that, M. canis followed by T. rubrum are potent producers for keratinase (26.32 and 22.00U/ml, respectively) while M. gypseum and T. interdigitale are weak producers (8.21 and 9.14U/ml, respectively). Treatment with MIC of ozonized oil induced steady reduction in keratinase activity in the case of M. gypseum and M. canis reaching 1.05 and 6.11U/ml as compared to their corresponding controls with reduction percentage of 87.21 and 76.79% respectively. Ozonized oil exhibited moderate reduction percentage of 67.49% for T. interdigitale while a reduction around 50% was recorded in the case of T. rubrum.
Effect of the minimum inhibitory concentration (MIC)a of ozonized oil on enzymes activity of selected dermatophyte fungi.
| M. gypseum | M. canis | T. interdigitale | T. mentagrophytes | T. rubrum | ||
|---|---|---|---|---|---|---|
| Keratinase | Control | 8.21±3.86 | 26.32±3.86 | 9.14±2.06 | 18.98±4.50 | 22.00±4.70 |
| Ozonized oilb | 1.05±0.98 | 6.11±3.33 | 4.43±3.04 | 6.17±2.70 | 11.08±5.74 | |
| % reduction | 87.21 | 76.79 | 51.53 | 67.49 | 49.64 | |
| Urease | Control | 1.74±0.12 | 0.97±0.07 | 0.56±0.04 | 0.48±0.09 | 0.88±0.15 |
| Ozonized oil | 0.82±0.37 | 0.204±0.05 | 0.16±0.03 | 0.16±0.07 | 0.20±0.33 | |
| % reduction | 52.87 | 78.97 | 71.43 | 66.67 | 77.27 | |
| Alkaline phosphatase | Control | 28.12±3.00 | 35.92±4.30 | 254.00±12.98 | 229.43±8.60 | 89.98±11.0 |
| Ozonized oil | 1.66±0.52 | 3.83±5.38 | 16.52±7.71 | 18.98±14.87 | 9.93±5.09 | |
| % reduction | 94.10 | 89.34 | 93.50 | 91.73 | 88.96 | |
| Amylase | Control | 59.11±2.22 | 22.07±3.31 | 11.01±2.52 | 8.58±2.49 | 7.77±2.01 |
| Ozonized oil | 21.11±1.21 | 3.61±1.81 | 4.82±1.49 | 3.59±1.30 | 2.82±1.13 | |
| % reduction | 64.29 | 83.64 | 56.22 | 58.16 | 63.71 | |
| Lipase | Control | 4.41±0.96 | 5.71±0.88 | 8.52±1.31 | 9.41±1.09 | 13.53±1.40 |
| Ozonized oil | 1.81±1.11 | 2.75±0.99 | 3.71±1.33 | 4.11±1.87 | 6.65±2.83 | |
| % reduction | 58.96 | 51.84 | 56.46 | 56.32 | 50.85 | |
For urease, the effect of MICs of ozonized oil led to a marked reduction in the enzyme activity ranging from about 71 to 78% in the case of T. interdigitale, T. rubrum and M. canis was observed. Ozonized oil was less effective in the case of M. gypseum, where the urease activity reached 0.82 as compared to 1.74U/ml in the case of the control with a reduction percentage of 52.87%.
Alkaline phosphatase production in the case of T. interdigitale and T. mentagrophytes reached 254.00 and 229.43U/ml, respectively. Application of MIC of ozonized oil induced high levels of reduction for all tested dermatophytes ranging from 94% in the case of M. gypseum to 88.96% in the case of T. rubrum.
For amylase, M. gypseum was the most active in the enzyme production (59.11U/ml). Application of MIC of ozonized oil led to significant reduction in amylase activity for all dermatophytes. Amylase activity reduction in M. canis (83.64%) was greater than M. gypseum (64.29%) and that in T. rubrum (63.71%) was greater than T. mentagrophytes (58.16%) and T. interdigitale (56.22%).
The maximum activity of lipase was 13.53U/ml (reported by T. rubrum) and the minimum activity was 4.41U/ml (reported by M. gypseum). Treatment with MIC of ozonized oil led to reduction of lipase activity for all tested fungi ranging from 50.85% (in the case of T. rubrum) to 58.96% (in the case of M. gypseum).
