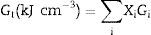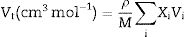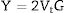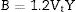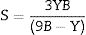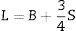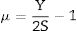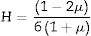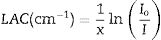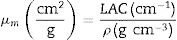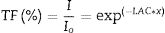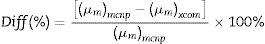The substitution of La2O3 by Tb2O3 was studied for glass samples with a chemical composition described by: 75P2O5+(25−x) La2O3+xTb2O3, where x=5, 10, 15, 20mol%. The mechanical properties were predicted via the Makishima–Mackenzie model. The study demonstrated that when increasing the Tb2O3 content from 5 to 20mol%, the elastic moduli of the glasses decreased, while the dissociation energy, packing density, and micro-hardness increased. In addition, the radiation shielding properties were studied using the MCNP5 code, which was utilized to simulate the linear attenuation coefficient (LAC) of the investigated samples. Furthermore, the mass attenuation coefficients (MAC) of the glasses were determined. The highest MAC was reported for the sample with 20mol% of Tb2O3 and decreased from 0.358 to 0.0515cm2/g between 0.184 and 1.408MeV. Furthermore, the effective atomic number (Zeff), equivalent atomic number (Zeq), exposure buildup factor (EBF), and the energy absorption buildup factor (EABF) of the glasses were calculated utilizing BXCOM software. The substitution of La2O3 by Tb2O3 was revealed to enhance the shielding features of the TLP samples.
Se estudió la sustitución de La2O3 por Tb2O3 para cinco muestras de vidrio con una composición química descrita por la fórmula: 75P2O5+(25-x) La2O3+xTb2O3, donde x=5, 10, 15 y 20%mol. Se evaluaron las propiedades mecánicas y de protección contra la radiación de las muestras de vidrio investigadas. Las propiedades mecánicas se predijeron utilizando el modelo de Makishima-Mackenzie. El estudio demostró que al aumentar el contenido de Tb2O3 de 5 al 20% en moles, los módulos elásticos (Young, Bulk, Shear y longitudinal) de los vidrios disminuyeron, mientras que aumentaron la energía de disociación, densidad de empaquetamiento y microdureza. Además, se estudiaron las propiedades de protección contra la radiación de las muestras de vidrio investigadas utilizando el código de transporte de partículas N de Monte Carlo (MCNP-5). Se utilizó MCNP-5 para simular el coeficiente de atenuación lineal (LAC) de las muestras investigadas. Con base en el LAC simulado, se determinaron los coeficientes de atenuación de masa (MAC) de las gafas. El MAC más alto se obtuvo para la muestra de vidrio con el 20% en moles de Tb2O3 y disminuyó de 0,358 a 0,0515cm2/g cuando se incrementó la energía del fotón gamma de 0,184 a 1,408 MeV. Además, el número atómico efectivo (Zeff), el número atómico equivalente (Zeq), el factor de acumulación de exposición (EBF) y el factor de acumulación de absorción de energía (EABF) de los vidrios se calcularon utilizando el software BXCOM. Se reveló que la sustitución de La2O3 por Tb2O3 mejora las propiedades de protección contra la radiación de las muestras investigadas.
As technologies keep beneficent, a large number of equipment that depend on radiation and radioisotopes has been manufactured and used. The great use of these devices in which different ionizing radiation types are used extends from medical, industrial and agricultural applications and even we have become using any radiation devices in homes, hospitals, and research centers [1–4]. Direct exposure to radiation or even indirect radiation for relatively long periods of time leads to a set of health problems such as skin burns or various cancers. Thus, a human can be protected from the radiation using various materials like lead, glasses and so on [5,6]. Therefore, we find many protocols that have been developed to regulate the use of radiation in various facilities such as hospitals, research centers, and various medical facilities. These protocols focus on three essential points: diminishing the radiation exposure time and increasing the distance between the radioactive source and the person and, most importantly, the use of protective materials, which are called radiation shielding materials [7–10]. These materials can absorb the incoming radiation and thus decrease the radiation's intensity to safe levels.
Therefore, researchers in the field of nuclear engineering are trying to design different types of radiation protection materials according to the application in which these materials will be used, but in all cases, these materials must have a high ability to absorb the largest possible amount of radiation. For instance, in the past few years, researchers succeeded in developing alloys, ceramics, polymers, lead composite, glasses, and concrete as shielding materials [11–14].
Through these different kinds of materials, glass has attracted researchers as a promising material for radiation protection due to its excellent physical, chemical, and optical features. Glass is also easy to manufacture in several ways, available in abundance, and at a relatively cheap cost compared to other types of materials. Moreover, one of the most important advantages of glass that encourages researchers and radiation protection materials developers is the ease of controlling the density of glass using heavy element oxides. It is known that the density is an important factor that enhances the performance of the glass to absorb radiation and thus improve the radiation protection properties of the prepared glass [15–17].
Several kinds of glass formers are usually utilized to create the glass network. It is well known that changing the glass formers will fundamentally change the final glass system's features. P2O5, B2O3, and SiO2 are typical glass formers that are used to fabricate phosphate, borate, and silicate glasses, respectively, and there are the earliest types of glasses and are still produced worldwide today [18,19]. Among several kinds of glasses, Phosphate glasses are featured compared to the traditional glasses, and this is due to some interesting features such as and high thermal expansion coefficient, high ultraviolet transmission, and low melting point [20]. Moreover, P2O5 has an excellent glass forming capability to dissolve high amounts of several types of glasses modifiers such as transition-metal, alkaline, and rare earth elements.
Previous studies on the possibility of using phosphate glass are still few and limited. Therefore, it is quite indispensable to examine the potential of utilizing phosphate glass as a new glass used in radiation protection. Thus, the mechanical properties and gamma-ray shielding capacity of the 75P2O5+(25−x)La2O3+xTb2O3 glass system were studied. The Makishima–Mackenzie model was utilized to estimate the mechanical moduli of the examined samples. The MCNP-5 code was applied to simulate the radiation attenuation factors for the TLP samples
Materials and methodsMechanical propertiesIn this work, the glass network's elastic properties with a chemical composition of 75P2O5+(25−x)La2O3+xTb2O3 are studied. TLP glasses are selected from reference [21]. Table 1 lists the nominal chemical composition and the density (ρ) of these TLP samples. Based on the packing coefficient (Vi) and dissociation energy (Gi) of the metal oxides constituting the TLP glasses, the mechanical moduli, such as Young's (Y), shear (S), Bulk (B), longitudinal (L), Poisson's ratio (μ) and micro-hardness (H) are detected [22–24]. According to the following equations, the dissociation energy (Gt) and packing density (Vt) can be calculated based on the ionic radius and the heat of formation of the constituting compounds.
where M represents the molecular weight of the examined TLP glasses.
