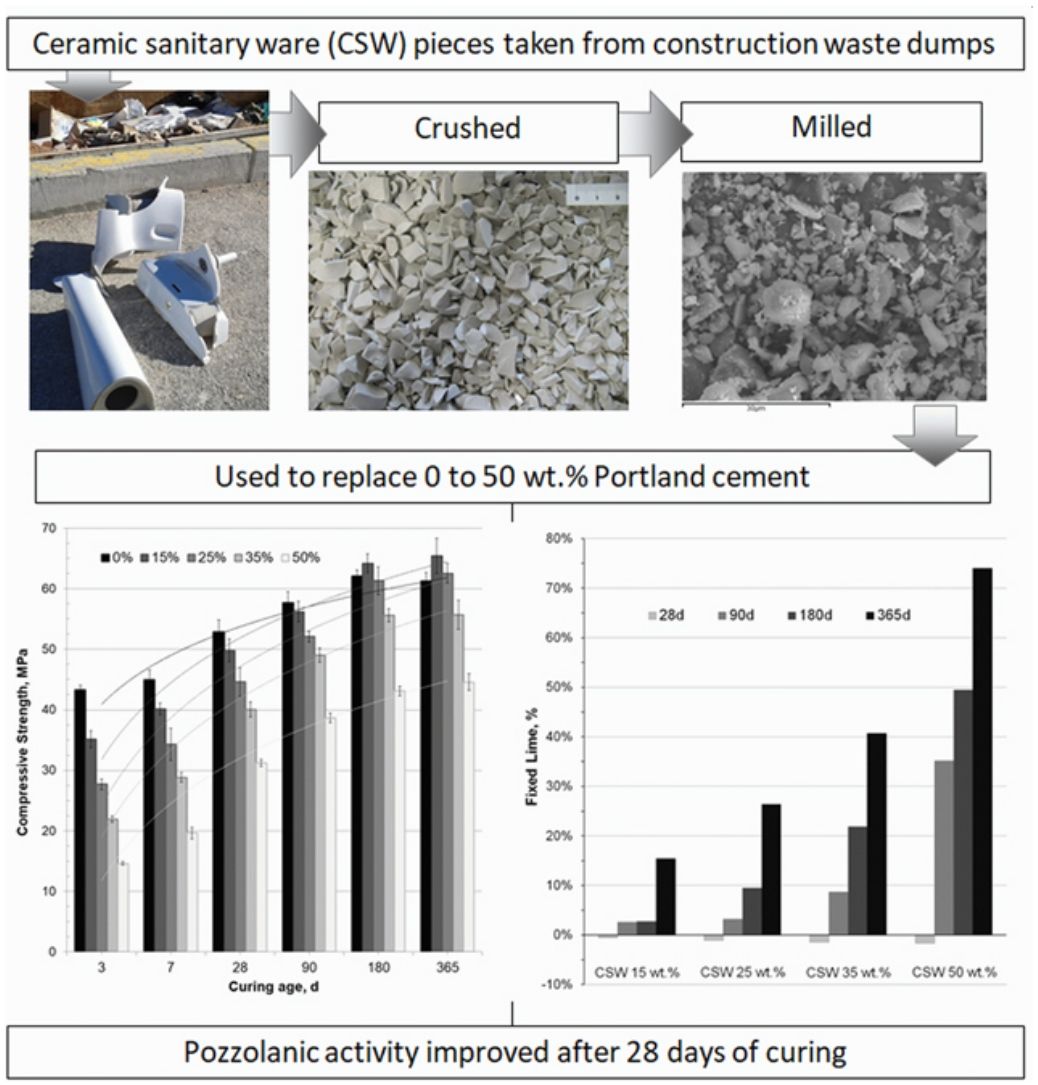
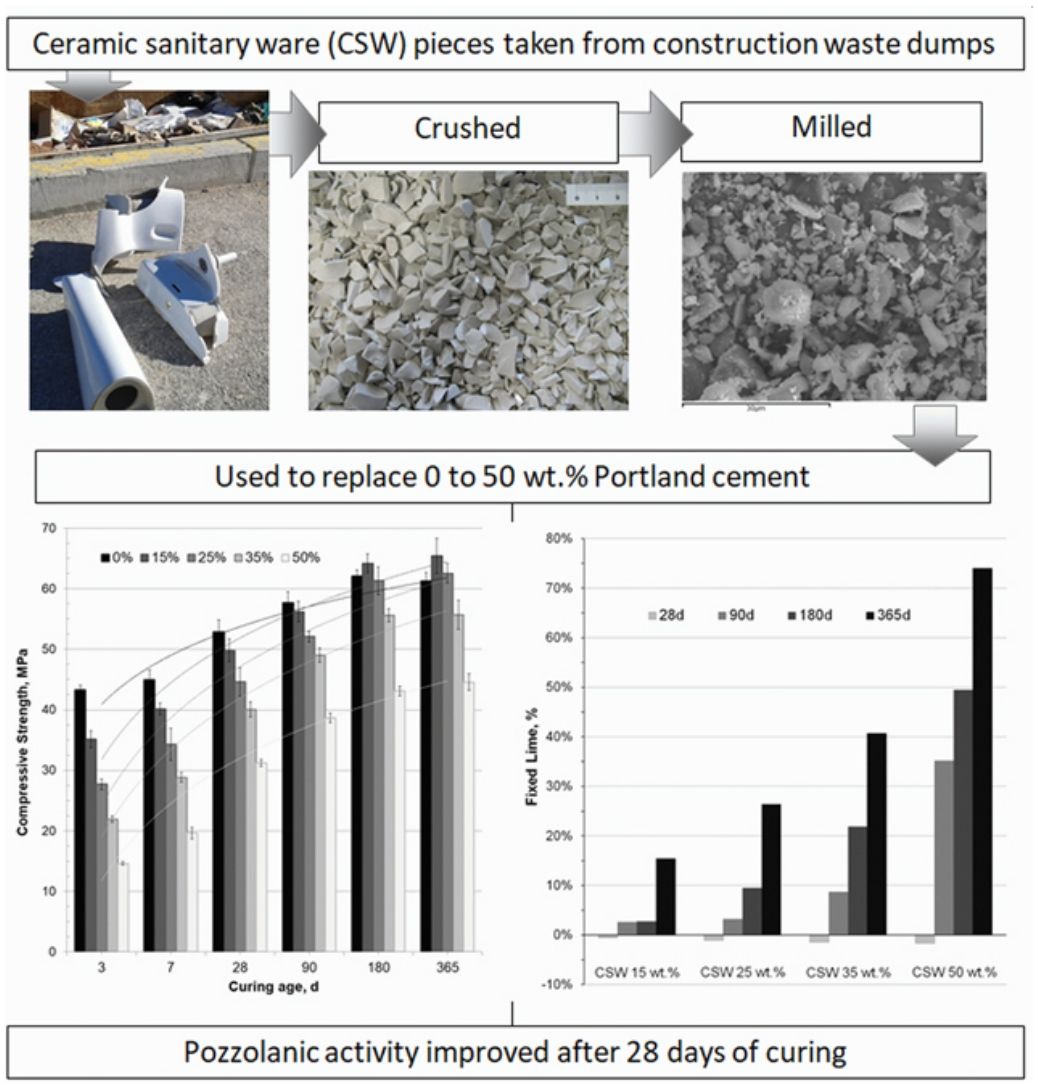
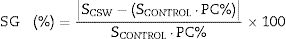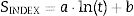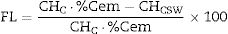This paper evaluated the pozzolanic activity of ceramic sanitary ware (CSW) waste when blended with Portland cement (PC). CSW waste units were broken, crushed and milled to reduce their particle size. These particles were characterized by scanning electron microscopy (SEM-EDX), laser granulometry, X-ray fluorescence (XRF) and X-ray diffraction tests (XRD), and were then used to replace 0–50wt.% PC CEM I 42.5R in pastes and mortars. Workability of the fresh mortars was assessed by the flow-table spread test, and the mechanical properties and microstructure (thermogravimetry, SEM-EDX, XRD and Fourier transform infrared spectroscopy tests) were investigated in samples cured at 20°C for up to 365 days. No significant workability variations were observed with increasing waste contents and, although pozzolanic activity of CSW was relatively slow, it improved with the curing time, and mortars prepared with up to 25wt.% ceramic waste satisfied the requirements established for other pozzolanic materials, such as fly ash.
