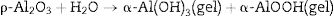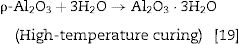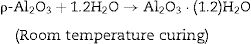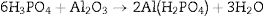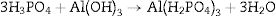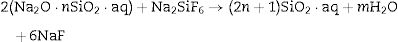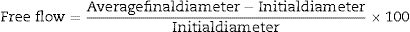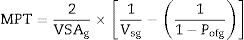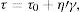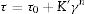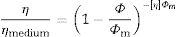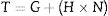Ceramic engineers have investigated the rheology of the refractory concrete to keep the balance between the desired characteristics of castables and its flow demeanor. The rheology of refractory concrete determines their application manner and a considerable fraction of its properties are largely affected by its flowability. This article gives a brief introduction to refractory concretes. It discusses the variable determinants of castables’ rheology according to their significance and their relation to each other. The measurements of rheology were examined by conventional techniques and the mathematical models of viscosity and rheometry approaches are also used for clarification. Insights into the rheology of alumina–silica containing castables were speculated through exploring submicron and nano-sized particles. The rheology of refractory concrete can be adapted properly according to the application manner and the specified requirements.
Refractories are inorganic non-metallic material capable of bearing high temperatures without damaging. They should also perform other purposes which include minimization of heat loss through kilns, transfer of heat to the materials inside kilns, and smooth flow of material through them. They are classified based on different bases: (1) According to the chemical structure, they are classified based on their major constituents e.g. silica brick: a refractory that contains at least 93wt.% silica. Fireclay brick: an aluminum silicate refractory with silica content up to 78% and alumina amount up to 44%. High alumina refractory: an aluminum silicate refractory with a dominant alumina phase reaching more than 45%. Mullite refractories: consist of 71.8wt.% alumina and 28.2wt.% silica. Corundum brick: a refractory structure with a single-phase polycrystalline α-alumina (more than 99wt.% Al2O3). Magnesite refractories: at least contain 85wt.% magnesium oxide. Dolomite refractories: usually contain less than 2.5 impurities and greater than 97.7% (CaO+MgO). (2) Based on the chemical behavior: refractories are classified to (a) acidic refractories: they are chemically noble toward acidic compounds but are attacked by alkalis e.g., silica. (b) Basic refractories: they are non-reactive toward basic compounds but are attacked by acidic compounds e.g., magnesite and dolomite. (c) Special refractories: like silicon carbide, zirconia, and carbon refractories. (3) Based on the physical state: refractories are classified into shaped refractories (bricks) and unshaped refractories (monolithics) [1]. A simple comparison between refractory bricks and refractory monolithics is shown in Table 1[2].
Simple comparison between refractory bricks and refractory monolithics.
| Refractory brick | Refractory monolithic | |
|---|---|---|
| Definition | Fired pre-casted refractories in a fixed shape prior to installation | Unfired materials with different particle sizes that are directly poured into the installation site |
| Uniformity | More uniform because they do not contain cement and fired at high temperatures without using anchors | Less uniform and is used by using anchors |
| flexibility | More flexible due to more lining joints | Less flexible |
| Ease of use | Labor intensive which Requires time and highly skilled technicians | Simple and less time consuming but carful curing is required |
| Pore size | Pore size ranges from 20 to 25μm | Micro porous structure with pore diameter 1–2μm |
| Strength and thermal shock resistance | Low strength and TSR due to relatively large pore diameter size | High strength and TSR due to small pore diameter |
| Thermal conductivity | Higher thermal conductivity | Lower thermal conductivity due to lower radiation heat transfer through small pore size |
| Corrosion resistance | Low corrosion resistance | Higher corrosion resistance |
| Deformability | Less flexible | More flexible especially phosphate-bonded castables |
| Dimensional stability | More stable as it is a fired product | Less stable due to shrinkage during firing in the installation place |
Refractory castables can be classified according to its lime (CaO) content [3] into conventional castables (CC) that contain 15–30% calcium aluminate cement (more than 2.5% CaO), low cement castables (LCC) where 2.5>CaO>1.0, ultra-low cement (ULLC) where 1.0%>CaO>0.2%, and no cement castables (NCC) where CaO<0.20 [3].
Different types of castables require different proportions of water during their preparation. The amount of mixed water during the pouring of castables increases with the lime content. The significant water part reacts with the cement to form hydration products and the remainder part aids for proper installation and did not take part in the reactions generating porosity after firing [3] result in lowering the high-temperature characteristics of castables. In this sense, the high water content in the conventional castables (about ∼8–15%) deteriorates their high-temperature properties; thus, low cement (LCCS) and ultralow cement castables (ULCCS) were then developed to minimize the water required for placement [3] while holding the strength. LCCS and ULCCS usually require 3–7% water content depending on the grade so they are characterized by high density, low porosity, high hot strength, and corrosion resistance [4].
The amount of added water determines the left porosity after drying and firing. A conventional castable has a porosity ranging from 9 to 17% after drying and from 20 to 30 after heat treatment at 1000°C otherwise LCCs with porosity not exceeding 10% after drying and 16% after firing with finer pore size distribution. However certain problems could emerge from reducing cement content such as longer working time when the castables are applied in-site [5].
Refractory castables compositionCastables are composed of refractory aggregates with different sizes from 300μm to 20mm for strength and hot properties beside matrix components, bonding agents, modifiers, and admixtures [6] as shown in Table 2[7]. The most abundant aggregates are fused or sintered alumina because they have low thermal expansion coefficient and high strength combined with good insulation properties. The aggregate Skelton is filled by finer particles (modifiers or fillers) which are responsible mainly for rheology and flow characteristics while the structure is held together by using a binder or bonding agent. Table 3 lists the widespread common modifiers/fillers used in refractory castables. The properties of the castables which include expansion control, bond enhancement, and mineralogy/chemistry adjustment can be controlled by using additives or admixtures in small amounts ranging from 0.05 to 0.5wt.% [3]. They can be organic, inorganic, or a combination of them [3]. Table 4 lists the widespread common additives used in refractory castables.
Common modifiers used in refractory castables.
| Filler/modifier | Chemical formula | Function |
|---|---|---|
| Fine milled aggregates | Various | Chemistry, mineralogy and adjustment, bond modification and development |
| Calcined alumina | α-Al2O3 | Chemistry adjustment, bond modification and development |
| Reactive alumina | α-Al2O3 | Flow/rheology control, bond modification and development |
| Silica quartz | SiO2 | Shrinkage control |
| Kyanite | 3Al2O3·3SiO2 | Shrinkage control (1325–1410°C), chemistry and mineralogy adjustment |
| Clay | Hydrated alumino-silicate | Filler, flow/rheology control |
| Zircon | ZrSiO4 | Reduce metal, slag, attack |
| Graphite/carbon | C | Reduce metal, slag attack |
| Fly ash | Varies | Low-temperature filler |
Common admixtures in refractory castables.
| Additive | Function | ||||
|---|---|---|---|---|---|
| Accelerator | Retarder | pH control | Water reducer | Rheology modifier | |
| Lithium carbonate | × | ||||
| Calcium hydroxide | × | × | |||
| Sodium carbonate | × | × | |||
| Sodium bicarbonate | × | ||||
| Sodium citrate | × | × | × | ||
| Sodium phosphate | × | × | × | ||
| Sodium polyacrylate | × | × | |||
| Polycarboxylate | × | × | |||
| Citric acid | × | ||||
| Boric acid | × | ||||
The first successful attempt to produce low cement castables with cement content from 5 to 8% and significant mechanical properties was granted in 1969 by Pros and Pauillac [3]. Another two patents in 1976 and 1977 succeeded to reduce the cement content less than 3% by using dispersants such as sodium tripolyphosphate in amounts (0.01–0.05wt.%) and fine matrix [3,8]. They replaced part of the cement with sub-micrometer particles ranging from 0.1 to 0.01μm which could easily be dispersed without forming a gel or a sol. The reduction in the amount of water used can be done by carefully grading the particles so optimum particle packing can be obtained which ceases the amount of water used to fill the voids between particles.
Cement free castables were then developed with further higher refractoriness due to the very low calcium oxide where they have superior corrosion resistance toward metals and slags but have lower physical and mechanical properties compared to LCCs and ULCCs [3,9]. A multitude of bonding agents was used including hydratable alumina, silica gel, clay, and phosphates [3,10,11]. The most common bonding agent of non-cement castables is hydratable alumina which is produced from the rapid dehydration of gibbsite Al(OH)3. When γ-Al2O3 reacts with water, it forms pseudo-boehmite (AH1–2) and bayerite (AH3) and gives the stable form (α-Al2O3) upon dehydration and then forms ceramic bonds at high temperatures [3,10]. This reaction can be speeded by alkali metal salts addition or slowed down by carboxylic acids and the silica could be added to form mullite or 0.5wt.% of cement is used with hydraulic alumina to control the setting time. The drawbacks of using hydratable alumina are explosive spalling around 200–300°C and curing must start at 18°C minimum beside the expensive cost [3,10].