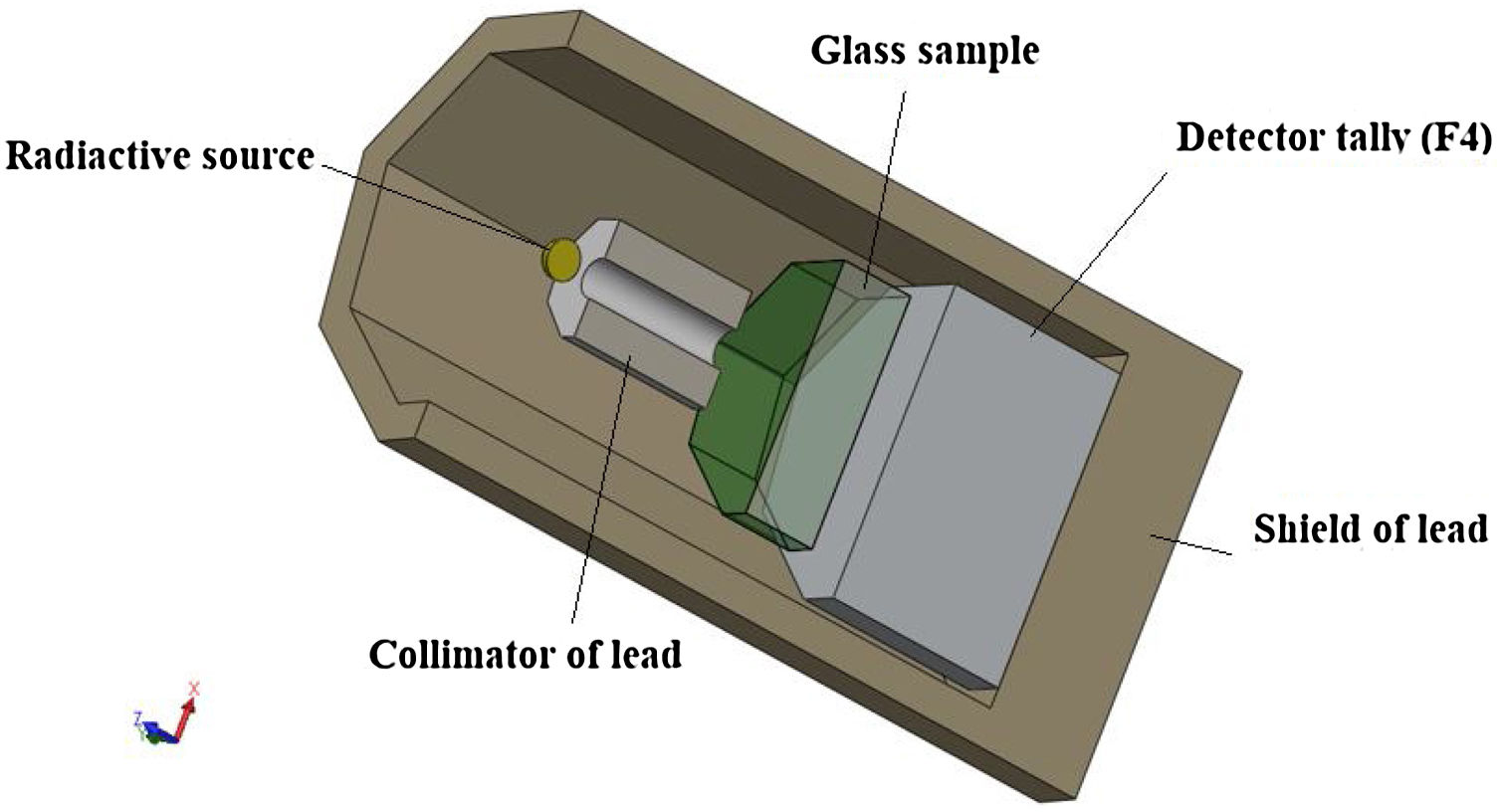
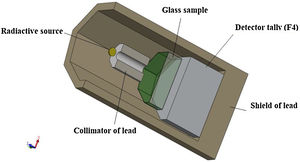
Gamma-ray shielding featuresThe MCNP-5 code is a simulation program used to detect shielding features [25]. The input file used to examine the photon attenuation competence for the TLP glasses is exhibited in Fig. 1. The details about the input file is mentioned in our work [26]. The identified sources in this investigation are 60Co with 1.173 and 1.332MeV energy, 137Cs with 0.662MeV energy, and 106Ho 0.184 and 0.28MeV energy, respectively. In order to assess the average track length (ATL) of the incident photons in the tested TLP samples, the detector was presumed to be an F4 tally. Based on the ATL, some shielding parameters like the mass and linear attenuation coefficients (we will use the abbreviations MAC and LAC for these two quantities) were simulated. The simulated results of these two quantities were then utilized to predict further important quantities, such as mean free path (MFP) and transmission rate (TR) [26–28].
The Io and I denote the incoming and transmitted photon intensities. Where x represents the thickness of the sample.
The recent BXCOM software was used for the evaluation of the effective and equivalent atomic numbers (Zeff and Zeq). Also, it helped for the evaluation of the buildup factors for the examined TLP specimens [29].
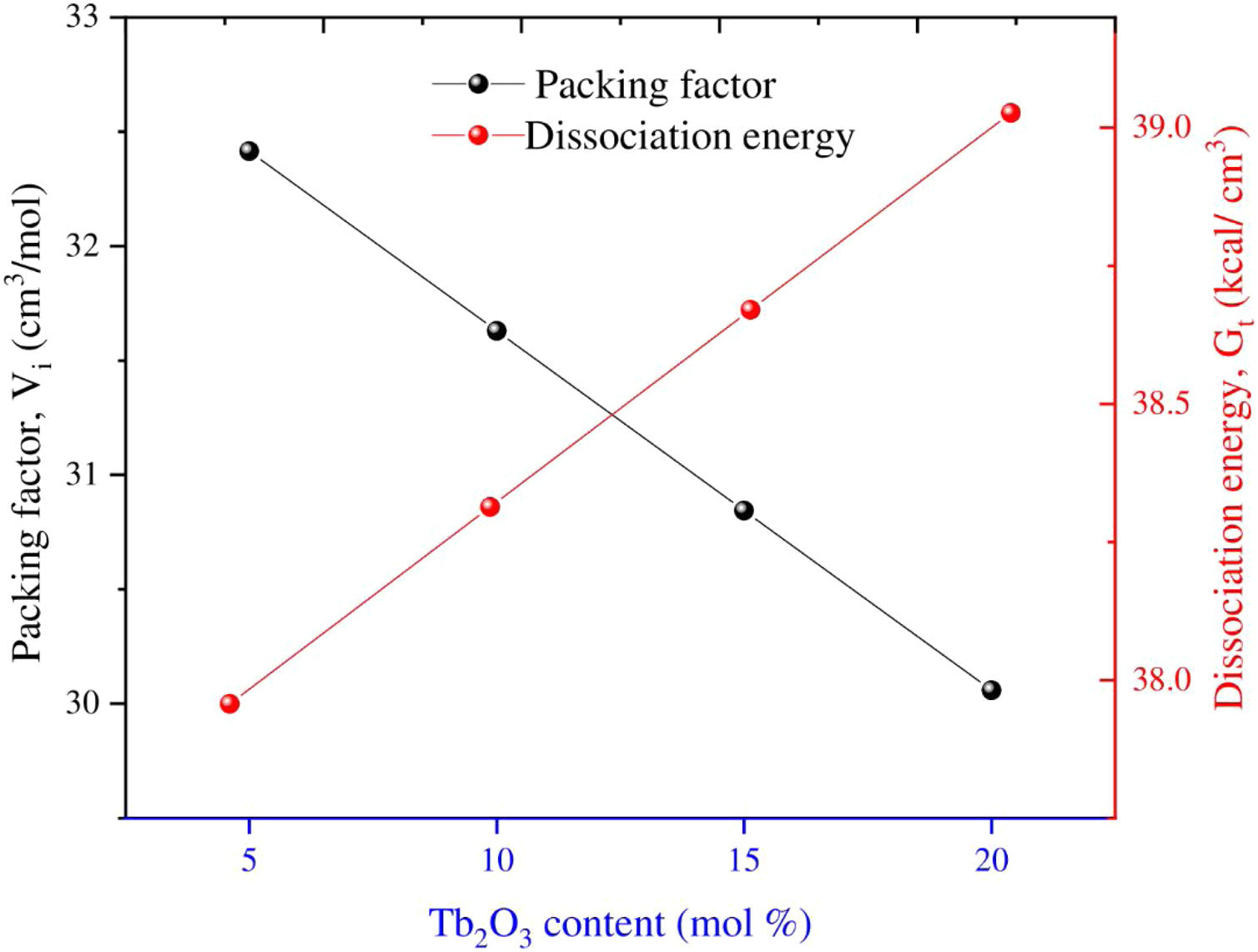
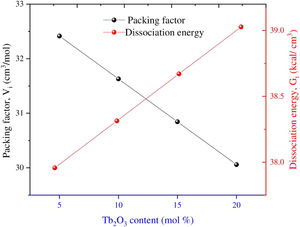
Results and discussionElastic propertiesThe Makshima–Makenzie model was used to determine the mechanical parameters of the fabricated TLP samples based on the Pauli ionic radius and the heat of formation (ΔHf) of each compound. Fig. 2 illustrates that the fabricated TLP glass samples’ packing factor (Vi) reduced from 32.415 to 29.273cm3/mol for TLP5 (with 5mol%) and TLP20 (with 20mol%) of the Tb2O3 content. This attributed to the replacement of La with a highly ionic radius (RLa=1.032Å) by Tb with a lower ionic radius (RTb=0.923Å). Fig. 2 also shows that the dissociation energy Gt increased from 37.957 to 39.384kcal/cm3 with increasing the Tb2O3 concentration between 5 and 20mol%, respectively. The mentioned increase in the Gt is due to the substitution of La2O3 with dissociation energy (Gi=65.8kcal/cm3) by Tb2O3 content with dissociation energy (Gi=72.934kcal/cm3).