En este artículo se evaluó la actividad puzolánica de residuos de cerámica sanitaria (CSW) en mezclas con cemento Portland (PC). Para reducir su tamaño de partícula se rompieron, trituraron y molieron piezas residuales de cerámica sanitaria. Las partículas resultantes fueron caracterizadas mediante microscopía electrónica de barrido (SEM-EDX), granulometría láser, fluorescencia de rayos X (XRF) y difracción de rayos X (XRD), utilizándose posteriormente para reemplazar entre un 0 y un 50% de cemento CEM I 42.5R en pastas y morteros. La trabajabilidad de los morteros frescos se evaluó mediante el método de la mesa de sacudidas, y las propiedades mecánicas y microestructura (análisis termogravimétricos, SEM-EDX, XRD y espectroscopía infrarroja por transformada de Fourier) se investigaron en muestras curadas a 20°C hasta 365 días. No se observaron variaciones significativas en la trabajabilidad al incrementar el contenido de residuo cerámico y, aunque la actividad puzolánica de la CSW fue relativamente lenta, mejoró con el tiempo de curado. Así, los morteros desarrollados con hasta un 25% en peso del residuo cerámico cumplieron con los requisitos normativos establecidos para otros materiales puzolánicos, como las cenizas volantes.
Portland cement (PC) is the binder that is most commonly used in concrete. However, its production requires high temperatures (approximately 1400°C), consumes natural raw materials (mainly clay and limestone), and emits large amounts of CO2 into the atmosphere (about 0.83kg per kg of cement produced) [1]. These emissions mainly originate from the decomposition of limestone and the energy needed to achieve the temperatures required in the kiln. On the other hand, reutilization of waste materials in the construction industry allows for energy saving and environmental protection [2]. In this sense, different studies have explored the potential use of industrial waste at different stages of the Portland cement manufacturing process: as raw materials [3,4], alternative fuels or pozzolanic admixtures [1,4–6]. All these alternatives benefit the environment, since they reduce both the visual impact resulting from the accumulation of deposited waste and the mining of natural aggregates [1,7].
Among the different types of waste that may be reused in concrete, ceramic materials deserve special attention since, as explained by Halicka et al., although they are usually chemically inert, they are non-biodegradable (biodegradation period up to 4000 years), and their accumulation has a significant visual impact. More specifically, sanitary ware waste is generated both during the demolition or refurbishment of existing buildings and when producing units such as washbowls, bidets or bathtubs. As reported by Baraldi [9], world output of sanitary ware grew by more than 60% in only ten years (2004–2014), reaching 349.3 million pieces produced (equivalent to 7.7 million tons) at the end of this period. Almost 50% of these pieces were produced in Asia, the largest production area, followed by the European Union, which accounted for 11.9% of the global production (41.5 million pieces produced, equivalent to 0.91 million tons) [9]. The exports and imports of sanitary ware units also grew significantly (75.2%) from 2009 to 2019, when 3.5 million tons of this ceramic product were traded worldwide [10]. According to Medina et al. [11], approximately 8% of the over seven million units manufactured yearly in Spain cannot be sold because of deficiencies such as nicks, cracks or glaze imperfections. An advantage of ceramic sanitary ware (CSW) waste, from the point of view of recycling and reuse, is that it can be easily separated from other construction materials, and generally does not contain any impurities, such as adhered cement, gypsum or metallic reinforcements.
