In the current study, 18 halotolerant and halophilic bacteria have been investigated for their plant growth promoting abilities in vitro and in a hydroponic culture. The bacterial strains have been investigated for ammonia, indole-3-acetic acid and 1-aminocyclopropane-1-carboxylate-deaminase production, phosphate solubilisation and nitrogen fixation activities. Of the tested bacteria, eight were inoculated with Triticum aestivum in a hydroponic culture. The investigated bacterial strains were found to have different plant-growth promoting activities in vitro. Under salt stress (200mM NaCl), the investigated bacterial strains significantly increased the root and shoot length and total fresh weight of the plants. The growth rates of the plants inoculated with bacterial strains ranged from 62.2% to 78.1%.
Identifying of novel halophilic and halotolerant bacteria that promote plant growth can be used as alternatives for salt sensitive plants. Extensive research has been conducted on several halophilic and halotolerant bacterial strains to investigate their plant growth promoting activities. However, to the best of my knowledge, this is the first study to inoculate these bacterial strains with wheat.
All forms of life are dependent on plants as they synthesise oxygen and form the staple food for humans and animals. According to literature, 98% of the world's food requirements are satisfied by 12 plant species and 14 animal species. More than 50% of the world energy intake is met by crops such as wheat, rice and maize.1,2 In addition, none of the 14 animal species can supply the necessary components and nutrition without addition of plants. Therefore, reduction in plant productivity severely affects the lives of many organisms that depend on plants for food and nutrition. Soil salinisation is defined as process of increasing dissolved salts in the soil profile. It severely affects soil health (socio-economic wellbeing) which in turn affects crop productivity.3 Arid and semi-arid lands worldwide have been increasingly facing the issue of soil. Saline soils are estimated to increase at a rate of 7% in the world.4 At a global level, the total amount of saline soils is around 15% in arid and semi-arid regions and approximately 40% in irrigated lands.5 High soil salinity adversely affects the physical and chemical properties of soil, thereby directly affecting the growth and diversity of organisms that live in or on soil such as plants, microbes, protozoa and nematodes. In plants, long-term high soil salinity conditions cause ionic and osmotic stress that adversely affects the functioning of various biochemical processes.6 Under high salinity conditions, plants cope with stress which in turn limits the expansion of the leaves. This indicates that in addition to the closure of stoma, processes such as cell division and expansion are severely affected initially.7,8 During ionic stress premature ageing of leaves occurs, which affects process such as photosynthesis and result in stunted growth.9 Further, excessive sodium and chloride concentrations adversely affect the energy production and physiology of the plants by interfering with various enzymes activities.10 Salt stress results in a significant decrease in productivity of salt-sensitive and salt-tolerant crops. Most the cereal crops have low salinity or salt stress thresholds. For example wheat can tolerates salinity up to 6dSm−1, while the salinity threshold for maize is three times less (approximately 2dSm−1).11 Kotuby-Amazher et al.,12 revealed that, beneficial microorganisms can reduce salt stress in maize and wheat by approximately 50%. In addition, it has been demonstrated that beneficial microorganism play a significant role in alleviating salt stress in plants, resulting in increased crop yield. Plant-growth-promoting (PGP) bacteria are a group of microorganisms that colonise the root of plants or free-living organisms that directly or indirectly enhance the growth of plants.13,14 In direct growth promotion, they produce some compounds (indole acetic acid, siderophore, HCN, etc.), solubilise minerals and break organic matters for easy uptake by plants and for their own use. They also fix atmospheric nitrogen and produce siderophores that enhance the bioavailability of iron and synthesise phytohormones such as cytokinins, auxins and gibberellins which have beneficial roles in various stages of plant growth.15–17 Indirectly, they aid in decreasing or inhibiting the detrimental effects of pathogenic organisms by enhancing the host resistance to pathogenic organisms.18,19
In this study, PGP activities of halophilic and halotolerant bacteria isolated from salt-affected soils of the East Anatolian region (Iğdır and Erzincan provinces) were investigated. To achieve this, 18 bacterial strains for their ability to produce indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylate (ACC) deaminase and ammonia and to fix atmospheric nitrogen and solublise phosphates were tested. Following this, seven PGP bacterial strains (EN1, E3, EN4, EN6, EN8, IA and ID) and one non-PGP bacterial strain (IE) were tested for their effects on the growth of Triticum aestivum in a hydroponic culture.