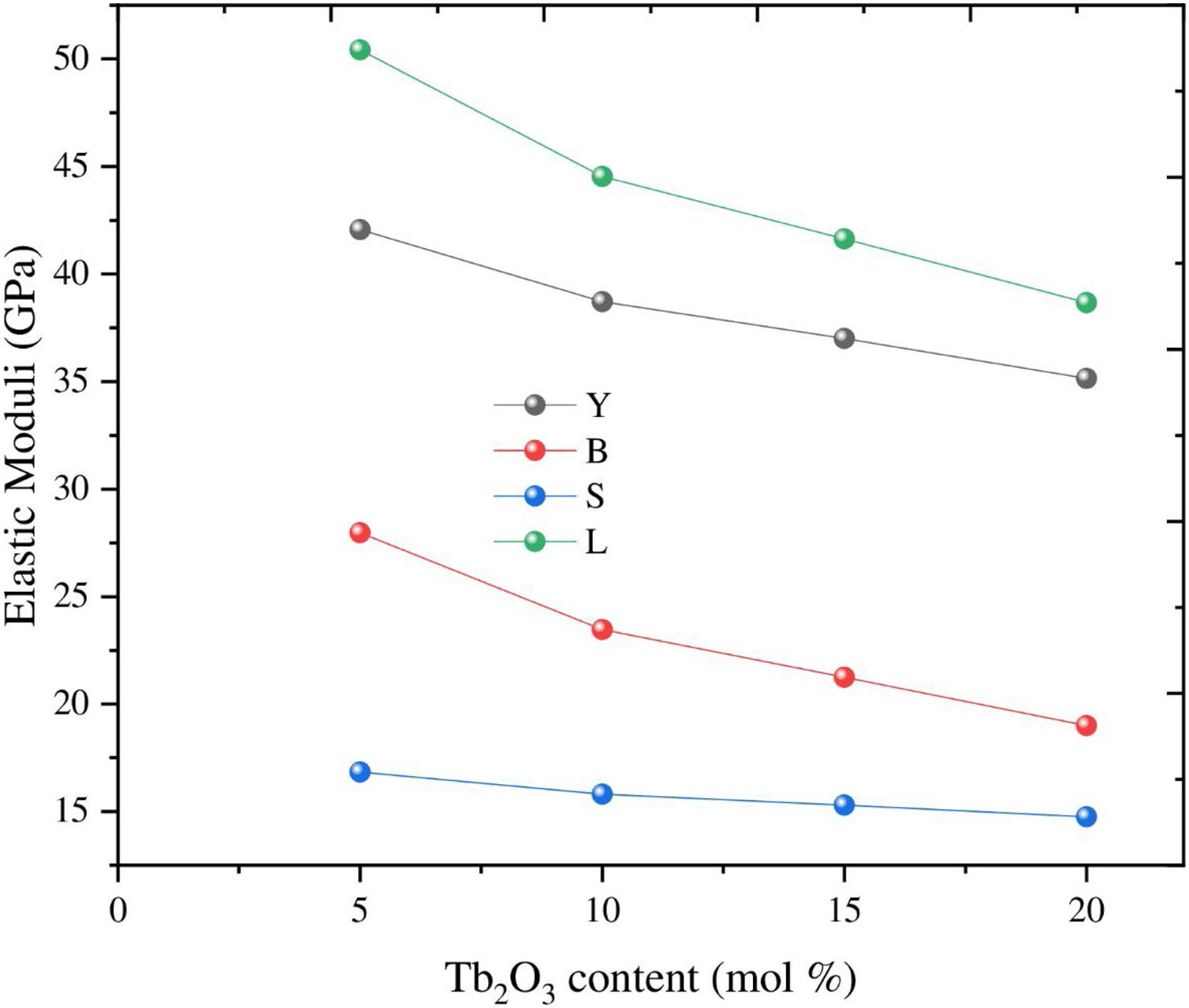
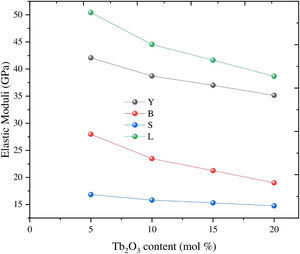
Based on the calculated Gt and Vi values, the mechanical moduli (Young, Longitudinal, Shear, and Bulk) were also predicted. Fig. 3 depicts the elastic moduli versus the Tb2O3 substitution ratio. The elastic model (E) decreased from 42.063 to 31.637GPa, Bulk (B) model reduced from 27.969 to 15.248GPa, Shear (S) declined from 16.834 to 13.705GPa, and Longitudinal (L) model decreased from 50.414 to33.521GPa with increasing the Tb2O3 substitution ratio from 5 to 20mol%. These reductions are due to the LaO bonds’ replacement with high bond stretch and formation energy with a new TbO bond.
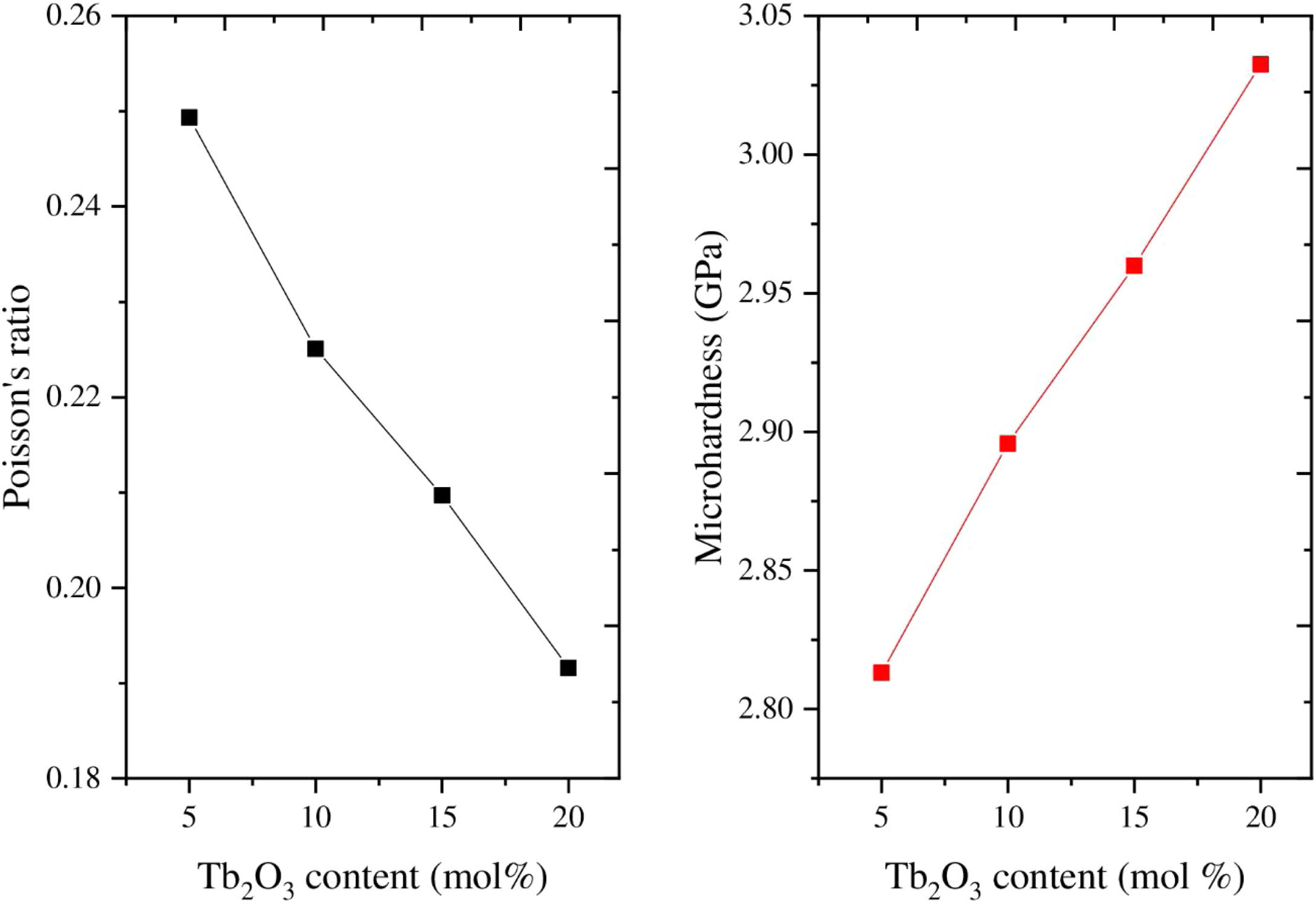
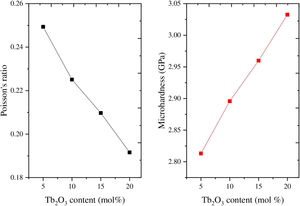
As mentioned in Eq. (9), the Poisson ratio was calculated. The Poisson ratio takes values 0.249, 0.225, 0.210, 0.192, and 0.154 for glass samples TLP5, TLP10, TLP15, and TLP20, respectively. Fig. 4 illustrates the Poisson ratio's reduced with increasing the Tb2O3 between 0 and 20mol%. The mentioned reduction is due to the detected decrease in the packing density Vi and Young model (E).