Several authors have successfully investigated the use of CSW waste as recycled aggregate in concrete production [8,12,13]. After replacing 20 and 25% of natural siliceous gravel with recycled sanitary ware aggregates, Medina et al. [13] noted that the density of the recycled concrete diminished and the compressive strength improved with increasing waste contents. Moreover, the recycled concretes thus developed were found to be as durable as the conventional ones. Halicka et al. [8] replaced natural sand and gravel with CSW aggregates and observed that, after heating at 1000°C, the loss of strength of the recycled concrete developed was similar to that registered for other types of concrete. Vieira et al. [12], who investigated the durability of concrete incorporating different amounts of fine recycled aggregates from crushed bricks and sanitary ware, concluded that addition of CSW increased the water demand due to the formation of agglomerated particles. New sustainable binders were also developed by the alkali activation of this ceramic waste in Ref. [14]. Compressive strength values ranging from 15 to 36MPa were achieved in mortars cured for 7 days at 65°C, activated with sodium hydroxide and sodium silicate solutions. Medina et al. [11] conducted a study on the use of CSW and construction and demolition waste as a pozzolanic admixture. After exploring the rheology and hydration kinetics (conduction calorimetry tests) of Portland cements blended with 10 and 20% of each of these waste materials, they concluded that, although CSW strongly influenced the rheology of the paste and retarded cement hydration, using low concentrations of this waste as a supplementary cementitious material was feasible. Preliminary studies on the hydraulic activity of CSW waste were also performed in Ref. [14], where PC was blended with 15wt.% and 25wt.% CSW and samples were cured at 20°C for 28 and 90 days. Promising results were obtained, since reactivity of CSW with the Ca(OH)2 liberated during PC hydration improved with the curing time and the mortars developed satisfied the minimum strength activity index (SAI, ratio between the strength of the pozzolanic mortar and that of the control mortar) established for other pozzolanic materials, such as fly ash [15].
Although promising results were obtained in previous work by Medina et al. [11] and Reig et al. [14], both studies used low percentages of substitution (up to 25wt.%) and relatively short curing times (28 and 90 days). This paper aims to complete the previous research performed on the use of ceramic sanitary ware as a pozzolanic admixture, investigating the evolution of the microstructure and compressive strength with higher amounts of waste (up to 50wt.%) and a wider range of curing times (from 3 to 365 days). Providing new knowledge on the use of CSW waste as supplementary cementitious material aims to help minimize landfill settlings, to conserve natural nonrenewable resources, and to reduce energy costs and emissions of CO2 and other greenhouse gases associated to the production of Portland cement.