Cardoso [12] compared the drying behavior of hydratable alumina-bonded (HAB) refractory castables to ultra-low cement compositions and found that HAB evaporation peak is lower than ultra-low cement castables (ULCC) with calcium aluminate cement (CAC) which indicate low permeability of HAB due to the formation of gel phases which restricts water vapors escape. Zhang et al. [13] experimented with the addition of different proportions (2, 4, and 6wt.%) of hydratable alumina (HA) to no cement bauxite–andalusite based castables and investigated castables performance. They found that the hot modulus of rupture, the cold crushing strength, and the thermal shock resistance were improved as HA increased from 2 to 6% meanwhile the permanent linear change decreases. Recently, Studart et al. [14] succeeded to develop cement free self-flowing castables through precise control of particle size distribution and rheology.
Bonding mechanisms in refractory castablesThe bonding system could be based on carbon from pitches and phenolic resins, mullite, and glass from clay, and/or alumina from calcium aluminate cement [15]. The bonding agents can be classified as hydraulic, chemical, coagulation bonding, and sol–gel.
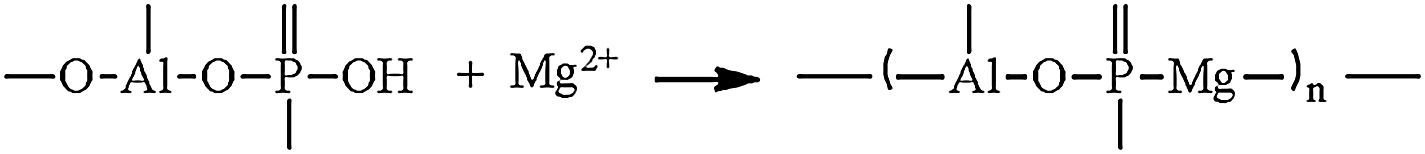
Hydraulic bondingHydraulic bonding is where the binders react with water at ambient temperature causing hydrate formation which gives the castables the green strength at lower temperatures. The binders include calcium aluminate cement (CAC), silicate cement, and hydratable alumina. In CAC, the green strength is supplied by coagulation of a crystallized network through the formation of C2AH8, C3AH6, and alumina gel (AH3) which then dehydrates at intermediate temperatures up to 900°C accompanied by a loss in mechanical strength due to bonds degradation and formations and finally strength increases again at high temperatures >1000°C as a result of ceramic bond formations. Hydratable alumina is low crystalline intermediate transition hybrid aluminas (e.g. κ, θ, ρ) which have hydraulic reactivity when reacting with water [16]. It is manufactured via flash calcination of gibbsite [Al(OH)3] through the formation of high specific surface intermediary compounds ρ-Al2O3[17,18]. The hydration occurs by reaction with water through the formation of pseudo-boehmite and bayerite which is responsible for the castable green strength. A general mechanism of hydration is given in Eq. (1)[7].
Different compounds (AH3) or (AH) gel could be formed depending on the curing temperature as shown below in Eqs. (2) and (3):
The developed osmotic pressure between alumina core and water surrounding the gel layer causes micro-cracks of the gel layer with the formation of boehmite (Al2O3·1.2H2O) and mostly bayerite (Al2O3·3H2O). When the temperature is raised, it loses its chemically binding water giving the stable form of alumina α-Al2O3 then a ceramic bond is formed when the temperature is further increased.
Chemical bondingThis category includes binders that form chemical bonds between the castables constituents at ambient or slightly elevated temperatures. The bond could be formed by new product formation or by polymerization between the binder and oxide refractory aggregates. The binders include sodium or potassium silicates, phosphoric acid, or phosphate salts such as monoaluminium phosphates or alkali phosphates.
Phosphate bindersPhosphate binders containing castables are characterized by high corrosion resistance toward molten metals and slags. They can be applied to the installation site by casting, ramming, or gunning due to their availability in different grades. They work by the reaction between the castables components and orthophosphoric acid at temperatures higher than 127°C can be indicated by Eq. (4)[20]:
or by the direct reaction between acid derived component like monoaluminium phosphate and the matrix [21,22]. The matrix can be basic or amphoteric oxides such as alumina, magnesia, and zirconia [23]. The monoaluminium phosphate is synthesized via the reaction between phosphoric acid and aluminum hydroxide in the temperature range 25–90°C as shown in Eq. (5)[23,24].In alumina based castables, the phosphoric acid forms Al(H2PO4)3 which is subsequently converted to Al(PO3)3 in the temperature range 400–500°C. Upon heating to 1300–1400°C, the Al(PO3)3 is converted to AlPO4 and finally to alumina with the release of P2O5 when further heated to 1700°C [1,25]. Monoaluminum phosphate binder can be also used with alkaline metal oxide setting additives such as MgO that is added to infer the required setting characteristics at ambient temperature. The reaction between monoaluminum phosphate and magnesia forms Mg(H2PO4)2. The green strength is obtained from the dehydro-polymerization reaction between aluminum phosphate and magnesium oxide as illustrated by Eq. (6) [19].
(6)Calcium aluminate cement is another setting additive that can be used with monoaluminum phosphate forming a composite bonding phase of Ca(H2PO4)2 and 3CaO·Al2O3·6H2O. Also, alkali phosphates such as sodium phosphates and alkaline phosphates can be used as a binder. The disadvantage of monoaluminum phosphate binder is setting time control [26] besides its high reactivity toward atmospheric water when used in a spray-dried form which limits its shelf life so alternatives such as mono-magnesium phosphate can be used to overcome that problem but it shows low strength values due to its limited solubility in water which is counteracted by high water addition. Moreover, the release of P2O5 gases from phosphate binders harms the equipment and the environment [16].
Silicate bindersSilicate binders are characterized by superior workability and strength when setting is under atmospheric air. The alkali metal silicates such as sodium silicate are used in combination with other hardening agents such as boric acid or sodium borates (Na2B4O7) to infer some desirable properties such as plasticity or hydration resistance. Sometimes sodium fluorosilicate can be used with water glass as a refractory binder in castables composition [27]. The bonding mechanism of silicate binder depends on siloxane (SiOSi) network formation after firing and dehydration from the aqueous sol (SiO2·nH2O or Si(OH)4). The setting mechanism occurs through the gelification process in an acidic medium. The setting time of the reaction can be accelerated with certain compounds additions such as sodium fluoride, calcium silicate, aluminum polyphosphate. For instance, sodium silicate (Na2O·nSiO2·aq) reacts with sodium fluorosilicate to form bonds as the reactions indicated by Eqs. (7) and (8)[19,28]:
The resultant gel is completely dehydrated at 350°C. Other setting agents could be used such as aluminum polychloride, aluminum polyphosphate, sodium phosphate, magnesium aluminum polyphosphate, and calcium and magnesium hydroxides [29,30]. The drawbacks of sodium silicate binders back to low corrosion resistance toward molten.
Coagulation bondingThe driving force behind coagulation is induced by Van der Waal forces which act on the particles nearby making it coagulate. The common binders for coagulation bonding are fine clay powder, ultrafine oxide powders, and silica or alumina sols. In colloidal suspensions saturated by micron and submicron-sized particles, electric double layers are formed which could counteract the van der Waals forces by inducing a repulsive force between particles. To induce particles agglomeration these repulsive forces must be minimized through the addition of contra ions which enter into the dispersion layer of the electric double layer and reducing the repulsion forces through electrical neutralization which compress the dispersion layer until making zero potential which is known by the isoelectric point [1,19]. Recently, nanopowders were used to enhance the bonding and sintering of castables at low temperatures due to its high specific surface area prone to the reaction but its downside parts involve high preparation costs and poor dispersion resulted from particle interactions so its use is limited. Table 5 summarized the development of refractory castables bonding agents with the time through history.