Hardness is an essential mechanical feature widely utilized to predict a material's ability to withstand an input load. It is divided into microhardness and macro hardness. Glass samples are usually smaller and thinner. Therefore, in the current work, the study of microhardness (H) is more important. The data in Fig. 4 and Table 2 showed that the micro-hardness varied between 2.813, 2.896, 2.960, and 3.033GPa for glass samples TLP5, TLP10, TLP15, and TLP20 glass samples.
Different mechanical parameters for the examined samples.
| Glass sample | Vi (cm3/mol) | Gt (kcal/cm3) | Vt (cm3/mol) | E (GPa) | B (GPa) | S (GPa) | μ | H (GPa) | L (GPa) | Vl (m/s) | Vs (m/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TLP5 | 32.415 | 37.957 | 0.554 | 42.06374 | 27.969 | 16.834 | 0.2493 | 2.813 | 50.414 | 4008.871 | 2316.544 |
| TLP10 | 31.629 | 38.313 | 0.505 | 38.712 | 23.469 | 15.800 | 0.225 | 2.896 | 44.535 | 3860.200 | 2299.236 |
| TLP15 | 30.844 | 38.670 | 0.478 | 37.00357 | 21.245 | 15.294 | 0.210 | 2.960 | 41.638 | 3751.591 | 2273.722 |
| TLP20 | 30.059 | 39.027 | 0.450 | 35.152 | 18.997 | 14.750 | 0.192 | 3.033 | 38.664 | 3644.182 | 2250.832 |
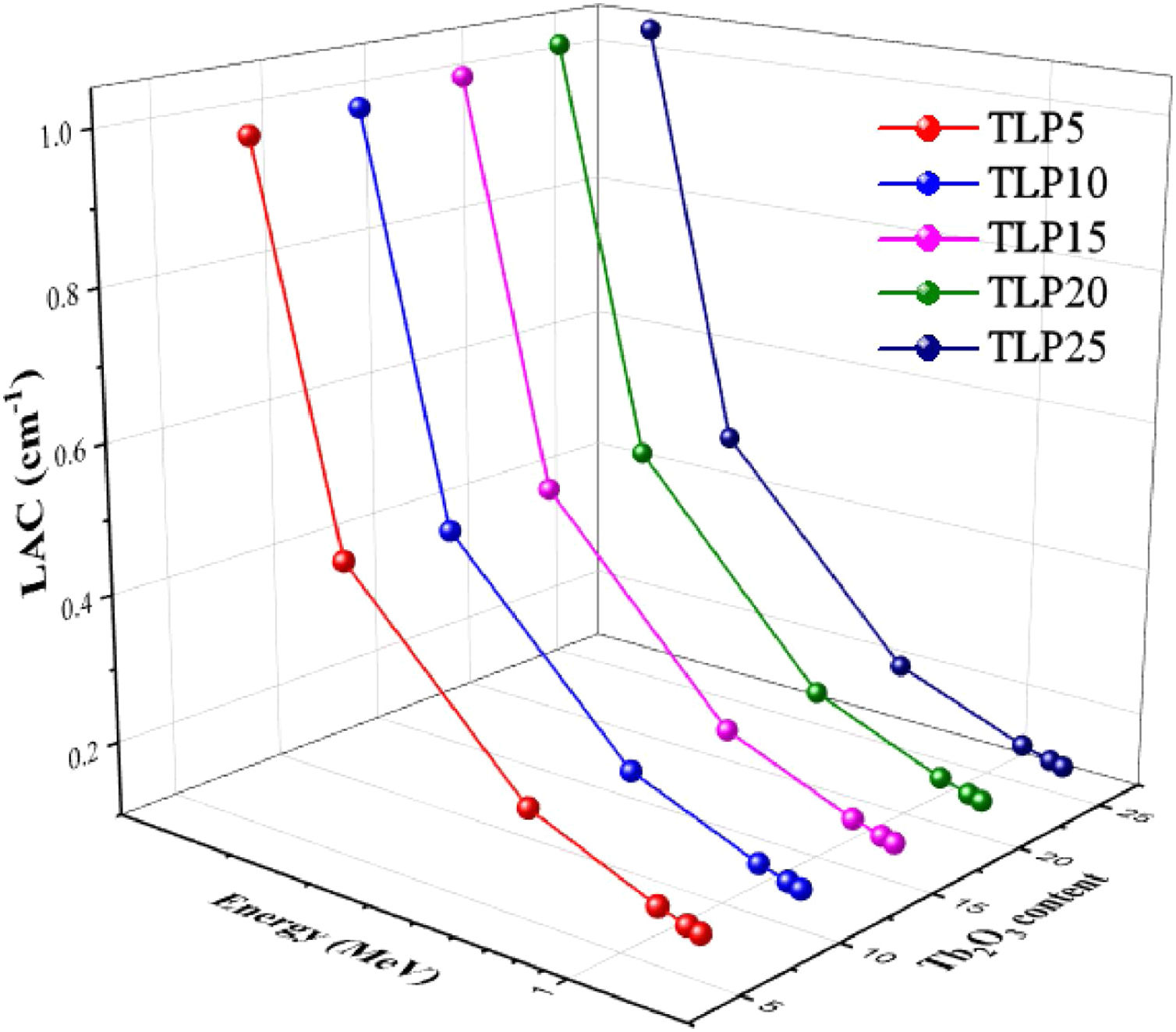
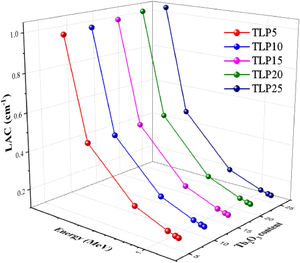
Fig. 5 displays the LAC for the TLP glasses. According to this figure, the LAC is affected by the incident energy. Also, it is affected by composition of the TLP glass. The highest LAC is found 0.184MeV, and increases from 0.99 to 1.024cm−1 for TLP5 with 5mol % and TLP 20 with 20mol%, respectively. It was also observed that with increasing the incoming radiation energy, the simulated LAC values decreased due to Compton scattering interaction (CS) [30]. The lowest LAC values were reported at 1.408MeV and equal to 0.159cm−1 for TLP5 and 0.149cm−1 for TLP20.
The LAC was also affected by the chemical composition of the TLP glasses. The molecular weight of phosphate glass affected increases with the insertion of Tb2O3. Therefore, Zeff progressively falls, decreasing by increasing the molecular weight of TLP glasses. The lowest LAC was observed at TLP5 with 5% mol Tb2O3 and suddenly increased to higher values for the TLP20 with 20% mol Tb2O3. The TLP20 has higher LAC values than TLP5, TLP10, and TLP15, respectively. The LAC values for TLP5 glass decrease from 0.99 to 0.159cm−1 and for TLP20 glass from 1.044 to 0.149cm−1 between 0.184 and 1.408MeV. The LAC is found to increase with the insertion of Tb2O3 into the phosphate specimens. The previous trend in the LAC is related to the CS.
