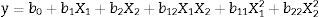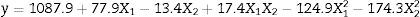Cyclodextrin glycosyltransferase (CGTase) catalyzes the conversion of starch into non-reducing cyclic sugars, cyclodextrins, which have several industrial applications. This study aimed to establish optimal culture conditions for β-CGTase production by Bacillus sp. SM-02, isolated from soil of cassava industries waste water lake. The optimization was performed by Central Composite Design (CCD) 2, using cassava flour and corn steep liquor as substrates. The maximum production of 1087.9UmL−1 was obtained with 25.0gL−1 of cassava flour and 3.5gL−1 of corn steep after 72h by submerged fermentation. The enzyme showed optimum activity at pH 5.0 and temperature 55°C, and maintained thermal stability at 55°C for 3h. The enzymatic activity was stimulated in the presence of Mg+2, Ca+2, EDTA, K+, Ba+2 and Na+ and inhibited in the presence of Hg+2, Cu+2, Fe+2 and Zn+2. The results showed that Bacillus sp. SM-02 have good potential for β-CGTase production.
Global market for the enzymes of microbial origin having environmental and industrial applications has significantly developed during the last decades. It has elicited a search for new microorganisms with biotechnological potential for enzymatic production. Brazilian tropical soils are bestowed with a microbiota that exhibits an exceptional capacity of enzyme productions; unfortunately, to a large extent the majority of these microbial communities are unknown.1–3 Several strains of bacteria producing cyclodextrin glycosyltransferase (CGTase; EC: 2.4.1.19) have already been isolated from different environments in different parts of the world, including Brazil.4–8 There are a few studies exploring the soils from the State of Bahia, Brazil, for the isolation of microorganisms with biotechnological potential for the production of this enzyme. CGTase is an extracellular enzyme that catalyzes the hydrolysis of α-1,4-glycoside bonds and subsequent intramolecular (cyclization) and intermolecular transglycosylation (disproportionation and coupling) reaction of α-1,4-glucane.9,10 This enzyme being amylolytic in nature is the only one capable of converting starch into a mixture of non-reducing sugars designated as cyclodextrins, besides linear sugars and dextrins.11 Cyclodextrins, the main products of the CGTase catalysis reaction, are cyclic oligosaccharides made up by d-(+)-glucopyranose units linked by α-1,4 linkages. These compounds have wide industrial applications due to their capability to form water-soluble inclusion complexes with several substances; this property modifies physicochemical proprieties of encapsulated host-molecules.12,13 The culture media required for the production of this enzyme usually includes carbon and nitrogen sources obtained by expensive processes. Therefore, approaches in pursuit of low cost substrates, especially from agro-industrial origins, have been developed with an objective to decrease production costs of CGTase at commercial scales.14–18 In this scenario, the use of cassava flour (Manihot esculenta Crantz) as a carbon source in fermentation processes for CGTase production, has gained an intense interest. Cassava flour, an agro-industrial product containing 60–80% starch, can be obtained by a simple low cost process.19 In Brazil, it is a widespread agronomic product specifically of the North and Northeast regions.20,21 Its quality is directly related to the physical and chemical characteristics of the cassava roots, plant genotype and age, favorable environmental conditions, harvest period, soil characteristics, genetic variability, and the processing method.22,23
Regarding the nitrogen source, corn steep liquor is commonly used in fermentation processes. It contains large quantities of nitrogen along with proteins, amino-acids and vitamins. It is also a low cost byproduct originating from corn processing.24 This study aimed to establish ideal conditions for CGTase production from Bacillus sp. SM-02 by means of experimental methods based on Central Composite Design (CCD), using agro-industrial substrates, cassava flour and corn steep liquor as alternative sources of carbon and nitrogen, respectively.
Materials and methodsBacterial strainBacillus sp. SM-02 was isolated from soil samples containing cassava waste water from a cassava flour factory in Cruz das Almas county, Bahia, Brazil. The bacterial isolates were preserved in cryo tubes containing 2mL of 20% glycerol and stored at −80°C.
Molecular identificationA pair of universal primers, 8F (5′ AGAGTT TGATCCTGGCTCAG 3′) and 1492R (5′ ACGGCTACCTTGTTACGACTT 3′) was used to amplify the 16S rRNA. The sequencing was carried out commercially by Macrogen Co. (Korea). The 16S rRNA sequence was used as query in BLASTN25 search against National Center for Biotechnology Information (NCBI) database. MEGA 5.05 software26 was used to construct an evolutionary model and to generate the maximum likelihood tree. The 16S rRNA sequences used in the phylogenetic analysis were retrieved from the site of List of Prokaryotic Standing Names (LPSN) during August, 2014.27
Preliminary tests for CGTase productionThe ability for β-CGTase production by Bacillus sp. SM-02 was evaluated by the degradation of starch forming a halo zone in solid media containing phenolphthalein.28 In the center of Petri dishes containing solid media, holes of 0.5cm diameter were prepared. These holes were filled with 50μL of culture containing 3×108 cells. Petri dishes were incubated at 37°C for 72h before measuring the halo zone.