Development of refractory castables.
| Bonding mechanism | 1920s | 1930–40s | 1950s | 1960s | 1970s | 1980s | 1990s | 2000s | 2010s |
|---|---|---|---|---|---|---|---|---|---|
| Hydraulic bonding | Traditional cement bonded castables [high SiO2, high CaO+H2O] | ||||||||
| Pure CA cement castables [high CA+H2O] | |||||||||
| High purity cement castables [low CA+H2O] rho-alumina bonded castables [ρ-Al2O3+ultra-fine powders+H2O] | |||||||||
| Chemical bonding | Water glass bonded castables [Na2O·nSiO2+Na2SiF6] phosphate bonded castables[H3PO4 or Al(H2PO4)3+MgO or CA] | ||||||||
| Sulphate, chloride bonded castables[Al2(SO4)3+CA, MgCl2 or MgSO4] | |||||||||
| Polyphosphate bonded castables[Na5P3O10 or (NaPO3)6+MgO, CaO or CA] | |||||||||
| Resin bonded castables [phenolic resin, etc.]Vinyl polymers, pitch, dextrin. | |||||||||
| Hydraulic and coagulation bonding | Low cement castables [CA+clay or ultra-fine SiO2+H2O] | ||||||||
| Coagulation bonding | Clay bonded castables[Ca- or Na-clay+CA+deflocculants] | ||||||||
| Ultra-low cement castables[ultra-fine powders+CA]Cement free castables[ultra-fine powders+electrolyte]Sol–gel bonded castables[silica sol+electrolyte | |||||||||
Nanotechnology was recently used in castables bonding to lower sintering temperature by 100–200°C but castables agglomeration should be controlled [31–33]. Particles which form a suspension where the gravity is not enough to make them settle, are termed a colloid or sol. The particles are very small with size ranges from 1 to 1000nm. When sol agglomerates under certain circumstances, it forms a gel so sol–gel definition was deduced [1,34]. The sol–gel mechanism depends on the formation of a three-dimensional polymeric network (gel) of colloidal particles that hold the refractory aggregates together during the green strength which after firing is replaced by ceramic bond formations. The risk of explosive spalling during drying is eliminated as a result of porous structure formation from the gelation process and the absence of hydrated phases. This technique was previously employed to produce ceramic powders at low temperatures with preferable properties compared to the solid-state method [35,36]. Its benefit includes low mixing time, self-flowing nature, and high refractoriness due to the absence of CaO, better corrosion and oxidation resistance, and extended shelf life because of the absence of hydratable phases [37]. Moreover, it has volumetric stability at high temperatures although expansion could occur in some cases, for example, mullite formation from colloidal silica in alumina systems [38].
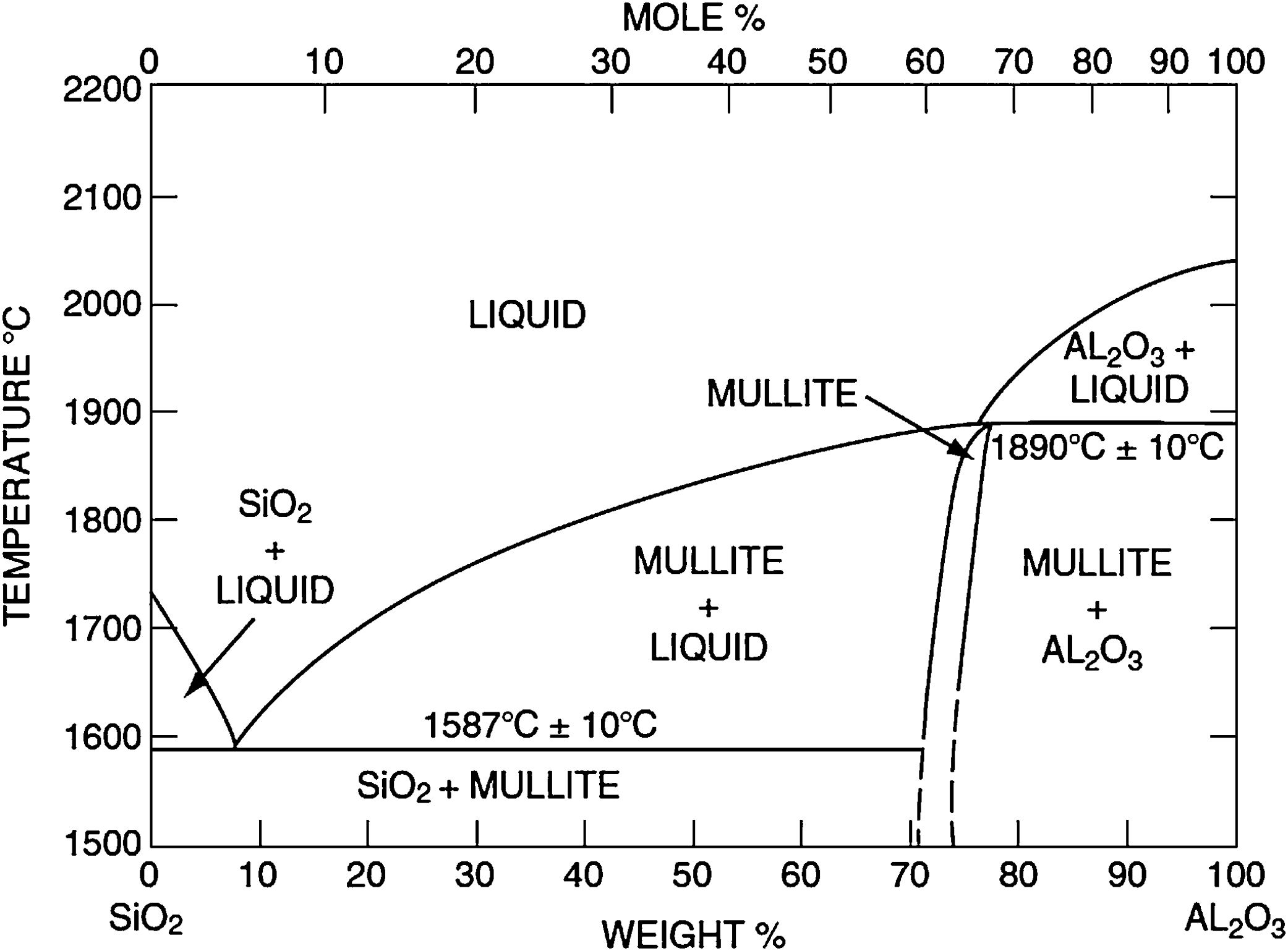
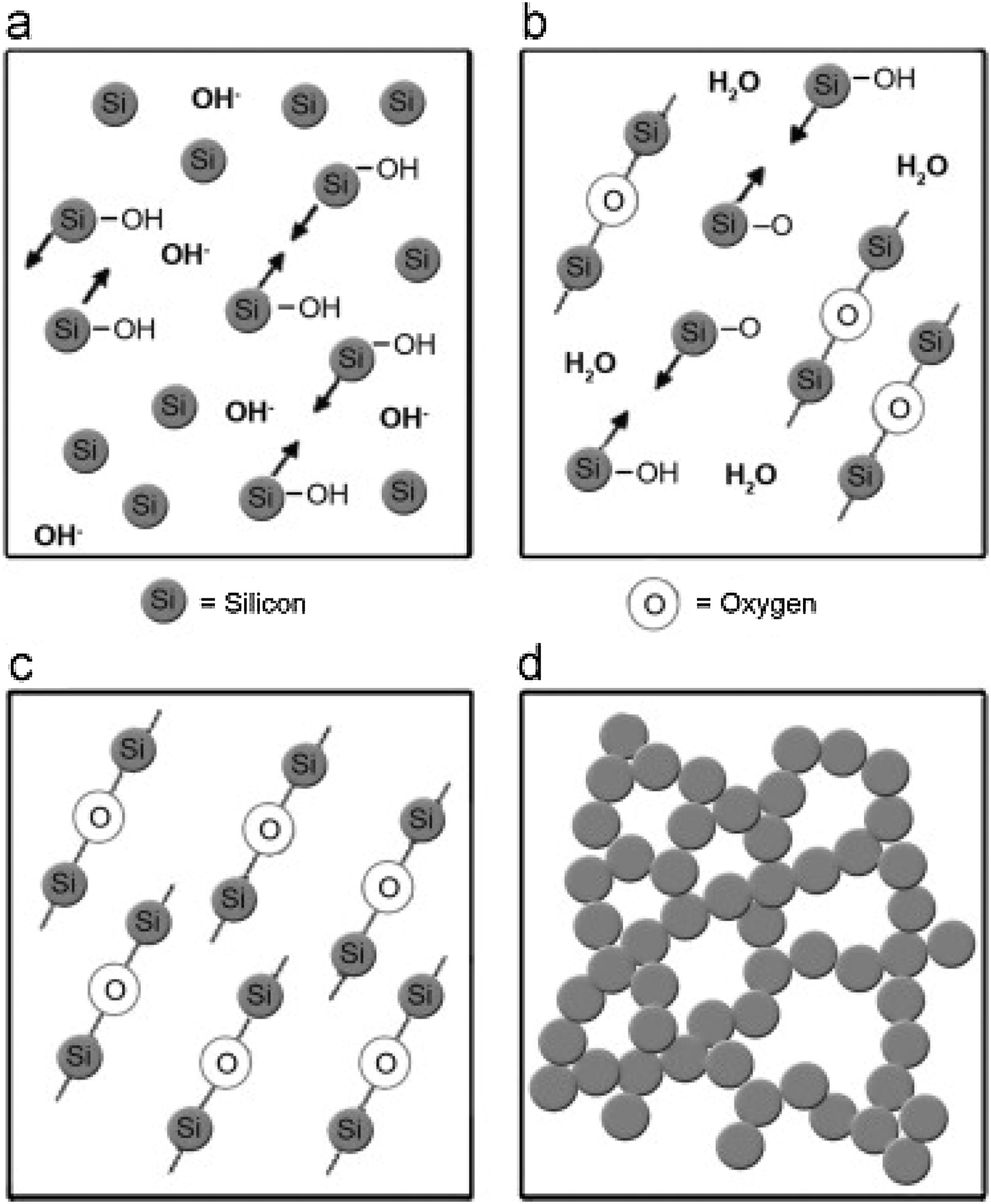
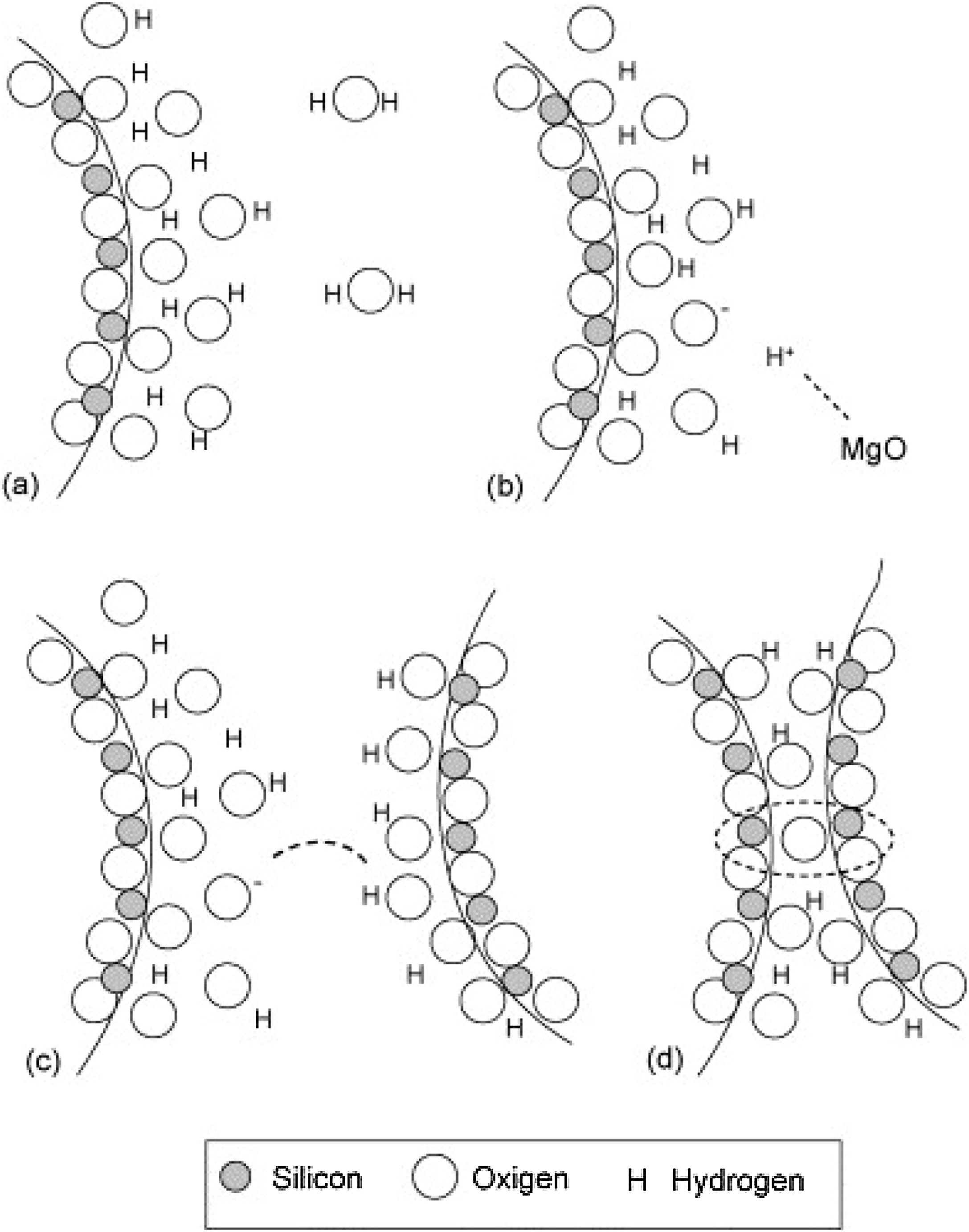
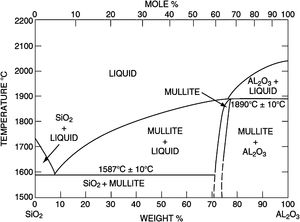
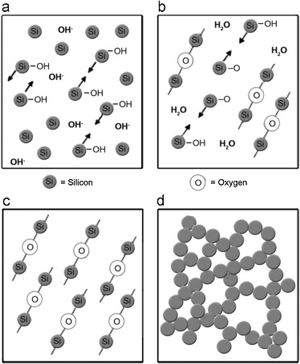
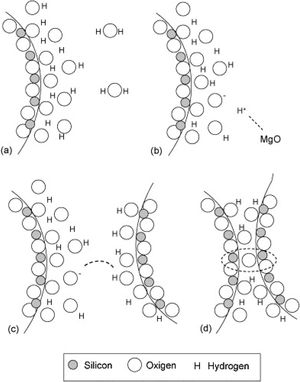
Silica solColloidal silica solution can be used to bind the castables which are hardened by using a small quantity of setting additive such as lime, magnesia, or calcium aluminate cement [29,39]. Dehydration of this system can take place easily at lower temperatures (<125°C). Silica sol is the only commercially available colloidal binder based on water suspensions containing spherical nanoparticle amorphous silica up to 50wt.