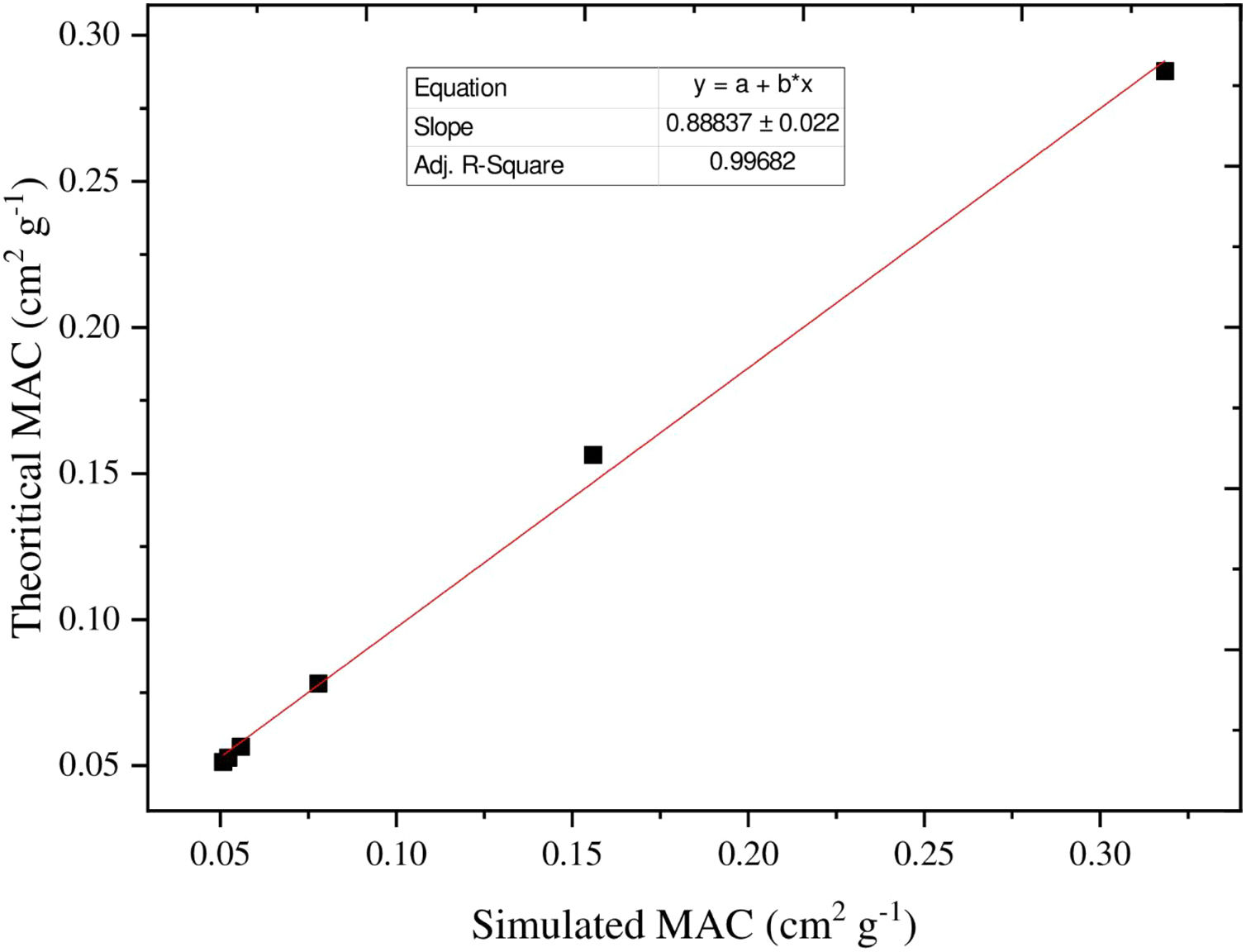
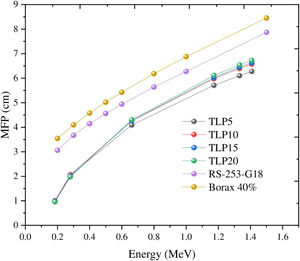
Additionally, the MAC can be estimated based on the values of simulated LAC for TLP glasses. The MAC's simulated values were compared with the theoretical MAC values, which were determined from the XCOM software. The strong correlation between the two approaches used to evaluate the MAC for the chosen TLP5 glass and is introduced in Fig. 6. We also found good agreement for all the remaining samples and a strong correlation between the MAC obtained from the two approaches. The difference (%) between the simulated and theoretical MAC was detected via the following Eq. (13) and documented in Table 3:
The simulated (MCNP-5) and XCOM MAC for the examined samples.
| Mass attenuation coefficient (cm2/g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glass sample | TLP5 | TLP10 | TLP15 | TLP20 | ||||||||
| E (MeV) | MCNP-5 | XCOM | Difference (%) | MCNP-5 | XCOM | Difference (%) | MCNP-5 | XCOM | Difference (%) | MCNP-5 | XCOM | Difference (%) |
| 0.184 | 0.3185 | 0.2877 | 9.6644 | 0.3374 | 0.3059 | 9.3317 | 0.3462 | 0.3236 | 6.5226 | 0.3585 | 0.3410 | 4.8939 |
| 0.28 | 0.1560 | 0.1563 | −0.2036 | 0.1619 | 0.1623 | −0.2340 | 0.1677 | 0.1681 | −0.2173 | 0.1734 | 0.1739 | −0.2712 |
| 0.662 | 0.0779 | 0.0780 | −0.2021 | 0.0785 | 0.0787 | −0.2172 | 0.0792 | 0.0794 | −0.2373 | 0.0798 | 0.0800 | −0.2462 |
| 1.173 | 0.0558 | 0.0564 | −1.0751 | 0.0560 | 0.0566 | −1.1316 | 0.0561 | 0.0568 | −1.1935 | 0.0562 | 0.0569 | −1.2423 |
| 1.332 | 0.0522 | 0.0527 | −0.9088 | 0.0523 | 0.0528 | −0.9505 | 0.0524 | 0.0530 | −0.9952 | 0.0525 | 0.0531 | −1.0436 |
| 1.408 | 0.0508 | 0.0512 | −0.8084 | 0.0509 | 0.0513 | −0.8459 | 0.0510 | 0.0514 | −0.8880 | 0.0511 | 0.0515 | −0.9335 |
The difference (%) was found lower than 10% for the TLP5, TLP10, TLP15 and TLP20 glasses.
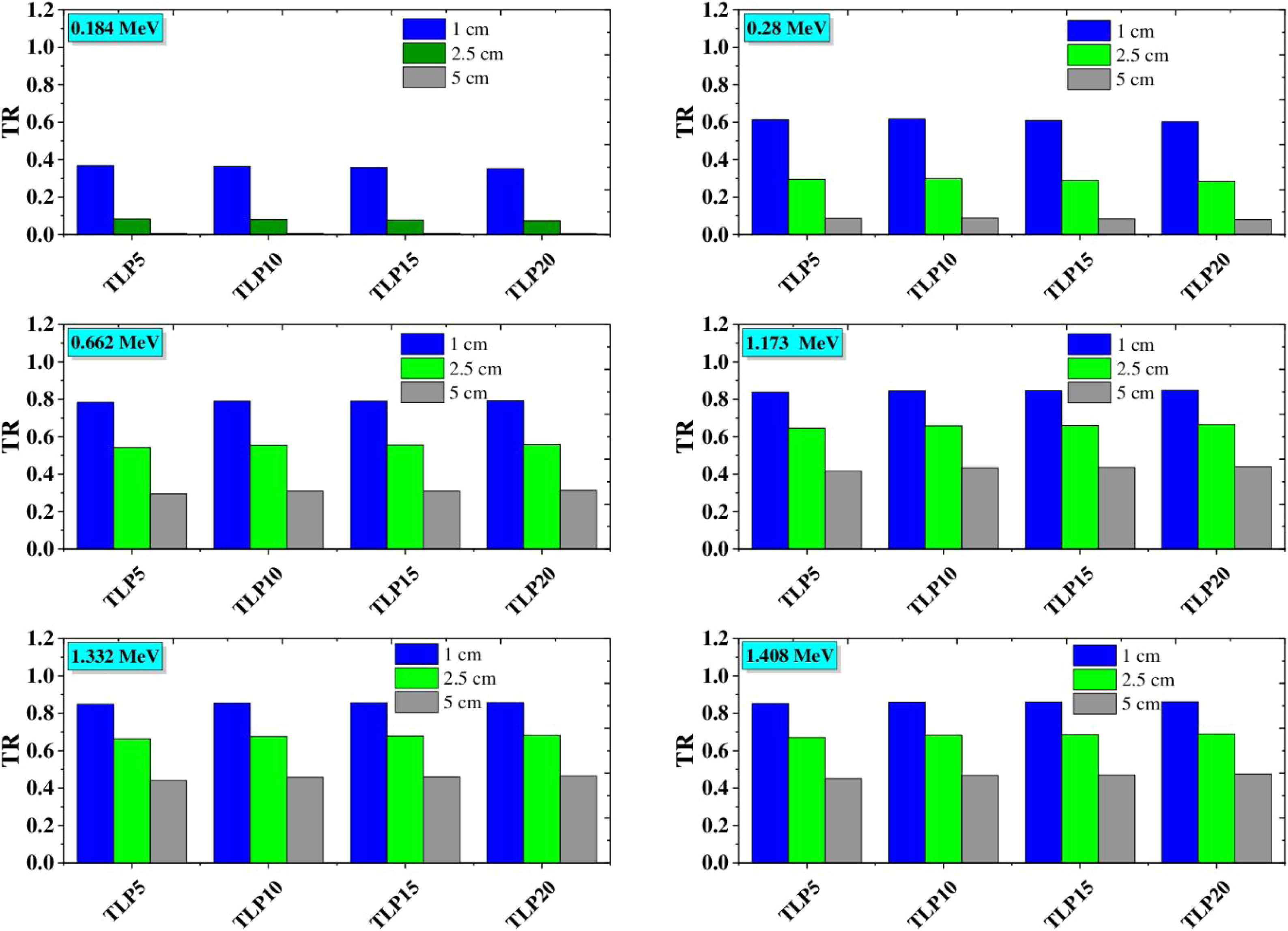
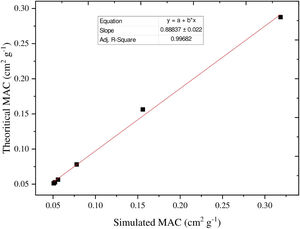
The transmission rate (TF) of incident gamma radiation through the investigated glass material was studied and presented in Fig. 7. The transmission rate is an indicator of the number of incident gamma radiation interactions with the glass materials. Therefore, Fig. 7 illustrates the variation of TR values with the incident gamma energy and the thickness of TLP glasses. First, At the same thickness of the investigated TLP glass, the TR varies linearly with the incident gamma energy. The highest values of TR were observed at high energies (1.408MeV) and decrease from 0.85 to 0.45 for TLP5 and diminishing from .0.86 to 0.47 While at low incident gamma energy (0.184MeV), the TR has low values and decrease from 0.37 to 0.007 for TLP5, and from 0.35 to 0.005 for TLP20. The transmission rate of incident gamma depends on the interactions inside the glass material, which led to a decrease in the gamma wavelength. Thus, the number of interactions of the gamma radiation decrease and can be penetrated the glass material. Therefore, the TF of incident gamma radiation increases. The second main factor is the thickness of TLP glasses. The variation of TR with the thickness of TLP glasses was presented in Fig. 7. The transmission rate at incident gamma energy (0.184MeV) decreases from 0.37 to 0.007 for TLP5 glass with thickness of 1 and 5cm, respectively. The increasing of the specimen's thickness leads to the TR of incident gamma radiation decrease. This is because the photons will spend more time passing through the glass material, and the number of incident gamma interactions with glass materials increase.