Fermentation assayIn order to re-activate the microorganism, sub-cultures were prepared from the stock culture in solid media developed by Nakamura and Horikoshi,29 containing (gL−1): soluble starch, 10; peptone, 5; yeast extract, 5; MgSO4·7H2O, 0.2; K2HPO4, 1; bacteriological agar, 1.5; Na2CO3, 10 (separately sterilized and added to the culture media at 60°C temperature) at pH 9.8. The inoculum was prepared after 72h of culture growth by transferring, with the help of a loop, of the culture to a 125-mL Erlenmeyer flask containing 50mL of liquid media supplemented with cassava flour and corn steep liquor for cell adaptation to the fermentation media at pH 9.8. After the incubation period of 24h, an aliquot of 1mL of the inoculum was standardized (OD600=0.1) and the final volume for each assay was made to 30mL by using of the culture medium, replacing soluble starch of the basal media by cassava flour and peptone and yeast extract by corn steep liquor (Sigma®). The cultures were incubated in shaker at 150rpm and 35°C. After 72h of fermentation, the samples were centrifuged at 5000rpm and 4°C for 30min. The cell-free supernatant was used to determine the enzymatic activity; and the precipitated cell mass was used for biomass determination.
CGTase production using the experimental designConcentration optimization of cassava flour as a carbon source and corn steep liquor as a nitrogen source was carried out using the response surface methodology (RSM). β-CGTase activity (UmL−1) was considered as dependent variable and substrate concentrations as independent variables. A factorial planning matrix 22 was performed by employing Central Composite Design (CCD) resulting in 11 runs.30 Two levels were selected, a superior (+1) and an inferior (−1), besides a central point (0) (the only one performed with three replicates to determine methodological strength) and two axial points (+1.41 and −1.41). This model is represented by a second order polynomial regression to generate response surface, Eq. (1):
where y is the predicted response of CGTase activity; X1 and X2 are the codified forms (cassava flour and corn steep liquor, respectively); b0 refers to the insertion point; b1 and b2 are linear coefficients; b12 is the coefficient of double interaction and b11 and b22 are quadratic coefficients. The results obtained by this experimental method were evaluated using the Software Release 7.1, Stat. Soft. Inc., USA.Enzymatic assayEnzymatic activity was determined by colorimetric method of the cyclodextrin-phenolphthalein complex (CD-PHE).31 The mixture containing 5mL of the crude enzymatic solution and 5mL of the soluble starch solution at 1% was incubated in a thermostated reactor at 55°C. Aliquots of 0.5mL from the reaction mixture were taken at 0, 3, 6, 9 and 12min and inactivated in a boiling water bath for 5min. To each inactivated aliquot, 2.5mL of a phenolphthalein alcoholic solution (3mM), diluted in 600mM Na2CO3 buffer at pH 10.5, was added. Absorbance of the samples was recorded at λ=550nm.