Material and methodsDetermination of the IAA productionSalkowski's colorimetric method was used to determine the IAA concentration produced by each isolate.20 The pure culture of each isolate was grown in a nutrient broth medium containing 0.1mgmL−1l-tryptophan and 5% NaCl and was incubated at 30°C for 2–4 days. After incubation, the broth was centrifuged, the supernatant was retained and 1mL of supernatant was mixed with 2mL of Salkowski's reagent (2% 0.5 FeCl3 in 35% HCLO4 solution) and kept in the dark for minimum 30min. Subsequently, the optical density (OD) was measured at 530nm.
Determination of ammonia potentialTo test the ammonia production activity, the bacterial isolates were added to peptone water (Peptone 20.0g/L and NaCl 30.0g/L) with constant shaking at 140rpm for 5 days at 30°C. After incubation, 0.2mL of the culture supernatant was mixed with 1mL Nessler's reagent. The OD of the mixture was measured at 450nm using a spectrophotometer,21 and an end point of a brown to yellow colour was evaluated as ammonia production.
Determination of the N-fixation potentialThe nitrogen fixing ability was determined using Burk's modified N-free medium, which contained the following ingredients per litre: sucrose, 10.0g; glucose, 10.0g; K2HPO4, 0.64g; KH2PO4, 0.16g; MgSO4·7H2O, 0.20g; NaCl, 30.0g; CaSO4·2H2O, 0.05g; Na2MoO4·2H2O, (0.05%) 5.0mL; FeSO4·7H2O, (0.3%) 5.0mL and agar, 15g.22
Determination of phosphate solubilisation abilityThe bacterial strains were incubated at 30°C for 7 days with Pikovskaya's modified medium to determine the phosphate solubilisation ability. Pikovskaya's modified medium contained the following per litre: glucose, 10g; Ca3(PO4)2, 5g; (NH4)2SO4, 0.5g; MgSO4·7H2O, 0.1g; KCl, 0.2g; yeast extract, 0.5g; MnSO4·H2O, 0.002g; FeSO4·7H2O, 0.002g, NaCl, 30.0g and agar, 15g.23
Determination of ACC deaminase activityACC deaminase activity was assayed according to a modified method proposed by Honma and Shimomura.24 The bacterial extracts were prepared in 1mL of 0.1M Tris–HCl (pH 7.6) and transferred to a 1.5-mL microcentrifuge tube. The contents of the microcentrifuge tube were centrifuged at 16,000×g for 5min and the supernatant was removed. The pellet was suspended in 600mL 0.1M Tris–HCl (pH 8.5). Subsequently, 30μL toluene was added to the cell suspension and vortexed at the highest setting for 30s. Then, 200μL of the toluenised cells were transferred to a clean 1.5-mL microcentrifuge tube; 20μL of 0.5M ACC was added to the suspension, vortexed for 5s and then incubated at 30°C for 15min. After the incubation, 1mL 0.56M HCl was added to the mixture, vortexed and centrifuged for 5min at 16,000×g at room temperature. To 1mL of this suspension, 800μL of 0.56M HCl was added and mixed in glass tubes. 300μL of 2,4-dinitrophenylhydrazine reagent (0.2% 2,4-dinitrophenylhydrazine in 2M HCl) was added to the glass tube, vortexed and then incubated at 30°C for 30min. The absorbance of the mixture was measured at 540nm after the addition of 2mL of 2N NaOH.25
Determination of the PGP potential of isolatesStrains and culture conditionThe pure bacterial cultures were grown in a nutrient agar for experiments. A single colony from each strain was transferred to a 50mL flask containing the nutrient broth. The colonies were aerobically grown in the flasks overnight on a rotating shaker (200rpm) at 30°C.