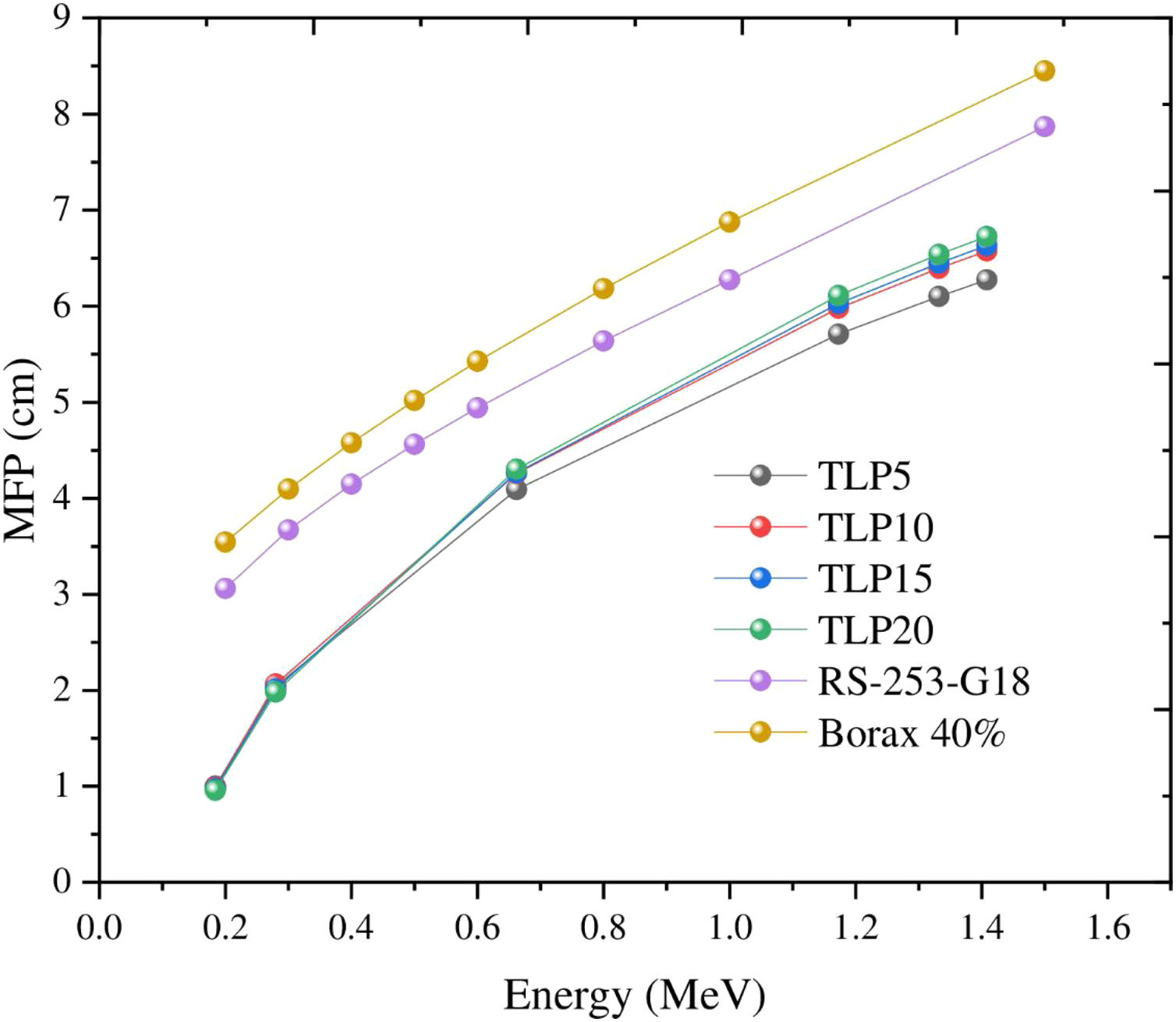
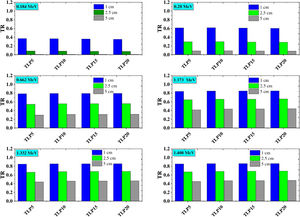
Fig. 8 depicts the mean free path for the TLP5, TLP10, TLP15 and TLP20 glasses. Also, in the same figure, the MFP for the aforementioned glasses is compared with the RS-253-G18 glass as well as the previously borax glass 40%. Fig. 8 displays the MFP of TLP glasses increases with increasing the energy. The highest and lowest MFP was found at 1.408MeV and 0.18MeV, respectively. The values of MFP for the TLP glasses are lower than Borax 40% and the RS-253-G18. Therefore, the TLP glasses are convenient high-density shielding glass.
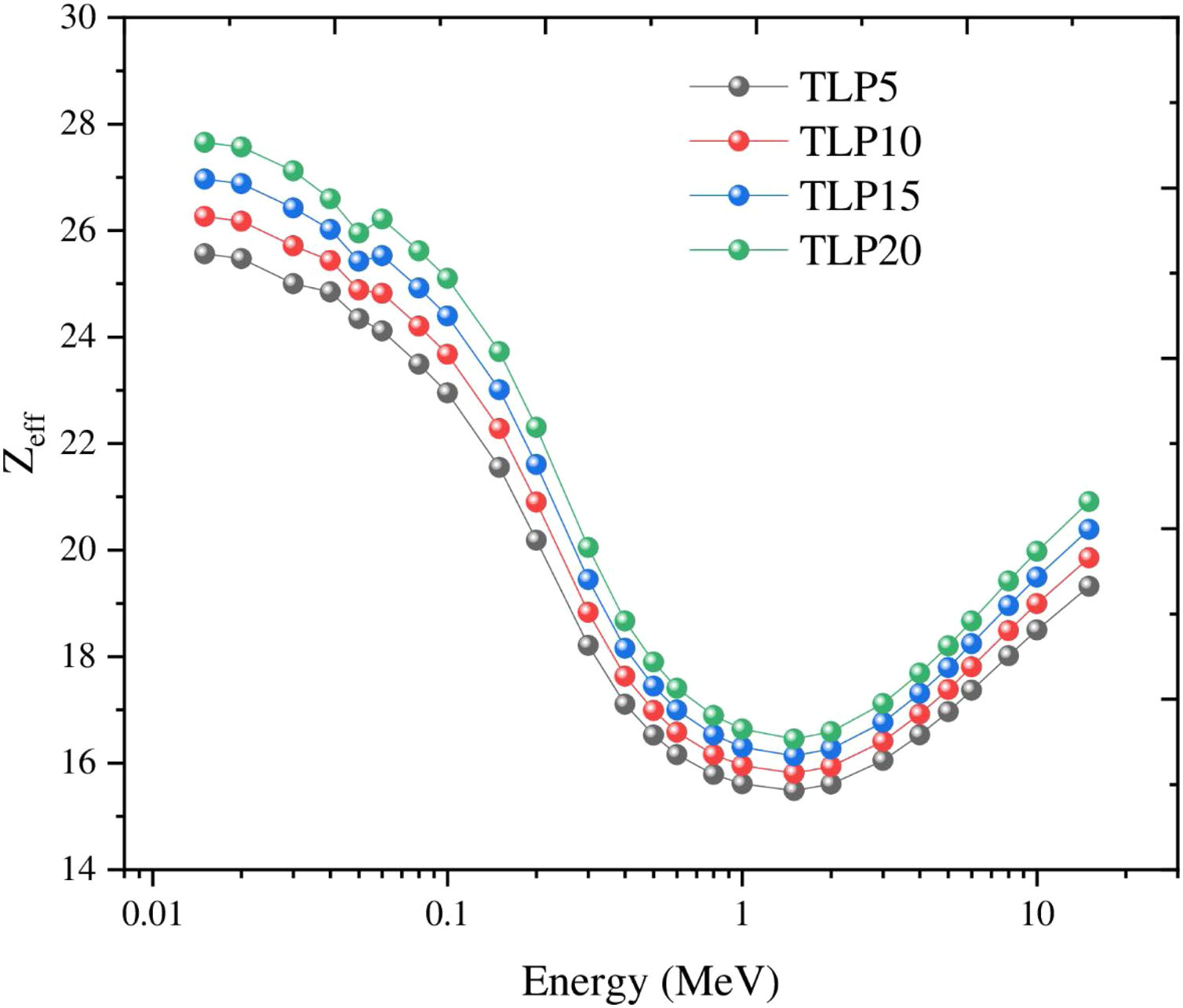
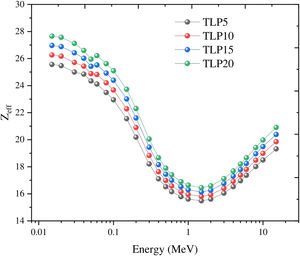
In this study, the other shielding parameters such as Zeff, Zeq, EBF, and EABF were reported via the BXCOM program database in the range of incident gamma energies (0.015–15MeV). Fig. 9 illustrates the Zeff for the tested TLP glasses. At low energies (<0.1MeV), the photoelectric effect (PE) is the prevalent process, with increasing the incident gamma energy, the Zeff values found drop progressively decrease, but can observe unexpected peaks at 0.0552MeV. After that, the Compton scattering interaction (CS) was observed at an energy range (>0.1–1MeV). The Compton scattering reduces with increasing the incident energy. This is attributed from the cross-section of CS where (σCSαE−1). Then it can be observed that the Zeff was raised with increasing the incident gamma energy above 1MeV.