Cell growthAfter fermentation, the biomass was separated from the supernatant by centrifugation at 5000rpm for 30min at 4°C. The biomass was re-suspended in 5mL distilled water and centrifuged again for cell washing. After removing the supernatant, 30mL of distilled water was added and the precipitate was re-suspended. Then, optical density was measured at 600nm and biomass was quantified by comparing with the standardized curve based in dry mass×optical density.
Total reducing sugars determinationDetermination of reducing sugars was performed trough 3,5-dinitrosalicylic acid (DNS) method of Miller.32 The samples were subjected to an acid hydrolysis in 2M HCl. After boiling for 20min, samples were neutralized with 2M NaOH.
Total protein determinationTotal protein concentration was determined by the Bradford method,33 by adding 0.2mL to the crude enzyme extract to 2mL of the Bradford reagent. Absorbance was recorded at λ=595nm.
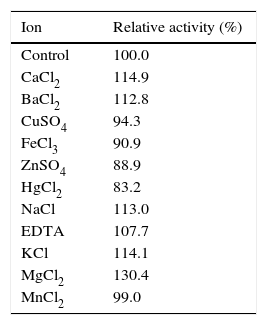
Crude enzyme partial characterizationTo evaluate the effect of pH on β-CGTase activity the following buffers (50mM) were used: glycine–HCl, pH 2.0–3.0; sodium citrate, pH 3.0–6.0; phosphate, pH 6.0–8.0; Tris–HCl, pH 8.0–9.0; and glycine–NaOH, pH 9.0–10.0. The influence of metallic ions on CGTase activity was determined by using following solutions (50mM): CaCl2, FeCl3, NaCl, ZnSO4, EDTA, KCl, MnCl2, CuSO4, BaCl2, HgCl2, and MgCl2. Optimal temperature of the reaction was determined by incubating the enzyme at 50–70°C. Thermal stability was accessed by incubating the enzyme at 50–65°C, for 5h at pH 5.0. The experiments were performed according to the standardizations of enzymatic activity according to the CD-PHE complexation method.31
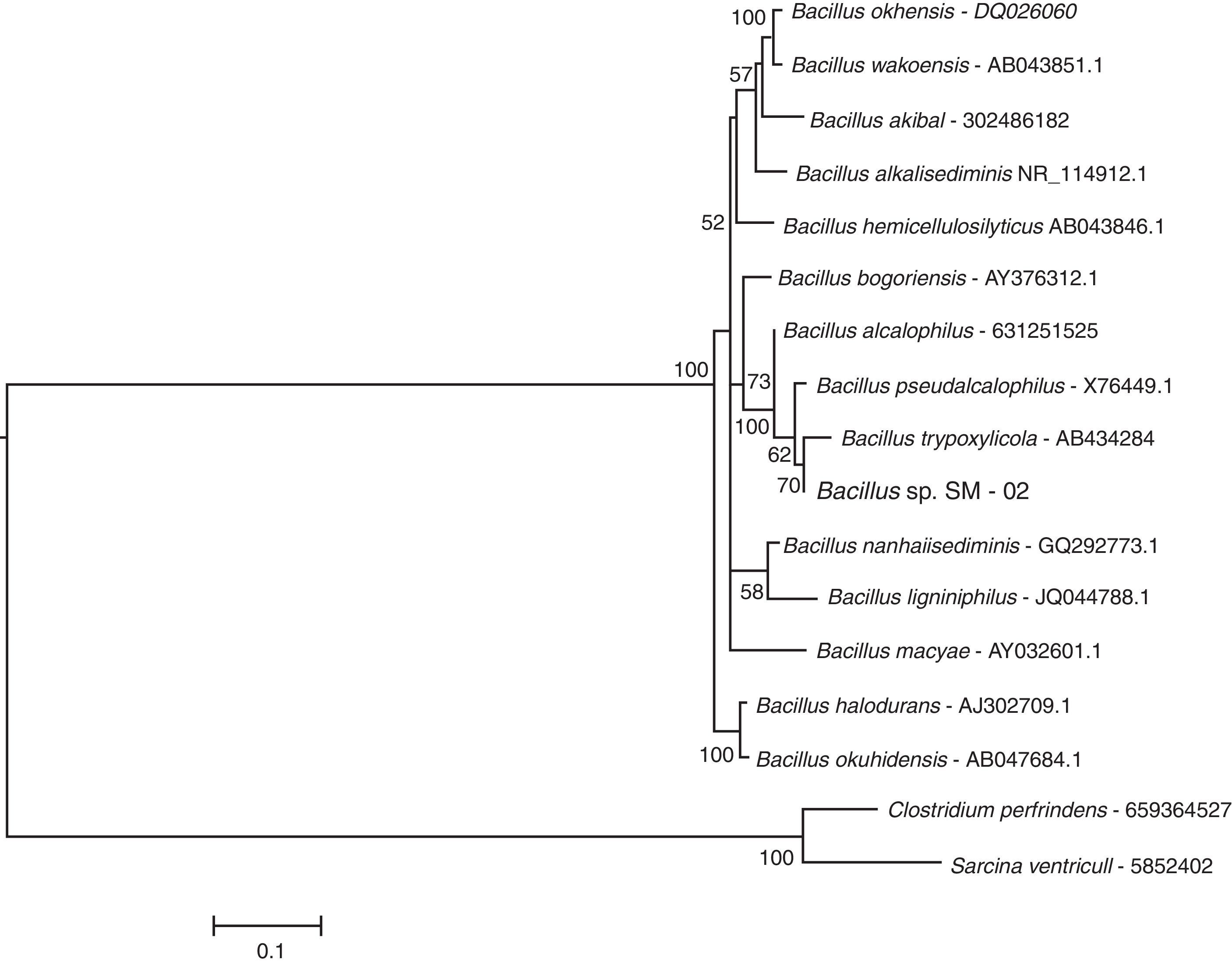
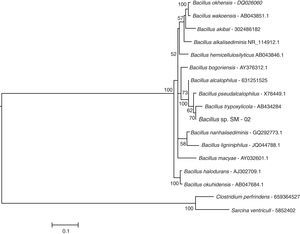
ResultsMolecular identification of strain SM-02The BLASTN search at the NCBI database indicated that the 16S rRNA sequence of bacterial strain SM-02 is 98–99% identical to 16S rRNA sequences of Bacillus species indicating that the strain SM-02 belongs to this bacterial genus. Therefore, we performed a phylogenetic analysis to understand the evolutionary relationship of Bacillus sp. SM-02 to other Bacillus species using 16S rRNA sequences of all Bacillus species available at the LPSN site. The analysis showed that the Bacillus sp. SM-02 is phylogenetically related to Bacillus trypoxylicola (Fig. 1), a xylanase-producing bacterium first isolated from the gut of Japanese horned beetle larva.34
The subclade of a maximum likelihood tree based on 16S rRNA sequences showing the phylogenetic relationship of strain SM-02 with the species of Bacillus genus. The tree was generated with 16S rRNA sequences from species of the genus available at the LPSN site (List of Prokaryotic Names with Standing in Nomenclature – www.bacterio.net/index.html) as on August, 2014. The phylogenetic analysis was performed using MEGA 5.1 software and employing the K2+G+I model. Numbers above the branches indicate bootstrap support; and the tree was rooted with 16S rRNA sequences of Clostridium perfringens ATCC 13124 and Sarcina ventriculi. The bar represents the number of expected substitution per sites under K2+G+I model.