% with diameter size (8–15nm). Its high specific surface area (200m2/g) can easily promote the formation of mullite during firing in alumina-based castables with supplementary mechanical strength fortification [16]. It is stabilized by sodium or potassium to pH 9–11 because of its anionic nature. It enhances mullite formation in an alumina-based system at low temperature but its usage is limited at higher temperatures >1600°C due to liquid phase formation in the Al2O3–SiO2 system as shown in Fig. 1[40]. There is a very limited solid solution of Al2O3 and SiO2 but at a higher temperature, the concentration of alumina increases in mullite otherwise binary metal systems which have significant solid solubility in pure components [41]. The mechanism of bonding depends on the formation of a 3-dimensional network of siloxane bonds (SiOSi) which entrap solid particles. First, hydroxyl groups (SiOH) are formed then water molecules are removed during drying resulting in siloxane bonds formation as illustrated in Fig. 2[31]. The isoelectric point of silica is at about pH=2 while increasing the pH number alters the surface area of silica by negative charges [42,43]. The expulsion of water can be assisted by pH variation or through adding gelling agents (salt, oxide, or organic solvent) for example addition CaO or MgO remove the hydrogen of hydroxyl groups through Mg(OH) or Ca(OH) formation [44]Fig. 3[45]. MgO is usually added in amounts from 0.3 to 0.6wt.% and the temperature of curing is around 25°C in micro silica and alumina-based castables [45]. Ca(OH)2 is more alkaline than Mg(OH)2 but Mg(OH)2 is more gelling than Ca(OH)2 due to less solubility of calcium hydroxide compared to magnesium hydroxide [45]. Also, the colloidal particles of opposite charges to silica like alumina can flocculate silica sols [42,46]. Phosphate can be used as a gelling agent with colloidal silica. Finally, the suspension can be aggregated by just drying without adding additives [31]. The obtained structure is substantially permeable which facilitates drying [16]. But Care should be taken when using silica sol in basic environments due to its acidic nature as the remaining free silica after firing sustain the acidic nature of the castables [47,48]. Other sols were then investigated to improve refractoriness, solid content, water requirement, flowability, and workability. Colloidal silica requires lower mixing energy compared to calcium aluminate cement and hydratable alumina due to large viscosity (10mPas) which hinders coagulation by keeping particles apart and lack of chemical reactions which interrupt ionic strength of the colloidal suspension [16]. Ismael et al. studied the mechanical behavior of silica-sol based refractory castables and found that its values are less sensitive toward curing conditions which accelerate the setting time of castable processing. Also, they investigated the combined effect with silica sol and hydraulic alumina as a binder in alumina based castables and found that they reduced the risk of explosive spalling and lowered the mixing time besides enhancing mechanical strength at intermediate temperatures [49]. The drawbacks of using silica sol bonded no cement castables are low green strength, long setting time, frost sensitivity, and transportation and storage of the liquid sol. Moreover, the high porosity generated can spoil the mechanical strength and make the castables prone to corrosion attack [50]. Recently, many experiments were conducted to develop highly efficient dispersants which gave appropriate setting time and fast dry out with enhancing green mechanical strength but still requires further researches and well documentation [51]. Recently, Elkem company innovated a binder composed of microsilica and alumina (SioxX-zero) which can be added to silica sol based castables which resulted in enhancements of green strength and setting. The reaction is accelerated further by adding small amounts (0.5wt.%) of alkaline compounds such as calcium aluminate cement where Ca2+ ions react with negative charges on the microsilica surface making a bridge between matrix and aggregates [52].