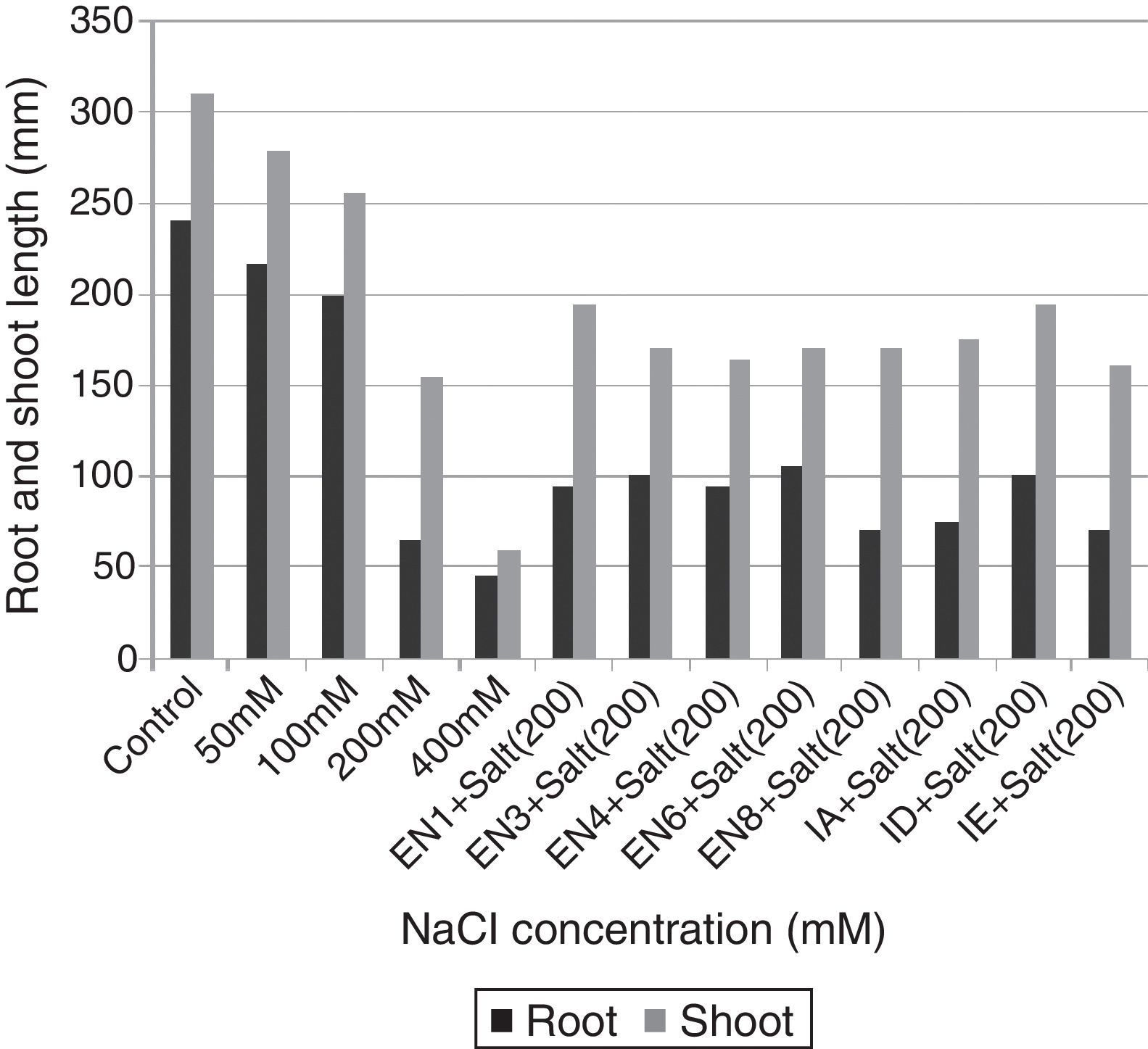
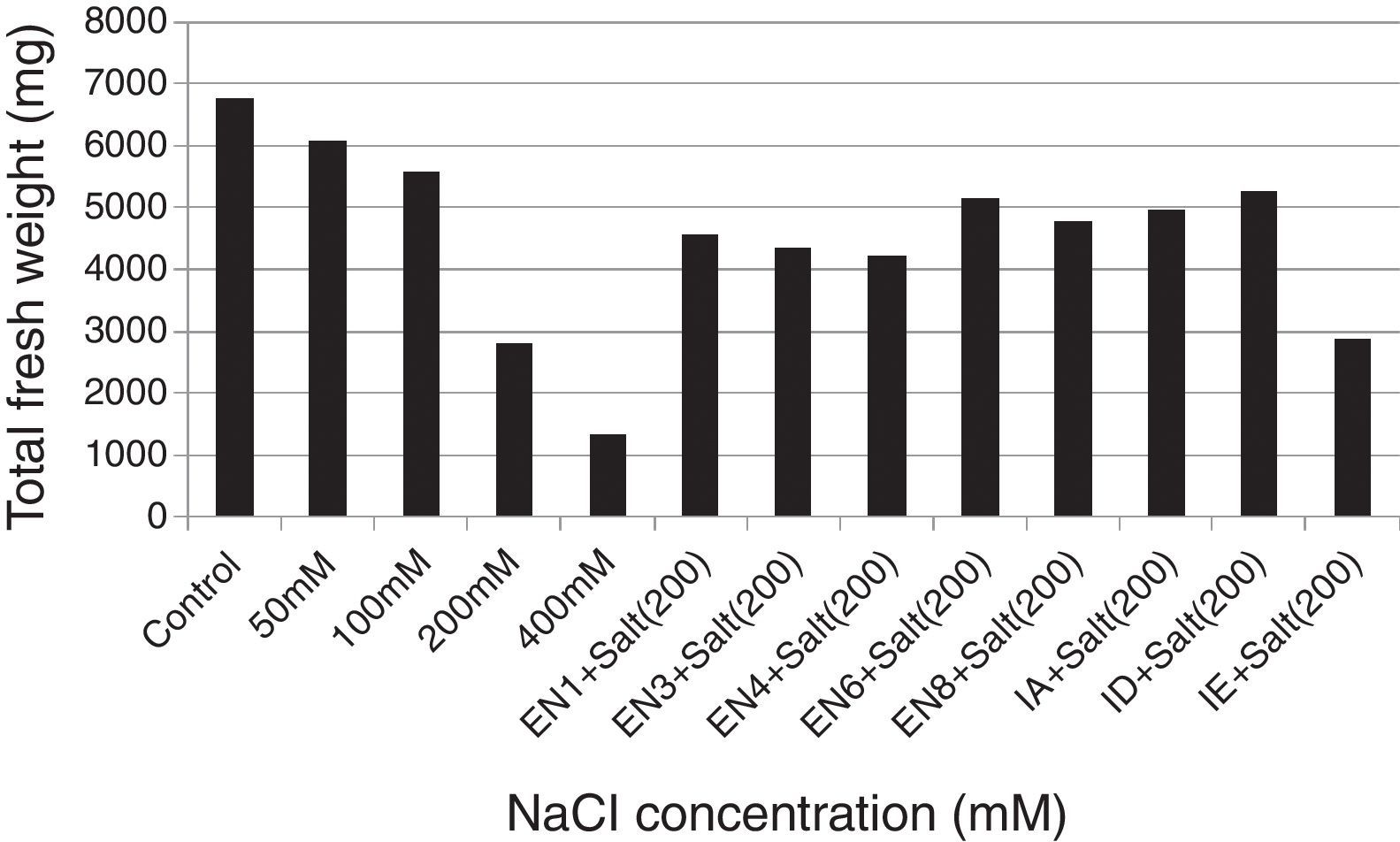
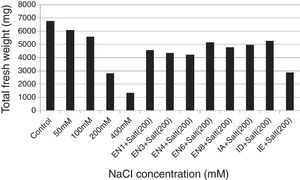
Plant materials and bacterial inoculationWheat seeds (T. aestivum cv. Yıldırım) were obtained from East Anatolian Agricultural Research Institute. The wheat seeds were surface sterilised with 3% sodium hypochlorite for 5min and then washed 5 times with sterilised distilled water. Following the sterilisation, the seeds were allowed to germinate in net cups filled with hydrotons at 30°C for 4 days. The seedlings were sown at a planting density of 25seeds/net cup (4 net cups/pot) containing half-strength Hoagland's medium (pH 6.0) for 10 days. The medium contains KNO3,18.05mg/L; K2SO4, 146.5mg/L; CaCl2·2H2O, 73.5mg/L; MgSO4·7H2O, 51.5mg/L; NaH2PO4·2H2O, 2.51mg/L; FeSO4·7H2O, 1.66mg/L; H3BO3, 0.47mg/L; MnCl2·4H2O, 0.19mg/L; ZnSO4·7H2O, 0.04mg/L; MnCl2·4H2O, 0.19mg/L; CuSO4·5H2O, 0.015mg/L; and H2MoO4, 0.11mg/L.26 To determine the effect of salt stress on plant growth, various concentrations of NaCl (50, 100, 200 and 400mM) were used. The results revealed that although the seedlings of T. aestivum cv. Yıldırım were affected at 50 and 100mM NaCl, the plants growth was the most retarded at 200mM NaCl. Therefore 200mM NaCl concentration was selected for all subsequent experiments. To eliminate the effect of nutrient broth medium on plant growth, the same volume of bacteria-free nutrient broth medium was added to the control and salt application groups. The experiments were designed as follow: Control: Hoagland's medium and 5mL of bacteria-free nutrient broth medium. Salt application: Hoagland's medium containing 200mM NaCl and 5mL of bacteria-free nutrient broth medium. Bacterial