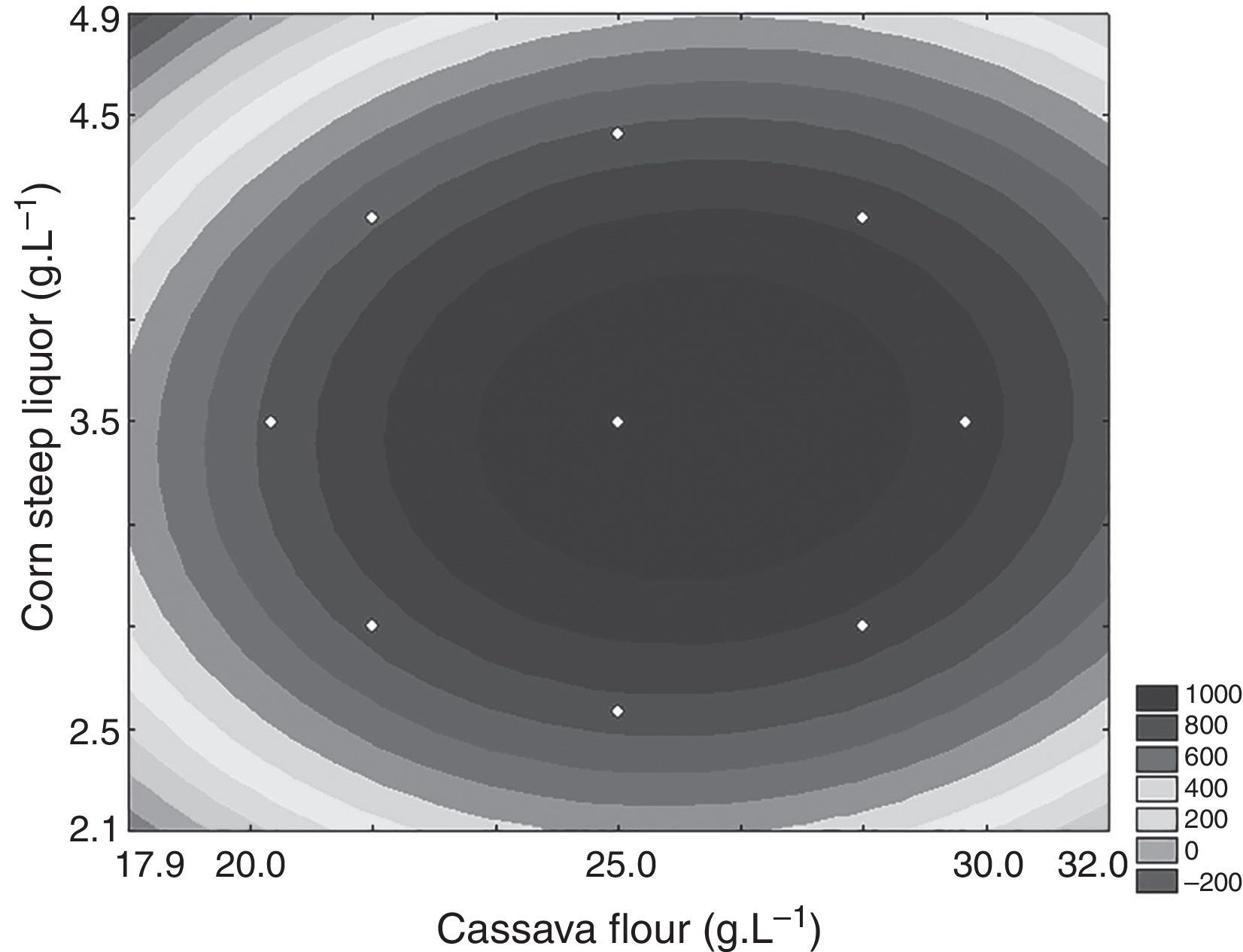
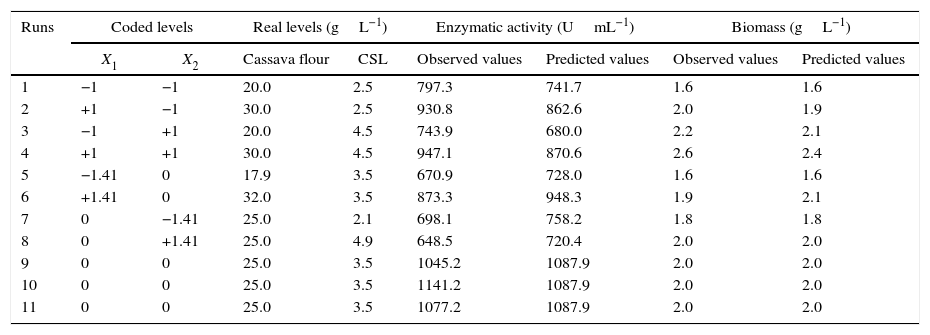
Through the obtained results of CCD 2,2 influences of the studied variables (X1: carbon source and X2: nitrogen source) over CGTase production by Bacillus sp. SM-02 were verified. Observed and predicted values by the experimental model are shown in Table 1.
Central Composite Design matrix for enzyme and biomass production.
| Runs | Coded levels | Real levels (gL−1) | Enzymatic activity (UmL−1) | Biomass (gL−1) | ||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | Cassava flour | CSL | Observed values | Predicted values | Observed values | Predicted values | |
| 1 | −1 | −1 | 20.0 | 2.5 | 797.3 | 741.7 | 1.6 | 1.6 |
| 2 | +1 | −1 | 30.0 | 2.5 | 930.8 | 862.6 | 2.0 | 1.9 |
| 3 | −1 | +1 | 20.0 | 4.5 | 743.9 | 680.0 | 2.2 | 2.1 |
| 4 | +1 | +1 | 30.0 | 4.5 | 947.1 | 870.6 | 2.6 | 2.4 |
| 5 | −1.41 | 0 | 17.9 | 3.5 | 670.9 | 728.0 | 1.6 | 1.6 |
| 6 | +1.41 | 0 | 32.0 | 3.5 | 873.3 | 948.3 | 1.9 | 2.1 |
| 7 | 0 | −1.41 | 25.0 | 2.1 | 698.1 | 758.2 | 1.8 | 1.8 |
| 8 | 0 | +1.41 | 25.0 | 4.9 | 648.5 | 720.4 | 2.0 | 2.0 |
| 9 | 0 | 0 | 25.0 | 3.5 | 1045.2 | 1087.9 | 2.0 | 2.0 |
| 10 | 0 | 0 | 25.0 | 3.5 | 1141.2 | 1087.9 | 2.0 | 2.0 |
| 11 | 0 | 0 | 25.0 | 3.5 | 1077.2 | 1087.9 | 2.0 | 2.0 |
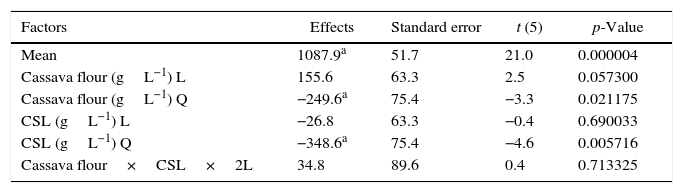
A gradual increase was observed from 648.5UmL−1 (run 8) up to 1087.9UmL−1 (mean of the central points), achieving the maximum production of CGTase at 25.0gL−1 of cassava flour and 3.5gL−1 of corn steep liquor. Under the same carbon source concentrations, there was an expressive negative influence of corn steep liquor over the enzyme production, with a decrease of almost 60%, when comparing values of the higher axial level (run 8) with the values of the central point runs (run 9, 10 and 11). This can be confirmed by the estimated effects (Table 2), where the quadratic terms of the concentration of cassava flour and corn steep liquor were statistically significant at 95% significance (p<0.05), showing negative effects over CGTase production. It means the increase in concentration of these variables caused a reduction in the enzymatic production. The adjusted mathematical model that describes CGTase production within the studied range can be expressed by equation Eq. (2):
Estimated effects for the cyclodextrin glycosyltransferase production.