Alumina has superior hardness, strength, and spalling resistance. Besides, the melting point of alumina 2050°C is much more than silica 1730°C so refractoriness is further enhanced [53]. Alumina sols can be synthesized by two methods which are polymerization and peptization. The polymerization process occurs through hydrolysis and polycondensation of precursor molecules such as alkoxide by organic solvents in the presence of a tiny amount of water. The most common addressed alkoxides are aluminum sec-butoxide, Al(OC4H9)3, and aluminum isopropoxide, Al(OC3H7)3[54]. The peptization method involves the hydrolysis of precursor molecules in a sufficient water medium with the formation of peptized colloidal particles from the formed hydroxides. Nitrate and chloride salts of aluminum are a well-known inorganic precursor for alumina sol making [55]. The disadvantages of using alumina are weak green strength due to low solid content <4% and the situation is further complicated when the solid content is increased due to more water requirements for workability which spoil the final strength. However, Braulino and others [56] concluded that colloidal alumina can have mechanical green strength equal to calcium aluminate cement and greater than hydratable alumina when cured at a temperature higher than 50°C. Not much literature is known for processing alumina sol in castables due to technical difficulties such as high-water requirement, low flowability, low solid content, weak stability of the sol and workability issues [57,58].
Mullite solMullite sol formation is a relatively low-temperature method (1000–1300°C) compared to solid-state synthesis which requires a higher temperature (1500–1700°C) due to homogeneity of the starting materials [59]. The precursors vary from inorganic salt to metal alkoxide but for silica, the alkoxide precursor is the preferred choice such as tetramethyl orthosilicate (TEOS) and tetraethyl orthosilicate (TEOS). The first trials by Roy [60] used TEOS with aluminum nitrate non-hydrate (ANN) as an alumina source. Another economic source of silica is silicic acid or aqueous suspension of silica [59,61]. The hydrolysis step of alkoxide can be promoted by partial displacement by some hydrated salt through reaction with water molecules of the salt or it can be controlled by pH variations thus acidic conditions enhance small particle formations in contrast to alkaline environments [62]. Mullite sol can be combined with CAC binders for further improved mechanical properties at low and high temperatures. The prepared mullite sol from ethyl silicate and aluminum isopropanol bonded corundum castables showed superior properties compared to conventional bonded corundum castables. Sarkar [63] studied the effect of different mullite precursors prepared from Al(OH)3 and silica sol on low cement high alumina castables and reported that no detrimental effects on strength occurred at intermediate temperatures from the dehydration of hydrates phases formed due to mullite formation.
Spinel solThe precursors used in sol making can be from the inorganic origin such as nitrate, chloride, or acetate metal salt or it can be organic such as metal alkoxide or a combination of them. Zhang et al. [64] succeeded in synthesis nano spinel at 600°C by using magnesium nitrate hexahydrate, Aluminum nitrate non-hydrate, and citric acid. The reaction occurs through decomposition of the precursors into γ-Al2O3 and MgO followed by solid state reaction. Olivier and others reported nano spinel formation through hydrolysis condensation reaction of a heterobimetallic aluminum magnesium n-butoxide modified by polyethylene glycol (PEG). Lepkova et al. [65] reported sol–gel route for spinel formation through reaction of aluminum sec-butoxide, Al(OC4H9)3 and magnesium nitrate hexahydrate by including additives such as B2O3 and TiO2 as alcoholates during hydrolysis to promote spinel formation. The advantage of using colloidal suspensions includes easy dry-out due to high permeable structure and volumetric stability at higher temperature due to the non-existence of CA2 and CA6 in the produced structure otherwise CAC based systems [66].
Rheology of refractory castablesRheology is defined as the science concerning the study of flow and deformation of materials [67]. It is termed as consistency when it is measured under a certain mode [68] and it characterizes the relevance between applied stress and deformability as a function of elapsed time [69]. The placing technology of refractory concrete takes an interest nowadays in demand for its huge applications in various industries. Many techniques were employed starting from a simple casting of conventional refractory concrete to more sophisticated methods such as gunning. Post-mortem investigations have focused on improving the mechanical properties through a gradual decrease of hydraulic binders used in castables. The recent trends gave great attention to packing technology as a novel approach for improving thermo-mechanical features. However, perfect packing counteracted the flow criteria of the applied refractory concrete. Refractory castables can be applied to the application site by different placing techniques and its rheology determines the most appropriate method (i.e., pumping, vibration, gunning, and self-flow) [69,40]. In addition to rheology, the amount of water plays a vital role in defining the type of castables and determining the most appropriate method for applying these castables; for example, vibratable castables can turn into self-flow if the amount of water increased to a certain limit.
Free-flow value is an important rheological parameter that determines the type of castable and accordingly specifies the appropriate method to apply this castable. The free values are measured according to the ASTM-C71 method by pouring a castable into a cone described in ASTM-C230; thereafter the cone is removed and the free values are calculated according to Eq. (9):
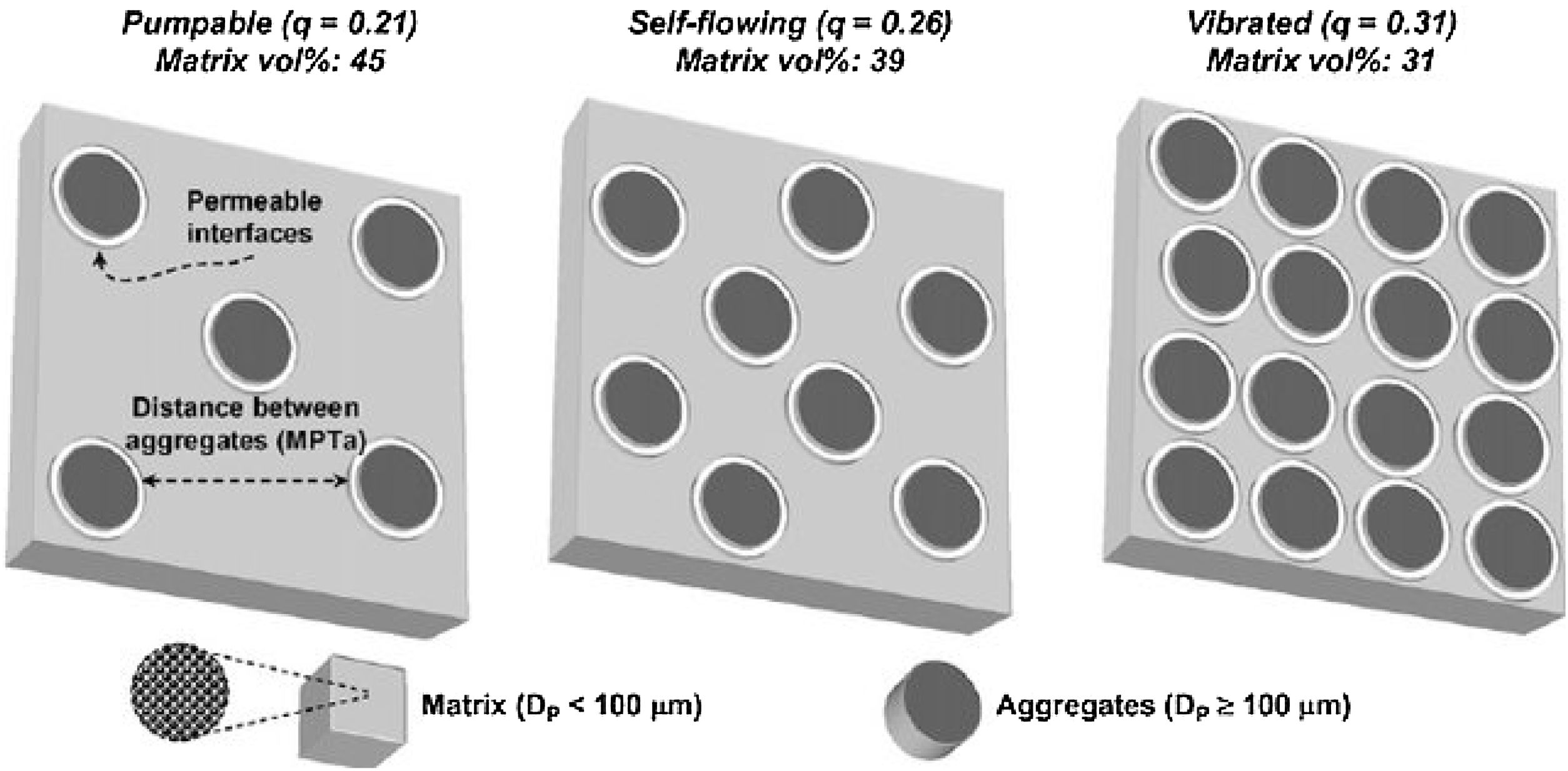
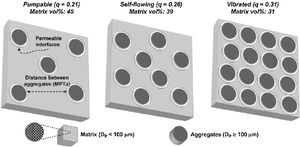
Studart et al. [70] prepared castables with different distribution coefficient (q) values and measured the free-flow values that were related to the appropriate application method. Self-flow castables have free-flow values from 80 to 110%, vibratable between 30 and 80%, and ramming mixture less than 30% [70]. As the free-flow values increase, the castables turn from ramming to self-flow. Even though self-flow castable is the preferable choice in the site, the castables with flow values greater than 120% are prone to segregation; a phenomenon that can be controlled if the matrix of these castables has a remarkable dilatant character.