application: Hoagland's medium containing 200mM NaCl and 5mL of nutrient broth containing each bacterial strain (the concentration of each strain was 1×109 colony forming unitsmL−1). To provide a homogeneous distribution of nutrients and oxygen for the bacterial strains and plant roots, the hydroponic systems were continuously aerated with an air pump during the experiments. Water lost by evapotranspiration was supplied with the same Hoagland's medium. Each treatment was replicated thrice (the effects of various concentrations of NaCl and bacterial application). On day 10 after the germination period, the plants were analysed for the root and shoot length and total fresh weight.
Statistical analysisThe results are presented as the average means and standard error (SE) of triplicate. The data were further analysed for statistical significance using analysis of variance (ANOVA), and the difference between means was compared by a high-range statistical domain using Tukey's test. A p-value <0.05 indicated statistical significance. The data were discussed in terms of percentage variation, with respect to the control plants.
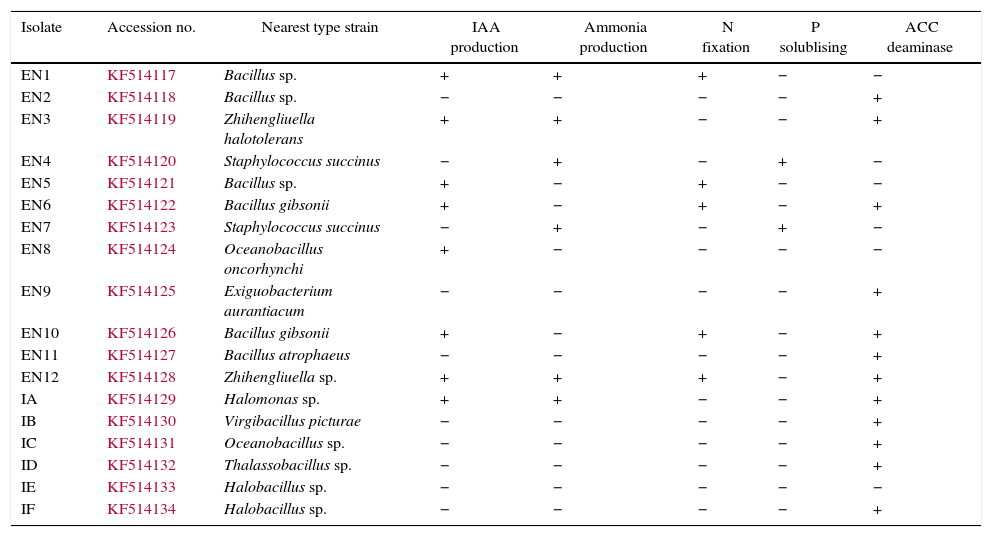
ResultsThe investigated bacterial strains were identified and characterised by conventional (morphology, physiology and biochemical tests) and molecular techniques (16 rDNA).27 In the current study, IAA, ACC deaminase and ammonia production, N-fixation and phosphate solubilisation activities of 18 bacterial isolates (three of these isolates were Bacillus sp., two isolates were Halobacillus sp., two isolates were Bacillus gibsonii, two isolates were Staphylococcus succinus and the others were Zhihengliuella halotolerans, Oceanobacillus