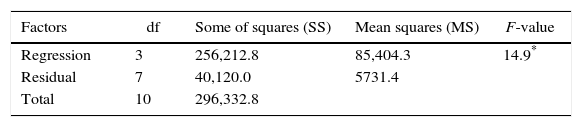
Table 3 shows the results evaluated by the analysis of variance. The F-value of 14.9 indicates that the model was significant at the confidence level applied (5% probability) with a good correlation between the experimental and predicted values (Table 1). Therefore, considering the p-values calculated by the analysis of variance, the most significant variable was corn steep liquor in linear terms, followed by cassava flour in quadratic and linear terms, respectively.
Analysis of variance for the regression model obtained from response surface experiment.
| Factors | df | Some of squares (SS) | Mean squares (MS) | F-value |
|---|---|---|---|---|
| Regression | 3 | 256,212.8 | 85,404.3 | 14.9* |
| Residual | 7 | 40,120.0 | 5731.4 | |
| Total | 10 | 296,332.8 |
According to ANOVA a variation coefficient (R2) of 0.8646 shows that 86.46% of the response variability can be explained by the model. Considering the results shown, the effect of the independent variables over the enzymatic production response was evaluated from a response surface (Fig. 2). The optimization area represents the best response for the enzymatic production, where the central point is located. The lowest CGTase production was observed at the inferior (−1.41 and −1) and superior (+1 and +1.41) levels, where the lowest and highest concentrations of carbon and corn steep liquor are found, respectively.
Besides, Fig. 2 shows that near the optimization point, there is a range of concentrations of the carbon and nitrogen sources that showed optimal production of the enzyme, between 22.5gL−1 and 27.5gL−1 and 3.0gL−1 and 4.0gL−1, respectively. Thus, any concentration found inside this optimal range may be used for enzymatic production, without influencing the process within the optimized condition.
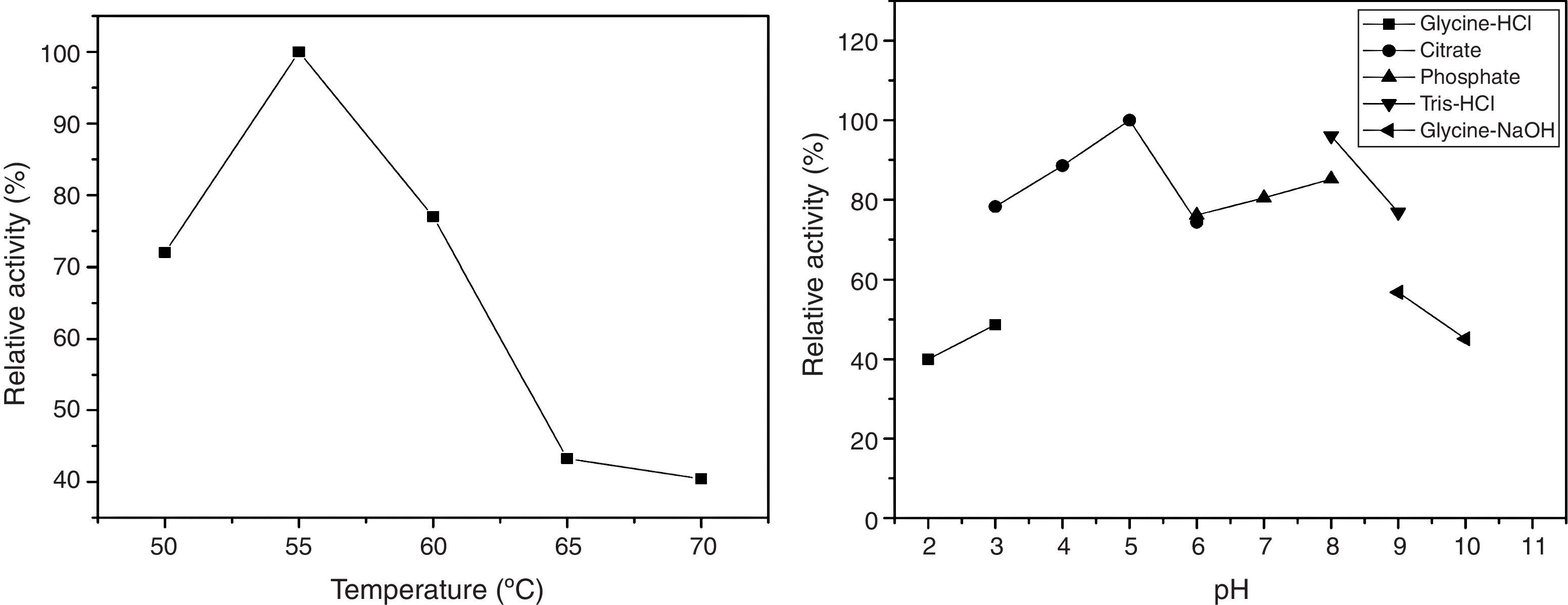
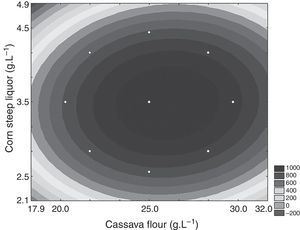
Partial characterization of the crude enzymeThe crude enzymatic extract showed optimal temperature of 55°C, but retained up to 70% activity in temperatures between 50 and 60°C. Relative activities at 50°C and 70°C were 72% and 40%, respectively (Fig. 3).