The distance between aggregates is defined as maximum paste thickness (MPT) which is another important rheological parameter that affects considerably the free-flow values and the rheology of castables as a whole. The MPT is expressed by the formula (Eq. (10)):
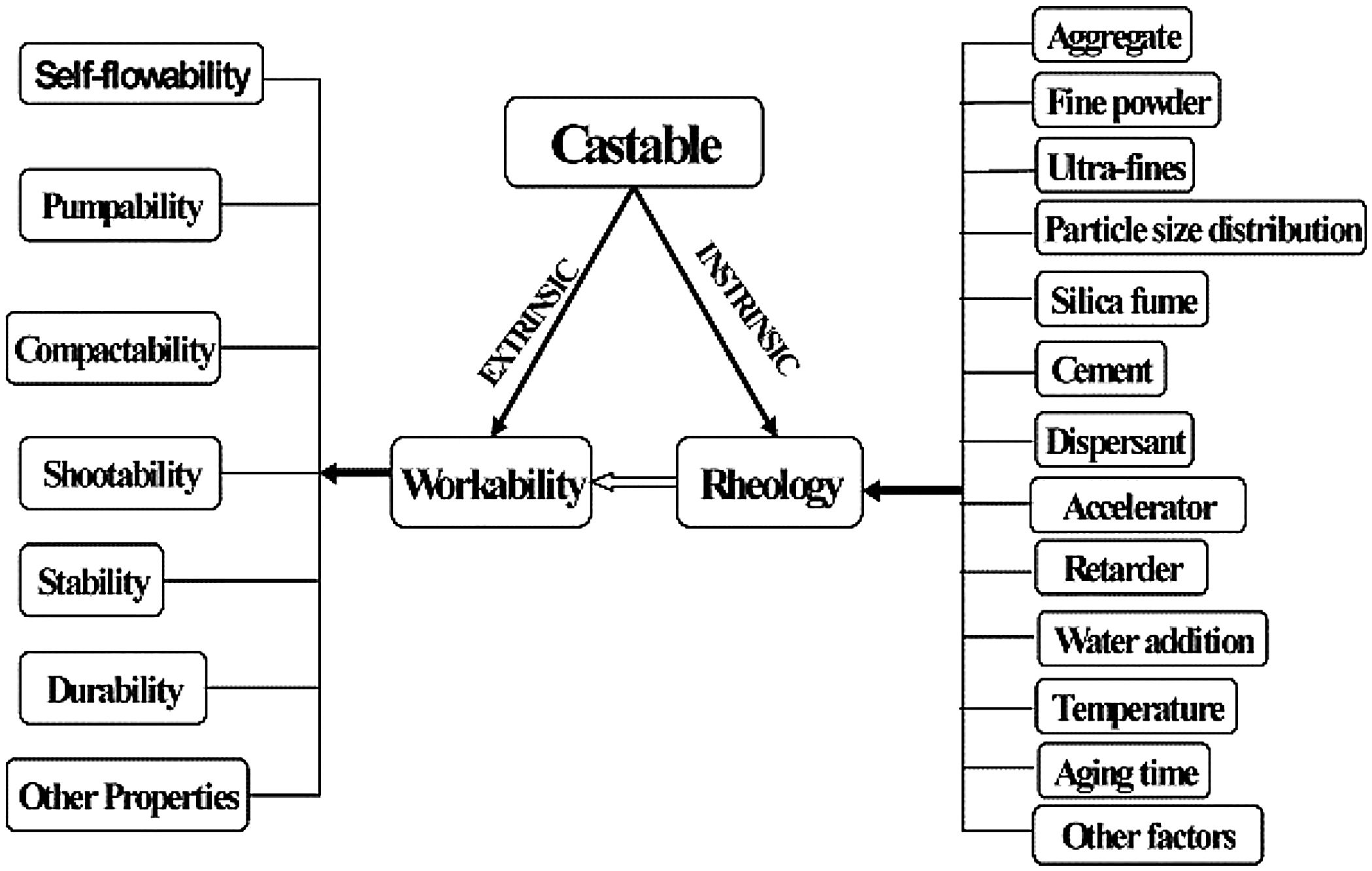
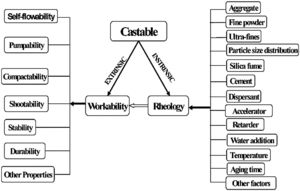
where VSAg and Vsg are the volume surface area and volume concentration of coarse particles, respectively; Pofg is the porosity of coarse particles distribution that is typically calculated in terms of the apparent volume (Va) via the following equation: Pofg=1−(1/Va)[71]. The flowability increases and in contrast strength decreases with the increase of the MPT. Flowability enhancement with the MPT can be explained in terms of the diminution of the friction forces with the MPT enhancement. In contrast, strength deterioration at high MPT values can be attributed to a decrease of the coarse to fine particles ratio. Silva and co-workers [72] studied how to optimize MPT for obtaining high flowability using statistical design methodology; this study concluded that optimum MPT value for a multitude of castables ranged between 100 and 150μm. Most of the recent researches is focused on maximizing the flowability of the castables, together with keeping high mechanical characteristics. Strength development together with flowability enhancement at the optimum MPT values has occurred through efficient particle packing via using multimodal particles with a broad size distribution [73]. Such broad particle size distribution helped greatly for achieving high strength characteristics alongside with further improving the flowability via the extra diminution of the friction forces happened by the wide range of particle size distribution.Even though the rheology of castables are mainly controlled by the MPT [74,75] and particle packing, other factors affect considerably the rheological behavior of the castables such as particle shape, mixing behavior, additives, binding agents, coarse to matrix ratio, water content, and aggregate attributes and many other factors as depicted in Fig. 4[9]. For example, the shape of the particles, whether they are sphere, angular or flaky types, was found to be highly effective in determining rheology and flowability characteristics of the castables [76]. In another example, Yang et al. [77] and Williams et al. [78] proved that the mixing process affected significantly castable viscosity and accordingly its rheological characteristics. Pileggi and others [79] revealed that the two-step addition of water to the castable has improved its fluidity more than the one-step scheme.
Also, for a definite amount of mixing water, the fluidity of the castables is boosted as the fine matrix fraction is enlarged over the coarse aggregates. This can be expressed by the exponent (q) in the Andresen equation where lower values are stating predominant fine particles. Nevertheless, the viscosity becomes a critical factor for flowability perseverance when fine particles are excessively increased. It was also verified that the flowability of refractory castables can be calculated by the classical flow-table method but a comprehensive study of rheology requires additional assisted technology advances that elaborate viscosity and yield strength [80,81].
Shear resistanceThe force which causes the deformation of materials by slipping alongside the shear planes parallel to the applied force is termed as shear stress. In the case of a slurry suspension, it is governed primarily by the interparticle interactions and the viscosity of the liquid in a well-dispersed system while the pressure imposed in processing has a negligible effect on interparticle stresses, however, in castables with more crowded slurry, it may be blocked by neighboring particles.
Viscosity is defined as the opposing of materials to flow. The resistance to shear flow is dependent on particle movement away from the plane of shear. When viscosity diminishes with time at a stable shear rate, thixotropic behavior occurs which is the common characteristic of suspensions containing irregularly shaped particles [82,67]. The shear rates are ranging from the low required for gravity leveling (10−1s−1) to the medium rate used in mixing and pumping processes (1–103s−1) and the very high rates characterizing for spraying (105s−1).