oncorhynchi, Exiguobacterium aurantiacum, Bacillus atrophaeus, Zhihengliuella sp., Halomonas sp., Virgibacillus picturae, Oceanobacillus sp. and Thalassobacillus sp.) were investigated. According to the result obtained, approximately 44% of the bacterial strains were found to have IAA production potential. Among the studied isolates, the following showed IAA production potential Bacillus sp. (EN1), Z. halotolerans (EN3), Bacillus sp. (EN5), B. gibsonii (EN6 and EN10), O. oncorhynchi (EN8), Zhihengliuella sp. (EN12), and Halomonas sp. (IA). Ammonia production potential was observed in approximately 33% of the bacterial strains including Bacillus sp. (EN1), Z. halotolerans (EN3), S. succinus (EN4 and EN7), Zhihengliuella sp. (EN12), and Halomonas sp. (IA). Approximately 28% of the bacterial isolates showed nitrogen fixation potential. They include Bacillus (EN1, EN5 EN6 and EN10) and Zhihengliuella (EN12). In addition, only one Bacillus isolate (EN5) was able to solublise phosphate. Approximately 66% of the bacterial isolates showed ACC deaminase potential. The isolates possessing ACC deaminase activity were Bacillus sp. (EN2, EN6, EN10, and EN11), Zhihengliuella sp. (EN3 and EN12), Exiguobacterium sp. (EN9), Halomonas sp. (IA), Virgibacillus sp. (IB), Oceanobacillus sp. (IC), Thalassobacillus sp. (ID) and Halobacillus sp. (IE and IF). Table 1 illustrates the 17 strains that possessed multiple PGP activities and one non-PGP stain. After determining PGP activities, seven different bacterial stains [Bacillus sp. (EN1), Zhihengliuella sp. (EN3), S. succinus (EN4), Bacillus gibsonii (EN6), Oceanobacillus sp. (EN8), Halomonas sp. (IA), and Thalassobacillus sp. (ID), Halobacillus sp.] were used to study plant