The effect of pH on CGTase activity was evaluated within the range of pH 2.0–10.0 (Fig. 3). The enzyme showed above 70% activity within the range of pH 3.0–9.0, with 100% activity at pH 5.0.
Enzymatic activity was enhanced in the presence of Mg+2, Ca+2, EDTA, K+, Ba+2 and Na+, with a maximum observed for Mg+2. The enzymatic activity was slightly inhibited in the presence of Hg+2, Fe+2, Cu+2 and Zn+2, with retention of at least 83% activity. The enzymatic activity of cyclization had no inhibitory significance in the presence of Mn+2 (Table 4).
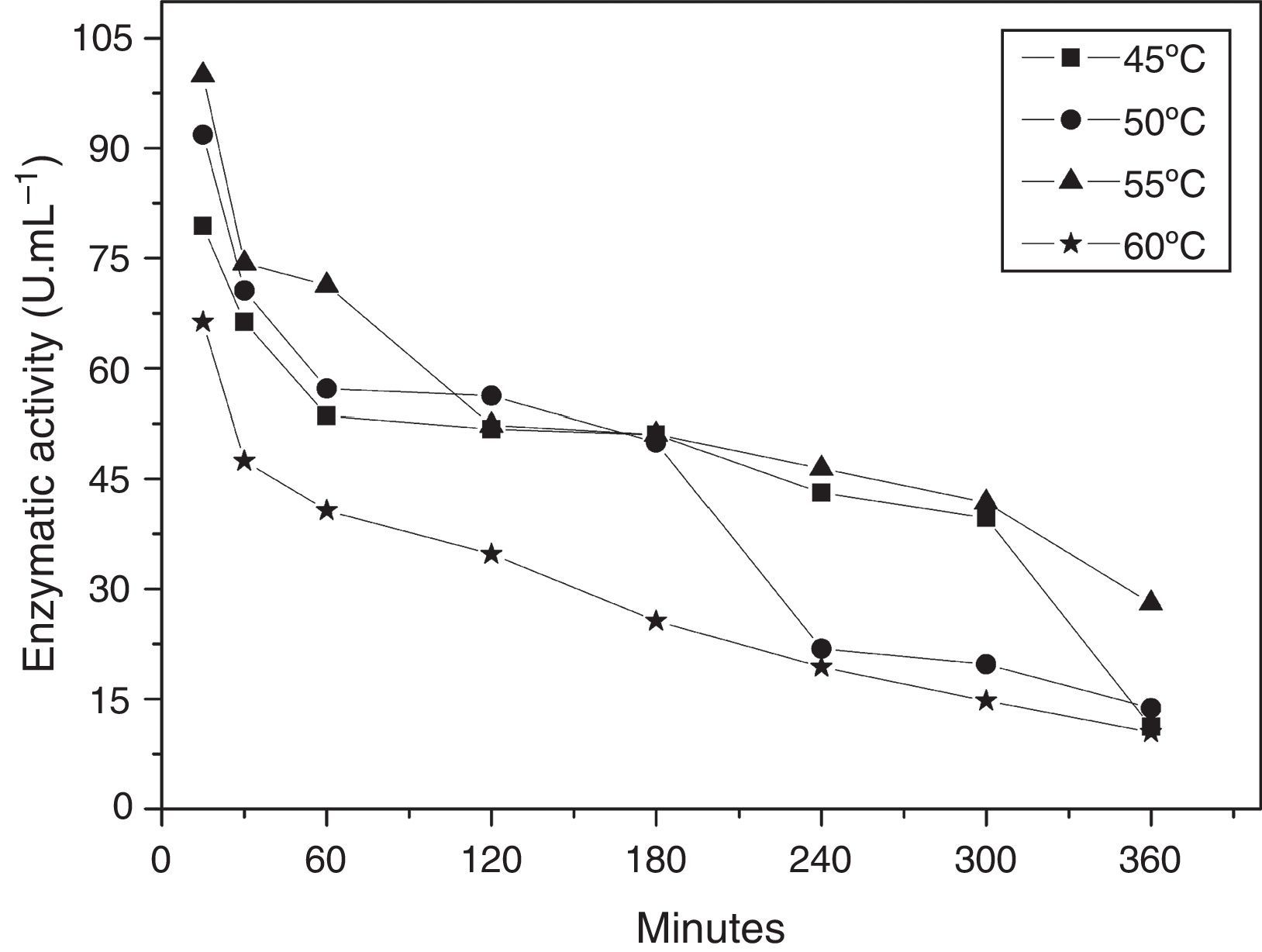
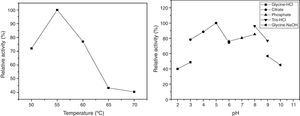
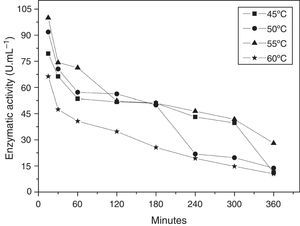
The thermal stability of the enzyme was evaluated in a temperature range of 45–60°C, pH 5.0, in the presence of Mg+2. The enzyme preserved its cyclization activity above 50% for 3h at 45, 50, and 55°C (Fig. 4), and the activity was steeply lost upon incubation at 60°C.