The yield stress τ0 (Pa) is bound up with the viscosity η (Pas) through Eq. (11):
where τ (Pa) is the shear stress and ′γ is the shear strain (s−1). The shear stress is increased as viscosity is raised and also the stress is intensified when high flow values are encountered. This equation depicts Bingham rheology behavior in ideal suspension but, it turns to Herschel–Bulkley equation (Eq. (12)) when a dilatant character occurswhere K and n are intrinsic material properties. When the factor n equals 1, the two equations are indistinguishable but as the factor n>1, shear thickening occurs and viscosity increase when the shear strain increase which results in difficult flowability. The third flow behavior occurs in diluted suspensions as a linear relationship between viscosity and shear strain known as Newtonian behavior which occurs when the suspension exhibits a very low shear rate [83]. Suspensions of low viscosity are obtained through optimum particle packing of a broad mesh of particle size distribution. This demonstrates the dependence of the suspension viscosity on the volume percentage of the solid content and the packing density. This relation can be demonstrated by the Dougherty Eq. (13)[84,85].where η represents the suspension viscosity, ηmedium is the base medium viscosity, Φ is the solids of the suspension volume fraction, Φmedium is the maximum volume fraction of solids in the suspension, and [η] is the viscosity of the medium. It can be inferred that as the solids’ concentration is found in the suspension increase, the viscosity rises. It is an advantage to get a matrix with intensified solid content and minimum viscosity.Dilatant behavior or shear thickening could appear above a particular shear rate due to particle interference and hindrance rotation. Dilatancy can be reduced by using fine particles like silica fume and ultrafine alumina (<0.5μm) characterized by pseudo-plastic behavior at a high shear rate. The dilatant behavior is increased by the narrower particle size distribution and it is believed that irregularly shaped particles affect viscosity besides dilatant behavior in a negative way due to larger hydrodynamic volume during flow caused by anisometric particles [86]. The shape of the particles also affects dilatancy thus the use of spherical reactive alumina in silica-free systems instead of platelets removes dilatancy [68].
Practical experimentation revealed that dispersing alumina with commercial names ADW/ADSH which are used for alumina-based systems or M-ADS/M-ADW for silica fume rich matrix boosted the flowability [83] where ADW has an accelerating effect and ADS a retarding effect or a combination of them can be used to control the setting time with amounts up to 1.00wt.% [87]. However, a trade-off is usually made to infer the desired properties and limit the deleterious effect of using fine spherical particles on other properties like erosion resistance, thermal shock resistance, and creep behavior. For example, fine particles are known to have greater creep rates at a higher temperature than coarse particles due to grain sliding and grain boundary movements. Therefore, castables that flow properly when installed without a dilatant manner are expected to deform easily than low flow types [69].
Surface chemistryRefractory castables are composed of aggregates and fine particles which are micron and submicron in size. The fine fraction of the castables is responsible for surface forces because it accounts for most of the surface area, so surface chemistry has a fundamental role in determining the flowing properties of the castables. Surface chemistry deals with suspensions (solid–liquid mixtures) containing colloidal particles (<1μm) of relatively high specific surface area (>1m2/g), which are exposed to surface forces occurring at the solid–liquid interface. These forces control the dispersion and rheology of colloidal suspensions [14].
The counteracting capillary and the Van der Waals forces are fortified as the fine matrix gets smaller which are directly proportional to the area under the shearing torque of the mixing turning point (conversion of the powder to the fluid state). Castables with a high specific surface area (SSA) require a much larger amount of water to reach the turning point. The torque of the rheometer can be calculated from Eq. (14):
where G is the flow resistance, N is the angular speed of the impeller, and H is the torque viscosity [70]. The torque proportion and the mixing energy are inversely proportional to the q values because the SSA decreases as the q value increases [69].Aggregates are hard clusters of particles linked by strong covalent forces while Agglomerates could be found when the particles are poorly dispersed so weak clusters are formed. Alumina particles show a strong tendency to agglomeration compared to other materials so dispersants in low concentrations should be used [70]. To counteract agglomerate formations, the amount of water should be increased which harms the strength of the castable due to the larger number of formed pores and segregation may occur when the amount of water is excessively added. To handicap particle agglomerations, different dispersion mechanisms could be used. They are electrostatic, steric, and electro steric mechanisms.
The double-layer formed to neutralize the negatively charged particles that cause a voltage difference between the surface of the particles and the oppositely charged molecules in the suspending liquid. The voltage difference is of the order of millivolts and is termed as surface potential which depends on double layer thickness and the charge of the surface. The surface potential decreases linearly through the stern layer then exponentially through the diffuse layer. The charged particles can move with a fixed velocity under-voltage difference which is known as electrophoresis. This movement depends on the viscosity and dielectric constant of the suspending liquid and its magnitude is affected by the boundary potential difference between the slipping planes. The electrical potential at the slipping plane is related to particle mobility which is known by the zeta potential.
The particles tend to repel each other whether they have a large negative or positive zeta potential. On the contrary, they are flocculating together when the zeta potential values are low. The zeta potential is pH-dependent and the colloidal system at its isoelectric point is least stable due to zero zeta potential.
Otroj et al. [71] studied various deflocculates effects on the flowing properties of ultra-low cement alumina-based castables. They measured the change in pH values as a function of deflocculants concentration and they found that citric acid lowered the pH of the suspension to 6.5 while deflocculants such as sodium polyacrylate acid (Na-PAA), sodium tripolyphosphate (Na-TPP), and sodium hexametaphosphate (Na-HMP) did not change pH significantly. The pH of the matrix in alumina-based castables is higher than 7 and the charge zero point of high alumina castables ranges in the pH from 8 to 10, so it is important to add anionic molecules such as citric acid to increase the zeta potential. The anionic dispersants such as citric acid shift the zeta potential curve toward acidic values promoting negatively charged particles over a broad pH range while cationic molecules induce the opposite action [14].
The dispersion of refractory castables is promoted by generating electrical charges with the same signal on the particle surfaces but this cannot be accomplished by simply controlling the pH of the suspension because unlike materials offer different surface charges over a wide pH range and for simply a single material, it will require a substantial amount of acidic or basic components which will increase the ionic strength of the liquid medium. The simple solution for this dilemma is to provide dispersants that can adsorb on the particles surface providing them with the same signal charge [14].
DispersantsTo produce a low viscosity paste, the particles should be deagglomerated by dispersing agents. Generally, the phosphate such as sodium hexametaphosphate and sodium tripolyphosphates is used to disperse silica fume in amounts 0.1–0.30wt.% meanwhile polyacrylate level of 0.01–0.10wt.% is used for reactive alumina [73].
Organic additive known as super-plasticizers were recently used due to its high performance in the dispersion of the castable constituents. Investigation showed that polycarboxylate ethers (PCE) are more effective dispersants than polyacrylates (PA) in alumina–silica systems thus polyacrylates are working by electrostatic mechanism while polycarboxylates combine electrostatic and steric mechanisms [74]. Otroj et al. [75] studied the effects of different dispersants which are polycarboxylate ether (PCE), citric acid (CA), and sodium polymethylacrylate (PA) on the flow of high alumina low cement castables and found that PCE is the most activator in boosting flowability through 0.06wt.% addition.
Common deflocculants such as Calgon (hexametaphosphates) or Darvan 811D (polyacrylates) are added in amounts of 0.20 and 0.05wt.%, respectively. The dispersion mechanism may occur through curling up high molecular weight organic molecules between particles or surface absorption of phosphate molecules [88]. Dispersants such as Calgon could have optimum flow at the expense of longer setting time or it may affect the shelf life in micro silica-containing castables so a compromise is always encountered between the desired characteristics [89]. For example, electro steric deflocculants often slow down the anhydrous CAC dissolution which extends the working time for castables [10]. In micro silica-containing castables, a dispersant can be added according to the micro-silica surface where 1mg of dispersant per square meter of micro silica surface area [88].
Recently, Elkem company innovated a binder composed of micro silica and alumina (SioxX-zero) which can be added to silica sol based castables which resulted in enhancements of green strength and setting. The reaction is accelerated further by adding small amounts (0.5wt.%) of alkaline compounds such as calcium aluminate cement where Ca2+ ions react with negative charges on the micro-silica surface making a bridge between matrix and aggregates [51].
Nonetheless, the quantity of additives should not be added extremely thus significant quantities may create strong charged molecules adhesion which downsizes the double layer thickness or it may enlarge its thickness resulting in molecular water entrapment and hence flowability resistance [80]. Lately, many experiments were conducted to develop highly efficient dispersants which gave appropriate setting time and fast dry out with enhancing green mechanical strength but still requires further researches and well documentation.
Particle packingParticle size distribution or particle packing was first studied in 1930 but the successful trials to produce low cement castables by optimizing particle packing density was in the 1970s [90]. Afterward, Self-flowing castables were first developed in the mid of 1980 [81]. Smooth round or equiaxed particles make efficient packing density more than irregular rough shaped particles. Also, two different particles dimension gives intensified packing than monomodal packing structure. This bimodal type packing can be even densified as the difference in particle diameter increase up to 20 times assuming minimum 20% difference between the particle size ratios [91,92].