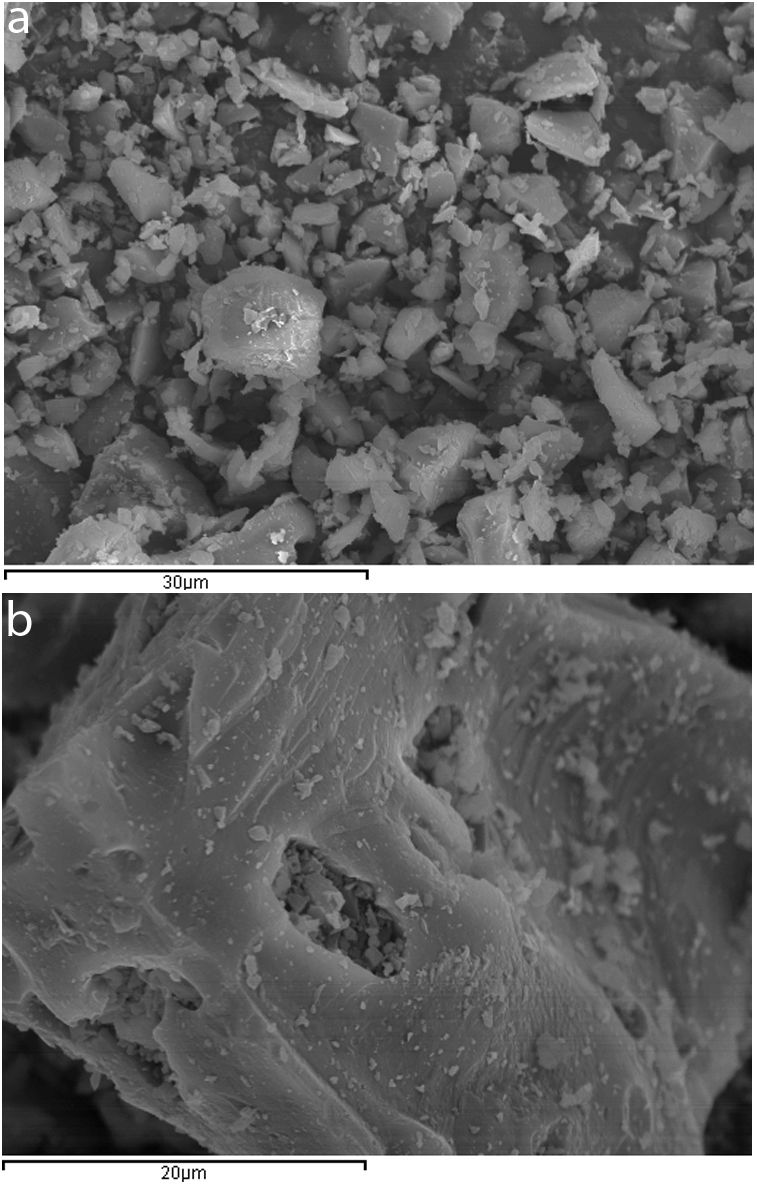
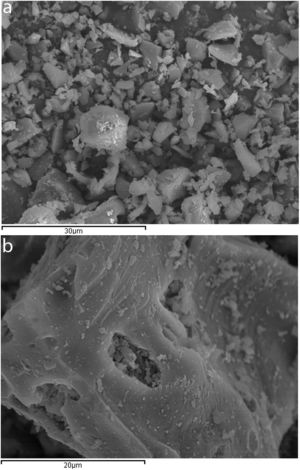
ExperimentalMaterialsCeramic sanitary ware (CSW) units (such as bidets, washbowls, bathtubs or lavatory pans) were taken from construction waste dumps. They were broken into pieces with a hammer, and crushed in a jaw crusher (BB200, Retsch). The crushed waste was then sieved and particles below 2mm were dry milled for 25min (450g of waste, 90 balls of alumina, Gabrielli Mill-2 ball mill). As Fig. 1 shows (scanning electron microscope SEM-EDX JEOL JSM-6300), milled particles were dense, irregular and presented a smooth surface with sharp edges.