growth in a hydroponic culture. To determine the detrimental effects of salt stress, various concentrations (50, 100, 200 and 400mM) of NaCl were used testing. The results showed that all the NaCl concentrations reduced the root and shoot length of the plants, and consequently, the total fresh weight of the plants (Figs. 1 and 2). The reduction rates of total fresh weight were 10.1%, 17.4%, 58.4% and 80.3% for 50, 100, 200 and 400mM NaCl, respectively. On the other hand, the supplementation of the PGP bacterial strains significantly increased the root and shoot length and total fresh weight of the plants. The results obtained from bacterial application on plant growth indicate that the reduction caused by NaCl was ameliorated with the application the PGP bacterial strains. The highest amelioration rate was obtained with the ID (Thalassobacillus sp.) followed by EN6 (Bacillus sp.), IA (Halomonas sp.), EN8 (Oceanobacillus sp.), EN1 (Bacillus sp.) EN3 (Zhihengliuella sp.) and EN4 (S. succinus). In other words, the growth rates of the plants were 67.5%, 64.4%, 62.2%, 76.3%, 70.6%, 73.5% and 78.1% for EN1, EN3, EN4, EN6, EN8, IA and ID, respectively. As mentioned previously, 200mM NaCl was used in all experiments. The growth reduction with 200mM NaCl was 58.4%. EN1 ameliorated growth reduction in plants to 15.58%. Similarly, the amelioration rates of the other bacterial strains were 10.27%, 6.50%, 30.65%, 20.89%, 25.85% and 33.73% for EN3, EN4, EN6, EN8, IA and ID, respectively. Only the non-PGP bacterial strain (IE) had no effect on the growth of wheat under salt stress. Figs. 1–3 illustrate the effects of bacterial inoculation on total fresh weight and length of root and shoot of wheat seedlings.