Two different techniques were employed for particle sizing which are random and ordered systems. The random system mainly depends on sizing aggregates in broad mesh distribution then blended with fillers/modifiers to get adequate Particle size distribution (PSD) used mainly to manufacture lightweight and conventional dense castables [14]. Ordered systems include the subsidiary two methods known by the gap and continuous sizing. Gap sizing works by mixing two, three, or more tightly graded aggregates to achieve optimum packing density at the expense of flow while continuous sizing optimizes flowing properties by sizing aggregate continuously in large-close size distributions [14]. The optimum packing density in continuous sizing can be achieved through extending the level of mean top particle size to larger values while maintaining the intermediate-sized particle fraction in low proportion [82].
The basic concept behind the refractory industry is to obtain the possible dense structure which can be accomplished by optimizing material packing through particle size grading and to get a low viscous suspension. Achievement of 100 packing density is impossible but careful particle size distribution (PSD) can control the rheology and govern the mechanical properties of the castables. Other properties such as the mixing behavior, water content, drying criteria, and the creep value at high temperatures are also affected by PSD [93]. Recently, Studart et al. [14] succeeded to develop cement-free self-flowing castables through precise control of particle size distribution.
Many theories were developed to control the particle packing and the most commonly known models are Furans, Anderegg, and Andreasen, and Dinger-Funk (modified Andreasen) [73,94,95]. Furnas developed two models based on discrete and continuous packing [96]. The Furans discrete model is based on filling the space of a definite component system with finer particles which intensify the packing density. Mac-Zura et al. [97] concluded that discrete packing gives better densification but at the expense of flowability criteria while continuous packing results in enhanced workability combined with high mechanical strength due to lower water addition. The Furans model is cumbersome and complicated while the Andreasen is simple but was attacked because it supposes infinitely small particle size although real systems are finite. On the other hand, it requires no shape factor in the condition that all the particles have the same shape. A new model was developed which include a minimum particle size by Dinger and Funk which is commonly known as modified Andreasen [73,88].
Particle size distributions are based on volume bases rather than mass values so different amounts of castables constituents must be converted to volume fractions to calculate PSD. There is always a porosity for a q value greater than 0.37 so it is important to lower the q values below 0.37 to get a dense packing. The Furnas distribution gives a curved line in the log–log plot while the Andreasen model gives a straight line and modified Andreasen shows a downwards curvature [73].
When the fine fraction of the castables like micro silica is removed then the Andreasen model is better expressed by the modified Andreasen and vice versa. There are two possibilities for the micro-silica added to castables thus it may replace water and keep the volume constant which then reduces the amount of required water for casting or it can be added to the total volume expanding the castables to counteract volume increase.
It is better to use the Andreasen model for micro silica-containing silica compared to the Furnas model and if the deviation at the very low sized particles <1μm is negligible. Self-flow castables can be gotten for q values lower than 0.25 while Vibra-flow castables are obtained when the q value near 0.30 [73,88] thus as the q value is lowered, the fine fraction is increased at the expense of the coarser part showed by Fig. 5[98]. Coarse materials have a lower surface area compared to fine materials so less water is required but the flow is affected due to friction forces. On the other hand, strength could be decreased due to larger cracks associated with increased particle size [88]. However coarser particles show less firing shrinkage when heated otherwise fine matrix which contracts upon firing so the difference in thermal expansion between aggregates and fine matrix occurs which generates micro-cracks enhancing the thermal shock resistance of the entire castables [99]. Moreover, the wearing rate resistance of castables is enhanced when it is increased at the expense of the fine matrix.
Submicron silica and aluminaMicro silica is used as a filler in refractory castables to minimize porosity and maximize flowability. In addition, its high reactivity enhance bonding strength at high temperature when combined with alumina particles at a stoichiometric ratio forming mullite with enhanced mechanical strength [100]. Elkem referred to micro silica as the trade name of fumed silica in the 1980s. Fumed silica is the intermediate product in the production of silicon and Ferro-silicon industries. It consists of an amorphous structure of SiO2 with a high specific surface area of 20m2/g so the surface chemistry is largely affected by micro silica [58,101]. Its specific gravity is 2.25g/cm3 so the calculated surface area per unit volume basis equal 45m2/cm3 which is high compared to reactive alumina 26.9m2/cm3 and micro alumina 30.7m2/cm3.
The d50 is 0.5μm deduced from PSD analyzed by laser diffraction as small spheres of around 0.15μm diameter could be combined forming the building nuclei of the micro-silica sphere.
Microsilica has a negative surface charge in aqueous medium 20–30mV with an isoelectric point in the pH range from 2 to 3 while alumina and cement have a small negative charge depending on the pH which could results in coagulation thus calcium ions are absorbed on the silica surface. The coagulation could be hindered by whether lowering the pH below 5 or adding a surface-active agent. The drawback of pH reduction involves setting problems so the addition of polyelectrolyte is the optimum solution [58]. Alumina has a positive charge when dissolved in aqueous media so it is expected that it flocculates on the surface of silica particles because of the existence of dissolved silanol groups with negatively charged anions but this does not occur due to the shift of zeta potential toward negative values in calcium aluminate cement-based suspensions [102].
Different microstructures of alumina forms are used at a larger fraction in combination with microsilica to strengthen the binding forces formed at elevated temperatures. Calcined alumina which is produced from heat treatment of bauxite from the Bayer process can be further ground to the desired particle sizes. Normal calcined alumina is usually milled below 45μm (95–99%). The reactivity of Calcined alumina can be increased and the sintering temperature can be decreased by grinding finer to sub-micrometer which is reactive alumina.
Reactive alumina is fine alumina powders with small primary crystal size <1μm, high specific surface area between 5 and 11m2/g, specific gravity (SG) approx. 3.95g/cm3 and low soda levels <0.15% [87]. Its particle size distribution follows multi-modal character which reduces the dilatant character but its plate-like morphology makes interlocking structure which makes paste layers stiff to move relating to each other. The volume micro silica can be replaced by reactive alumina thus if 4.0wt.% of silica is used so 7.0wt.% should be added to compensate for the difference in specific gravity but setting manner should be taken into consideration [88]. Experiments showed that micro silica has a retarding effect on the setting of refractory castables because it covers cement and slows down its dissolution while reactive alumina accelerates setting reactions of CAC [88].
Microalumina with a SG 8.6m2/g of spherical shaped particles can act as a lubricating layer when the shear stress is applied to the concentrated suspension which accelerates flow and relieves dilatancy. Another kind of calcined alumina that is believed to contain organic ingredients is dispersing alumina with a bimodal PSD and better flow characteristics which can improve the castables flowability and packing density.
Extremely fine powders <0.10μm with huge surface area 200m2/g improves packing density to optimum but inter-particles distances will further decrease subsequently and friction forces between the particles will be maximized so excessive amount of water will be added to diminish that forces and allow flowability but this, of course, spoils the strength [88].
Nanotechnology and rheologyNanotechnology was recently used in castables bonding to lower sintering temperature by 100–200°C but castables agglomeration should be controlled [66,47]. Particles which form a suspension where the gravity is not enough to make them settle, are termed a colloid or sol. The particles are very small with size ranges from 1 to 1000nm. Nanoparticles have a huge surface area to weight ratio which ranges from 600–800m2/g in carbon nanotubes to 10–30m2/g of thick micro silica grades. They can be used as a binding agent like colloidal silica and alumina or as a modifier. The diminutive surface energy of nanoparticles creates agglomerates but on the other hand, it can be used as a lubricant in castables formulation due to its high molecular mobility and lower bonding energy [103]. The problem of agglomeration can be solved by a proper dispersion system used in an adequate amount. Badiee et al. [104] examined the silica sol volume to weight percent concentration (cm3/g) on the flow properties of self-flow tabular alumina castables and found that the flowability and the working time were increased as sol content increased from 9 to 11 but further addition should be limited because thermo mechanical properties go down. Parr and Wohrmeyer [105] compared the rheological properties of silica sol boned castables to other hydraulic bonded systems with the same water content and revealed that even though colloidal silica gives low flow value, its flow decay is the least among the others.
Concluded remarks- 1.
The thermo-mechanical belongings of refractory castables are dependent on its rheology which determines its application uses.
- 2.
The dilatant conduct occurs in concentrated suspension when the upward relationship between shear strain and viscosity occurs which turns to the opposite shear-thinning character when viscosity decreases with the increased applied stress.
- 3.
The viscosity can be relieved by a continuous distribution of closely sized particles of high mean top size through reducing the friction forces between them by using round or equiaxed molecules.
- 4.
The fine matrix controls the MPT between aggregates but viscosity becomes a crucial factor when it is excessively intensified.
- 5.
Submicron particles are prone to agglomeration but its surface forces can be directed through appropriate levels of dispersing agents.
- 6.
A refractory castables can be designed to have the desired flow degree counting on a multitude of parameters but it should be counterbalanced by other valuables.