DiscussionMost of synthetic antifungal drugs have side effects especially when used in long term application. An alternative to chemical drugs is the use ozone therapy. This study reports the evaluation of efficacy of gaseous ozone and ozonized oil as germicidal agents against a few common dermatophytes. We demonstrate the effect of different concentrations of gaseous ozone and ozonized oil on growth and spore germination of the most common five dermatophytes. Generally, we found that the application of ozone in the form of ozonized oil appears to be more efficacious than gaseous ozone. M. gypseum and M. canis were the most susceptible (the MIC for growth and spore germination was 4μg/ml for both fungi in the case of ozone applied as gas and was 0.5 and 0.25μg/ml for the same items in the case of ozonized oil), where T. interdigitale and T. mentagrophytes were relatively resistant (the MIC for growth was 16μg/ml in the case of gaseous ozone and 2.0μg/ml for ozonized oil in the case of both fungi). The increase in susceptibility might be due to the nature of their habitat being zoophilic for the former species and geophilic for the later ones. Weitzman and Summerbell stated that the resistance of T. rubrum to eradication is related to its cell wall. This protective barrier contains mannan, which may inhibit cell-mediated immunity, hinder the proliferation of keratinocytes, and enhance the organism's resistance to the skin's natural defenses.20
Ozonated vegetable oils have been attributed antibacterial and fungicidal effects. The higher toxicity of ozonized oil as compared with gaseous ozone is probably related to the gradual decrease of fatty chain unsaturation as result of long time ozonation, formation of ozonide, increase in peroxide and acid values.21,22 Ozonized oil has shown to be effective against staphylococci, streptococci, enterococci, Pseudomonas, Escherichia coli and especially Mycobacteria and has been utilized for the cure of fungal infections.23–25
The inhibitory effects of ozone on sporulation have considerable commercial potential, because the treatment breaks the infection cycle. The study revealed a steady decline in spore production of M. gypseum and M. canis reaching 98.71 and 97.05%, respectively on application of 0.5μg/ml of ozonated oil. Less reduction in sporulation was noticed for T. interdigitale and T. mentagrophytes and the least reduction was recorded for T. rubrum. This finding is consistent with the reported effects of gaseous ozone on sporulation of Penicillium spp. on citrus fruit.26,27
The present research indicates an increase in leakage of electrolytes and sugar after treatment with MIC of ozonized oil reaching 40.51, 34.87, 20.62, 21.17 and 11.52% of the total conductance for electrolytes and 102.6, 82.7, 47.2, 55.5 and 37.9% for sugar in the case of M. gypseum, M. canis, T. interdigitale, T. mentagrophytes and T. rubrum, respectively. The high leakage may be attributed to the impairment of the membrane permeability which greatly influences the normal physiological functioning of the cells and may result in a change in fractionating the cell into outer protein and/or plasma membrane protein and DNA damage mediated by singlet oxygen.
Mustafa attributed the biological effect of ozone to its stability to cause oxidation or peroxidation of biomolecules directly and/or via free radical reactions and to alteration of membrane permeability, and cell injury or death.28
The fungus–host interactions depend on enzymes produced by dermatophytes and other fungi that facilitate their multiplication within the host. Hence, the role of enzymes is to break down the fatty, protein and scleroproteinous substances present in skin tissues. The present data indicate that dermatophytes are mostly capable of producing different enzymes (keratinase, urease, alkaline phosphatase, amylase and lipase) which play an important role in the pathogenesis.29
The application of ozonized oil was efficacious in producing high loss in enzyme production reaching 78.97% and 83.64% for M. canis, in the case of urease and amylase, respectively, and 94.10% and 58.96% for M. gypseum in the case of alkaline phosphatase and lipase, respectively. Sugita et al. reported that 50% of α-amylase activity in seawater was lost after reaction with only 0.9mg TRO1−1 for 10min ozone showing that ozone treatment may influence the activities of many of the enzymes produced by microorganisms.30
Keratinases are considered the most important virulence factors of dermatophytes in skin infection. Keratinases have been partly isolated and characterized for Microsporum spp., Trichophyton spp. and Scopulariopsis brevicaulis.31–35
The present study revealed that M. canis and T. rbrum showed the highest keratinolytic activities, while T. interdigitale and M. gypseum were of low activities. Treatment with ozonized oil led to steady reduction in keratinase activity ranging from 87.21% in the case of M. gypseum to 49.64% in the case of T. rubrum. Cataldo demonstrated the action of ozone on invertase, pectinase and trypsine and found that ozone was able to show some degree of oxidation of the protein only after prolonged exposure that ozone causes denaturation of the proteins, i.e. introduces changes in their sensitive protein sites as well as secondary and tertiary structure. Cataldo suggested that the changes in protein enzymes may be connected with the partial oxidation of the aromatic monomeric units of the proteins and/or cysteine units.36
The results of the current study are promising and could be extrapolated to the establishment of an alternative anti-fungal strategy based on ozone therapy rather than on chemical drugs.
Conflicts of interestThe authors declare that they have no conflicts of interest in the research.