PGPR traits of the bacterial isolates.
| Isolate | Accession no. | Nearest type strain | IAA production | Ammonia production | N fixation | P solublising | ACC deaminase |
|---|---|---|---|---|---|---|---|
| EN1 | KF514117 | Bacillus sp. | + | + | + | − | − |
| EN2 | KF514118 | Bacillus sp. | − | − | − | − | + |
| EN3 | KF514119 | Zhihengliuella halotolerans | + | + | − | − | + |
| EN4 | KF514120 | Staphylococcus succinus | − | + | − | + | − |
| EN5 | KF514121 | Bacillus sp. | + | − | + | − | − |
| EN6 | KF514122 | Bacillus gibsonii | + | − | + | − | + |
| EN7 | KF514123 | Staphylococcus succinus | − | + | − | + | − |
| EN8 | KF514124 | Oceanobacillus oncorhynchi | + | − | − | − | − |
| EN9 | KF514125 | Exiguobacterium aurantiacum | − | − | − | − | + |
| EN10 | KF514126 | Bacillus gibsonii | + | − | + | − | + |
| EN11 | KF514127 | Bacillus atrophaeus | − | − | − | − | + |
| EN12 | KF514128 | Zhihengliuella sp. | + | + | + | − | + |
| IA | KF514129 | Halomonas sp. | + | + | − | − | + |
| IB | KF514130 | Virgibacillus picturae | − | − | − | − | + |
| IC | KF514131 | Oceanobacillus sp. | − | − | − | − | + |
| ID | KF514132 | Thalassobacillus sp. | − | − | − | − | + |
| IE | KF514133 | Halobacillus sp. | − | − | − | − | − |
| IF | KF514134 | Halobacillus sp. | − | − | − | − | + |
−: negative.
+: positive.
Extensive research has been conducted to unravel the beneficial effects of halotolerant and halophilic microorganisms on plant growth.28–31 IAA is a phytohormone that involved in root initiation, cell enlargement and cell division; therefore, the IAA production activity of halotolerant and halophilic microorganisms is crucial for plant growth. IAA-producing microorganisms increase the root growth and root length of plants, which constitutes a greater root surface area that enables the plant to get more nutrients from the soil.32 Another critical function of PGP bacteria include fixation of atmospheric nitrogen. Nitrogen plays an important role in limiting the growth and productivity of the plants. All organisms need nitrogen-containing compounds, which are essential components of various bio-molecules such as amino acid, nucleic acid, ATP and NADH. Ammonia production is as important as nitrogen fixation, as ammonia is taken up by plants as nitrogen source for the nitrogen containing bio-molecules. Previously research has been focused on the microorganisms that improve soil quality and fertility, but focus has now been shifted on microorganisms that can alleviate abiotic stress such as soil salinity. Of late, most research has been focused on isolating and investigating halotolerant and halophilic microorganisms for their PGP potential. A recent study has isolated 20 Halomonas strains from Southern Tunisia having resistance to a wide set of abiotic stresses and possessing PGP activities such as IAA production, phosphate solubilisation and potential nitrogen fixation.33 Kaydan et al.28 isolated IAA-producing bacterial strains belonging to Jeotgalibacillus sp., T. saccharophilus, T. goriensis, B. megaterium, B. simplex and B. aryabhattai. Rajput et al.34 showed that the halophilic bacteria Planococcus rifietoensis, has IAA producing activity, phosphate solubilising activity and ACC deaminase activity that enhance the growth and yield of T. aestivum under salinity stress. In the study by Siddike et al.,35 strains of Oceanobacillus sp., Halomonas sp., Exiguobacterium sp., Zhihengliuella sp. and several Bacillus sp. were found to have IAA production, nitrogen fixation, phosphate solubilising and ammonia production abilities. A study by Dasele et al.36 revealed that Halomonas sp. and Halobacillus sp. have IAA producing potential. Dias et al.37 have reported that a Virgibacillus strain has IAA producing and phosphate solubilisation potential. In general, soluble phosphates and ammonia directly support plant growth as they act as macro-nutrients.38 In addition to having beneficial effects such as IAA production, nitrogen fixation, phosphate solubilisation and ammonia production, halotolerant and halophilic microorganisms can accumulate osmolytes in stress conditions.39 According to a report by Qurashi and Sabri,29 endogenous osmolytes such as proline, glycine betaine and choline are accumulated in moderately halophilic bacterial strains (S. haemolyticus and B. subtilis) isolated from saline rhizosphere of chickpea. These osmolytes improve the growth of bacterial strains and plants