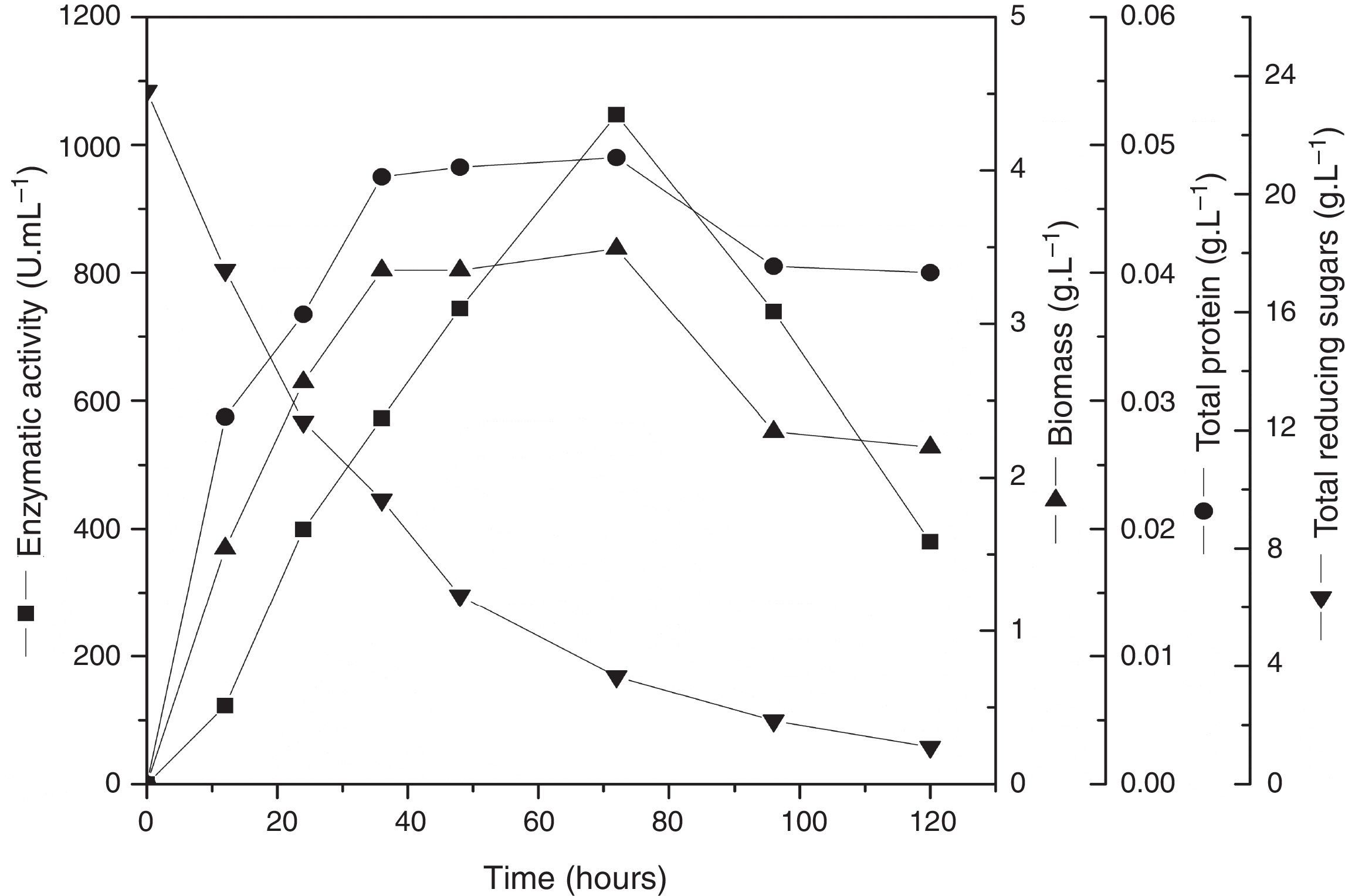
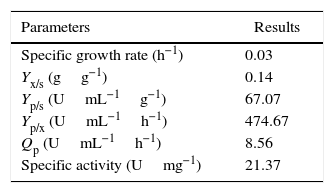
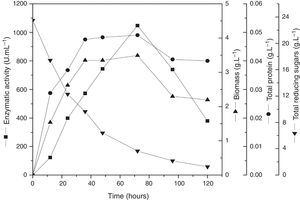
In order to validate the responses obtained through the DCCR, the behavior of the strain was evaluated by submerged fermentation using 25.0gL−1 of cassava flour and 3.5gL−1 of corn steep liquor at 35°C, 150rpm, for 120h. The enzymatic activity profile coincided with the cellular growth and the enzymatic synthesis was higher during the exponential stage of the growth, attaining the maximum activity after 72h of the beginning of fermentation (Fig. 5). Then, an abrupt reduction in the enzymatic production was observed, coinciding with the onset of the decline phase. The substrate was almost completely consumed after 120h of fermentation. The specific growth rate (u) was 0.03h−1 and the biomass yield as a function of the substrate was of 0.14gg−1. The enzymatic yield as a function of the substrate (Yp/s) was 67.07UmL−1g−1 and the productivity was 8.56UmL−1h−1, with a specific activity of 21.37Umg−1 (Table 5).