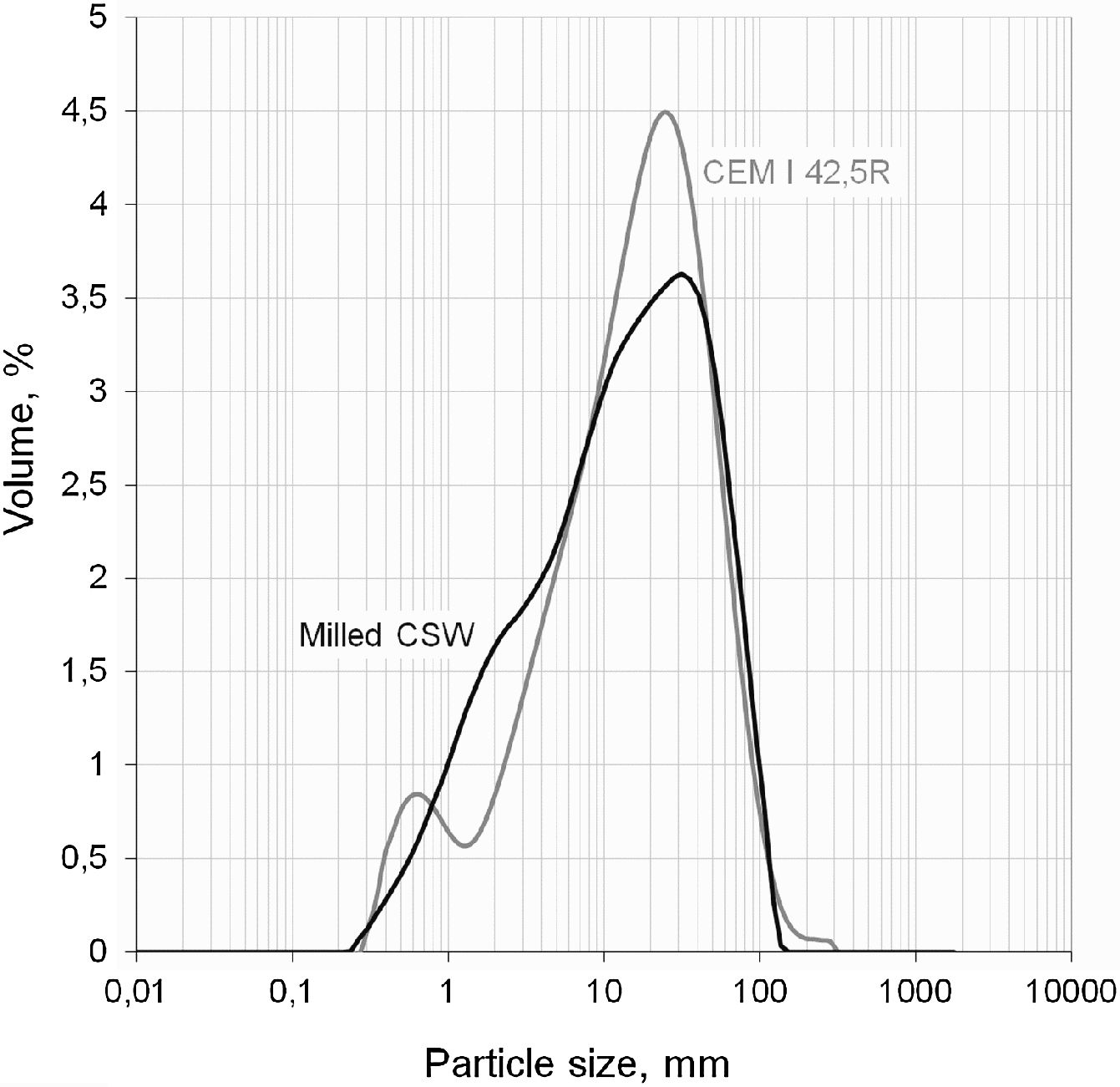
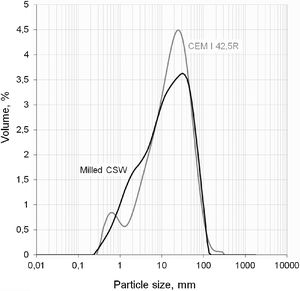
As plotted in Fig. 2, laser granulometry tests (Mastersizer 2000, by Malvern instruments) revealed that the milled powder had a mean diameter close to 24μm, with a d10 of 1.7μm (10vol.% under this size), d50 of 14.7μm, and d90 of 60.7μm. This particle size distribution was close to that presented by the PC used in this study, which was of the CEM I 42.5R type and had a mean diameter of 25.5μm (d10, d50 and d90 of 2.1μm, 17.3μm, and 58.1μm, respectively). Authors such as Payá et al. [16] and Mas et al. [17], who also used waste materials as pozzolans, reported a mean particle size close to 20μm, very similar to our value.