by alleviating salt stress. A more recent study has revealed that inoculation of two halophilic bacteria, (V. marismortui and T. halophilus) to tomato seeds improve the stem growth compared to the uninoculated control.31 In addition, these halophilic bacteria produce halotolerant and thermotolerant chitinases that help in decompositing chitin-based organic matters.31 Another halophilic microorganism, Microbacterium sp. isolated from rice rhizosphere showed ACC deaminase activity.30 Further, the halophilic bacterial strain Promicromonospora sp., isolated from agricultural field soil in Republic of Korea showed phosphate solubilising and gibberellin-producing ability.40 Consistent with the above results, Kavamura et al.41 have also reported that a Virgibacillus strain produces exopolysaccharide and has the ability to grow in a medium with reduced water availability. Another halophilic microorganism, Oceanobacillus, has been shown to possess phosphate solubilisation activity.33,42 The result obtained in the current paper revealed that the investigated bacterial isolates have PGP traits that can alleviate salt stress. The results are consist with previous results reported by Dias et al.,37 Siddike et al.,35 Mapelli et al.33 and Dasele et al.36 The PGP traits of the 18 isolates were determined. Further, The results of the current study showed that most of the isolated bacterial strains possess significant PGP traits which are thought to play a fundamental role in salt affected soil fertility. Furthermore, to the best of my knowledge this is first study to evaluate the PGP potential of halophilic and halotolerant bacterial species in salt affected soils of the East Anatolian region (Erzincan and Iğdır) in Turkey. The results of the present study will significantly contribute in expanding the available knowledge as most of the studied bacterial strains have high salt tolerance and fundamental PGP activities, which aids to the functioning plants under salt stress. It is well known that microorganisms having PGP activities can increase plant growth and yield. Halophilic and halotolerant microorganisms that have PGP activities are even more significant as they tolerate not only high salt but also increased crop yield.
Previous studies on plants performed under salt stress (in general with 100mM NaCl) have shown that plants inoculated with bacterial strains have higher growth rate than plants not inoculated with bacteria. In a study performed on peanuts (Arachis hypogaea), inoculation with Brachybacterium saurashtrense, Brevibacterium casei and Haererohalobacter increased the growth of plants in comparison to the control plants.43 Similar results have been reported for radish inoculated with Staphylococcus kloosii and Kocuria erythromyxa,44 lettuce inoculated with Bacillus subtilis, B. atrophaeus, B. spharicus, S. kloosii and K. erythromyxa,45 strawberry inoculated with B. subtilis, B. atrophaeus, B. spharicus, S. kloosii and K. Erythromyxa46 and wheat inoculated with P. rifietoensis under salt stress.34
In the current paper, it was shown that NaCl reduced the shoot and root weight of plants, but the presence of PGP ameliorated the stress caused by NaCl. Most of the studied bacterial strains promoted plant growth under 200mM salt stress and the results are in accordance with the previous studies.33–37,45,46 Among the investigated bacterial strains, EN1 (Bacillus sp.), EN3 (Z. halotolerans), EN4 (S. succinus), EN6 (B. gibsonii), EN8 (O. oncorhynchi), IA (Halomonas sp.) and ID (Thalassobacillus sp.) had the highest PGP potential under NaCl salt stress. However, the investigated strains have to be investigated with other crops to confirm the potential of these bacterial strains under salt stress. In conclusion, studies in evaluating the PGP potential of halophilic and halotolerant bacterial species will extend their application in biotechnology, agriculture practice and alleviation of salt stress and amelioration of salt affected soils. Furthermore, to the best of my knowledge, this is the first study to report that Z. halotolerans, S. succinus, B. gibsonii O. oncorhynchi, Halomonas sp. and Thalassobacillus sp. significantly improve growth in T. aestivum under salt stress (200mM NaCl).
Conflicts of interestThere is no financial or commercial conflict of interest to declare.
I thank Dr. Nashia ZİLBEYAZ for critically reading the manuscript and language corrections of the manuscript.