Based on statistical fundamentals, the method of factorial planning allied to the surface response analysis has been designated to study the optimization of parameters involved in industrial processes with the aim of minimizing costs and time, maximizing yield, productivity, and quality of production.30,35 In the present study, the use of factorial planning showed that Bacillus sp. SM-02 has a potential as CGTase producer, exhibiting top cyclization activity corresponding to 1087.9UmL−1, rarely found in other reported bacterial strains. Pinto et al.36 reported high enzymatic activity from the crude extract produced by Bacillus circulans ATCC 21783 in submerged cultures supplemented with fibrous industrial residue of soy. Using optimized conditions of pH, temperature, and aeration, the authors achieved 1155UmL−1 activity, with maximum yield of 32.8UmL−1g−1. In the present study, the variance analysis (ANOVA) indicated influence of cassava flour along with corn steep liquor over CGTase production in Bacillus sp. SM-02, showing that high concentrations of the carbon and nitrogen sources negatively influenced enzyme production. Similar results have been reported in difference studies, where maximum production of CGTase was obtained at low substrate concentrations. Rosso et al.37 optimized production media for CGTase from B. circulans DF 9R, by employing different sources and confirmed that 1.5% of cassava starch increased the enzymatic activity. Zain et al.17 performed optimization studies of the production media of CGTase by Bacillus sp. TS1−1 with 3.3% of tapioca starch and 0.13% of yeast extract achieving maximum production of 78.1UmL−1. Pinto et al.36 studied enzyme production from B. circulans ATCC using a soluble starch concentration five-times lower than the concentration used by Makela et al.,38 and obtained 21-times high enzyme production. Park et al.39 explained that the excess of substrate increases viscosity of the culture media, which interferes with oxygen absorption by the microorganism. Glucose and maltose may accumulate in the culture media due to high concentrations of starch and exert a catabolic repressor effect, influencing CGTase synthesis, as observed by Gawande and Patkar.40
Already published results suggest that the nature and concentration of the carbon and nitrogen sources used, and the microorganism strains, directly interfere CGTase production. Gawande et al.41 affirmed that concentration of the carbon source is important during enzyme production by many organisms, especially when this source is essential for enzyme induction. On the basis of these observations, the DCCR statistical methodology applied in the current study targeted a maximum enzyme activity, and consequently leading to an experimental setup to optimize the associated parameters. Different properties have been observed for CGTase obtained from different bacterial strains. The studies establishing the enzyme profiling of the CGTase producing microorganisms revealed that different biochemical characteristics, regarding the optimal temperature and pH, thermal stability, molar mass and capacity of cyclodextrin formation, vary depending on the microorganism. The stability of biological catalysts is a limiting factor while selecting them for industrial purposes due to high temperatures and extreme variations in pH used in the industrial processes.42 The enzymes might rapidly get inactivated. Therefore, in biotechnology, thermostability of microbial enzymes has major importance in bio-processes,43 especially, when considering storage of enzymes and enzyme-formulated products that require adequate temperature conditions. Bacillus sp. SM-02 showed high stability at 55°C during a 3-hour incubation period, a temperature previously reported for various species of Bacillus,44Bacillus stearothermophilus,45Bacillus lentus,46Bacillus firmus,47 besides the species Paenibacillus campinasensis H69-3.48 The vast majority of CGtase producing microorganisms investigated so far showed stability at temperatures between 40 and 60°C.48–53 However, few bacterial strains possess thermostable enzymes with optimal performance between 75°C and 85°C.54,55 Tachibana et al.56 reported CGTase producing species of Thermococcus showing functionality at 90–100°C. Optimal cyclization temperature of CGTase from Bacillus sp. SM-02 was 55°C. The same temperature was already reported for Bacillus sp. G1,50Bacillus agaradhaerens LS-3C,51Bacillus sp. G-825-653 and P. campinasensis H69-3.48 In general, optimal reaction temperature of CGTase from alkalophilic microorganisms has been reported among 45–60°C.18 Some rare strains as Thermoanaerobacter kivui.57 have considerable activity under extreme temperatures starting at 80°C.
CGTase from Bacillus sp. SM-02 showed an optimal range of pH varying between 3.0 and 9.0 for the cyclization reaction. These results are similar to those reported for Bacillus ohbensis C-1400,50B. firmus NCIM 5119,50Bacillus sp. 7–12,58Bacillus sp. G-825-6,53 and T. kivui.57 Moreover, the enzyme exhibited activity above 70% in a pH range of 3.0–4.0, rarely reported except that by Mora et al.,59 who observed enzyme activity above 70% within the same pH range for Bacillus sp. CGTase from Bacillus sp. SM-02 was stimulated by a variety of metallic ions, specially Mg+2, which showed an enhancement of about 30% compared to untreated controls. Atanasova et al.42 observed that activity of Bacillus pseudalcaliphilus 20R enzyme was strongly influenced by Mg+2 at 15mM. The implication of studying the influence of metallic ions over a biocatalyst's performance is related to the fact that these substances are able to modify enzyme reaction rates by inhibiting or activating enzymes; therefore, this information is important while choosing substrates that will be used in the fermentation process.
ConclusionIn the present study, we were able to establish optimized conditions, i.e., the best concentration of cassava flour and corn steep liquor that resulted in high CGTase production rates, while using the factorial planning and response surface methodology. The reported productivity, allied to the fact that an easy available and low cost agro-industrial substrate was tested, supports use of cassava flour and corn steep liquor in bioprocesses related to the cyclodextrin production. Bacillus sp. SM-02 appears to have an excellent biotechnological potential for CGTase production, under optimized conditions.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) for financial support.