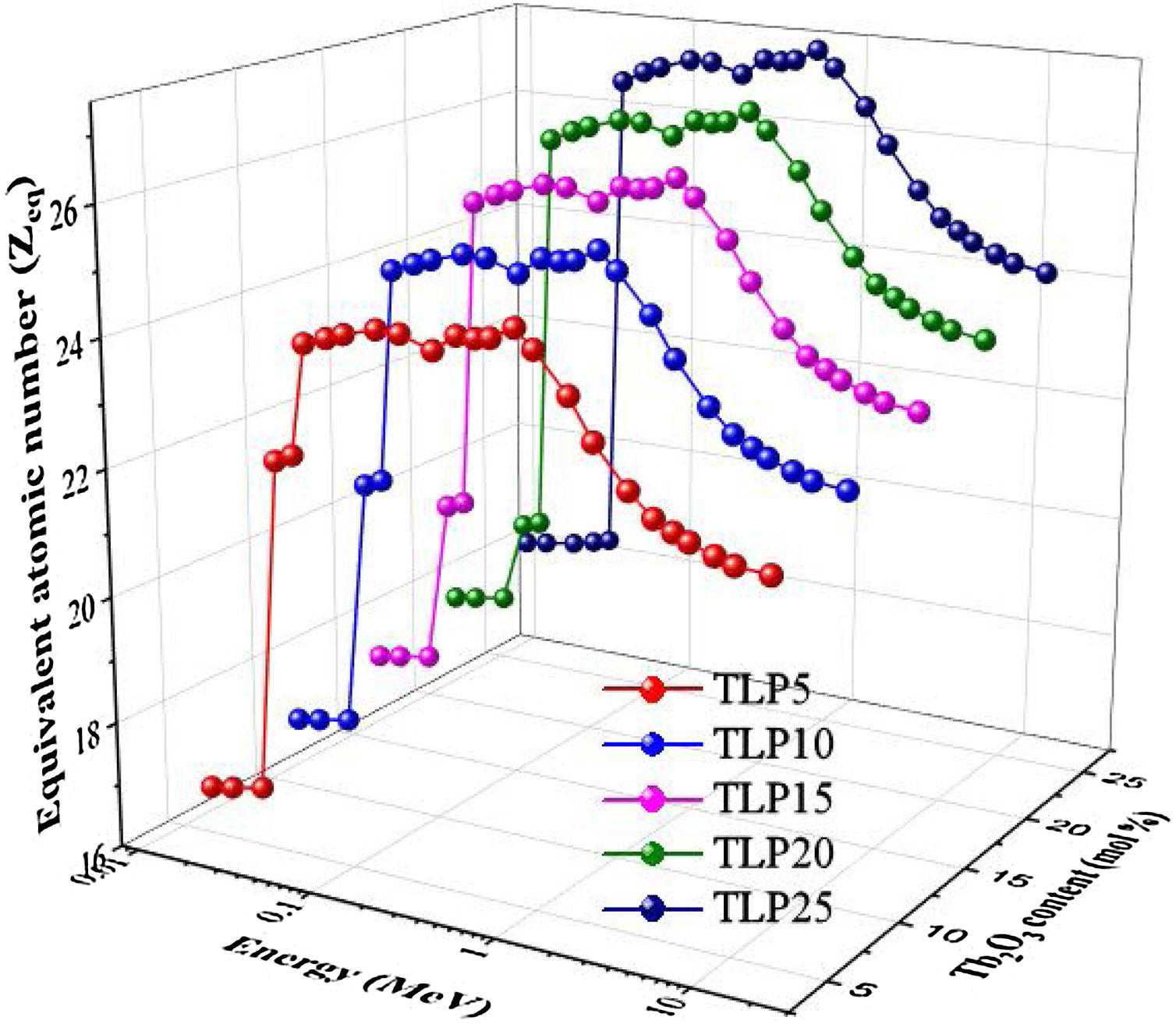
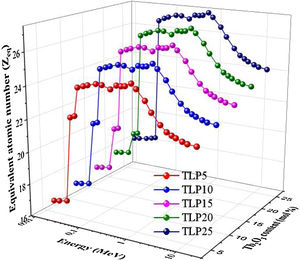
Fig. 10 represents the changes in the Zeq versus the incident energy for the TLP samples. The smallest Zeq is achieved at energies lower than 0.1MeV, where the PE region. Then, the Zeq values increased gradually with increasing the energy where the CS region was started. Then Zeq values speedily decrease with increasing the energies in the PP region.
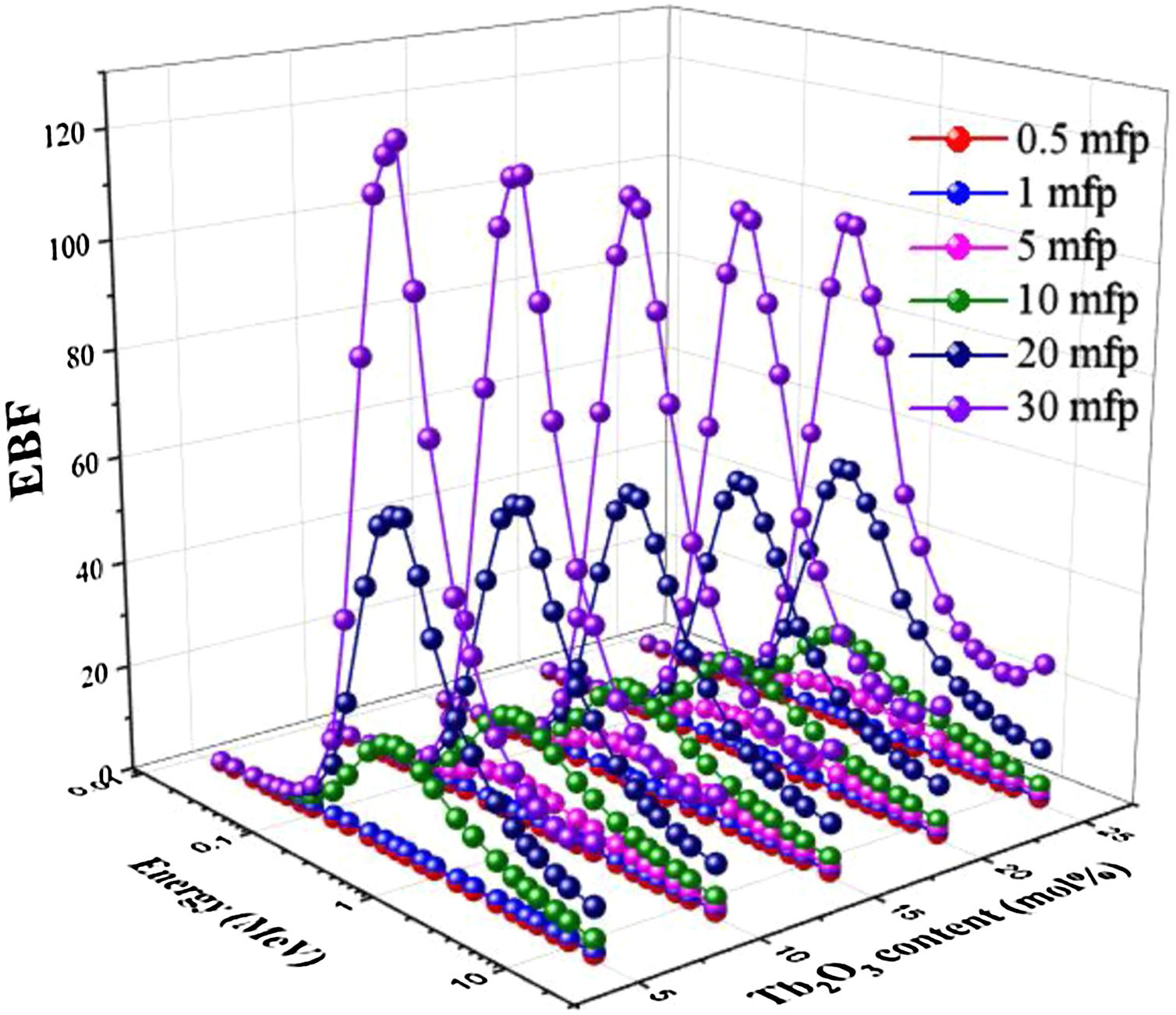
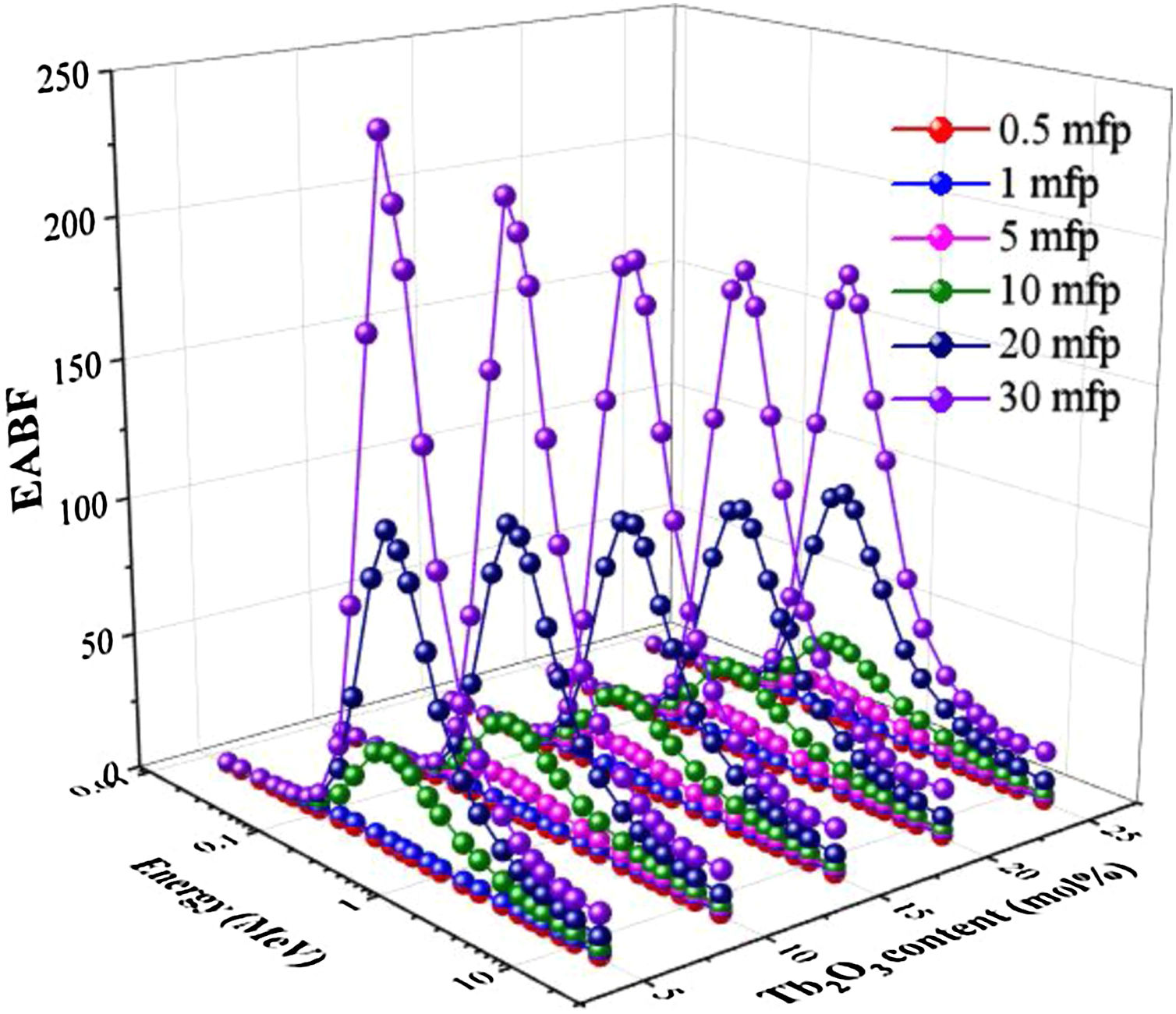
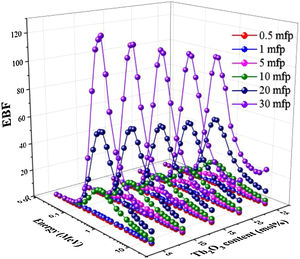
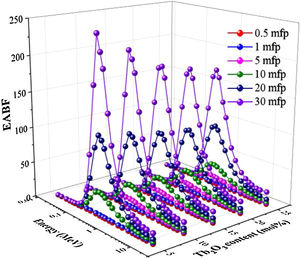
Two building factors that can describe photons’ accumulation inside the materials of TLP glasses are the exposure building factor (EBF) and the energy absorption building factor (EABF). Figs. 11 and 12 exhibit how the buildup factors change with the incident gamma energy, penetration depth in mfp unit, and the content of Tb2O3 for the TLP glasses. The EBF and EABF have low in the PE region, where the incident gamma's energy removes all incident gamma photons. Then, both factors given in Figs. 11 and 12 increase with increasing the energy. The CS interaction inside TLP glasses’ material increases and a small part of the incident photons passes through the material. Hence the rest part of the incident photons produce scattered photons that accumulated inside the investigated glass material. For E>1.5MeV, the PP interactions are dominant.
Furthermore, the EBF and EABF were raised regularly by increasing the PD up to 30 mfp. The photons spent more time to pass through the thickness material of TLP glasses. Thus, the more interactions will produce, and the photons accumulation will increase inside the TLP samples. The highest values were noticed at high penetration depth PD (PD=30mfp), while the lowest values were detected at 0.5mfp.
Figs. 11 and 12 display the variation of estimated EBF and EABF values with the insertion of the Tb2O3 content in the examined glasses. Apparently, both factors are diminish gradually with the increment of the Tb2O3 content (mol%) in the TLP glasses. The highest and lowest EBF and EABF are achieved at 5 and 20mol% of Tb2O3. Where the insertion of Tb2O3 content in the TLP glasses don’t leads to accumulate the photons in the examined glasses at the high content of Tb2O3.
ConclusionThe role of Tb2O3 on the mechanical and radiation protection characteristics was studied for 75P2O5+(25−x)La2O3+xTb2O3 glass systems. The results revealed that the dissociation energy (Gt) changes between 37.95 and 39.38kcal/cm3 with increasing the Tb2O3 substitution ratio between 5 and 20mol%. The packing density(Vt), Young's (E), shear (S), Bulk (B), and Longitudinal (L) moduli decreased between 0.554–0.402cm3/mol, 42.06–31.36 Gap, 27.96–15.24 GPa, 16.83–13.70GPa, and 50.41–33.54GPa, respectively. The shielding capacity against gamma-photons was evaluated using the MCNP-5 code between 0.184 and 1.408MeV. The best MAC achieved for the investigated glass sample TPL20 varied between 0.358 and 0.0515cm2/g in the examined energy range. The replacement of La2O3 with Tb2O3 results in an enhancement in the MAC values by 11.03% at 0.184MeV. The highest and lowest MFP was found at 1.408MeV and 0.18MeV, respectively. The values of MFP for the TLP glasses are lower than Borax 40% and the RS-253-G18. Moreover, the highest values of TR were observed at high energies (1.408MeV) and decrease from 0.85 to 0.45 for TLP5 and diminishing from .0.86 to 0.47. While at low incident gamma energy (0.184MeV), the TR has low values and decrease from 0.37 to 0.007 for TLP5 and from 0.35 to 0.005 for TLP20.
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program to support publication in the top journal (Grant no. 42-FTTJ-22).