As shown in Table 1, the CSW waste used was mainly composed of SiO2 and Al2O3 (89.6wt.%), and contained minor amounts of CaO (1.2wt.%) (X-ray fluorescence tests run in a Magix Pro spectrometer, by Philips). As previously reported in Ref. [18], the mineralogical composition denoted the presence of quartz (SiO2) and mullite (Al6Si2O13) as the major crystalline phases, together with minor amounts of microcline, a potassium feldspar (KAlSi3O8). In agreement with previous studies by Lavat et al. [7], the presence of amorphous clay compounds was corroborated by the deviation of the baseline in the 15–30 2θ degrees range. These disordered phases formed when sintering the sanitary ware units.
CSW chemical composition (wt.%).
| SiO2 | Al2O3 | K2O | Na2O | Fe2O3 | CaO | P2O5 | Other | LOIa | |
|---|---|---|---|---|---|---|---|---|---|
| CSW | 66.0 | 23.6 | 2.8 | 2.4 | 1.3 | 1.2 | 0.5 | 2.0 | 0.2 |
The aggregate used to prepare the mortars was siliceous sand (fineness modulus of 4.36 and maximum particle size of 2mm).
Sample preparationPastes and mortars were prepared to evaluate the pozzolanic activity of the CSW waste. Percentages of substitution of PC with the ceramic material ranged from 0 (control) to 50wt.%. Larger CSW contents were discarded because they would imply low amounts of portlandite, released during the hydration of Portland cement and required for pozzolanic reactions. Mortars were prepared according to the UNE-EN 196-1:2018 standard [19], using a binder:sand:water weight ratio of 1:3:0.5. Pastes were mixed in plastic containers, which were sealed after blending the binder (PC+CSW) with water (w/b: 0.5) for 4min. Both pastes and mortars were cured under controlled conditions (20°C and 95%) for up to 365 days. Table 2 summarizes the variables analyzed in this study.
Paste and mortar characterizationThe flow-table spread test (UNE-EN 1015-3:2000/A2:2007 [20]) was used to determine the workability of fresh mortars. The compressive strength was evaluated in mortars, according to the UNE-EN 196-1:2018 standard [19]. Strength values (in MPa) were completed with the strength activity index (SAI) and strength gain (SG). The SAI is the ratio between the strength of the pozzolanic and the control mortars (SCSW/SCONTROL). SG (%), which is calculated according to Eq. (1), illustrates the contribution of the pozzolan to the compressive strength of the blended mortar.
where:
SCSW=compressive strength of the PC/CSW blended sample.
SCONTROL=compressive strength of the control sample.
PC%=percentage of PC in the PC/CSW blended sample (per unit).
Pastes were prepared to investigate the microstructural evolution. Thermogravimetric analyses were run from 35°C to 600°C, at 10°Cmin−1, in sealed pin-holed aluminum crucibles and under a nitrogen atmosphere (75ml/min, TGA/SDTA851e/LF/1600 Mettler-Toledo thermobalance). Microscopic studies were performed by SEM-EDX (JEOL JSM-6300) and mineralogical phases were distinguished by X-ray diffraction (XRD), from 5° to 70° 2θ degrees, using CuKα radiation, at 20mA and 40kV (Brucker AXS D8 Advance diffractometer). Fourier transform infrared (FTIR) spectroscopy analyses were run from 400cm−1 to 2000cm−1 (Bruker Tensor 27 Platinum ATR FTIR).
Results and discussionMortar workabilityThe workability of all the mortars that were prepared fell within the 170±4mm range, whatever the degree of substitution of Portland cement by the ceramic waste (0–50wt.%). The flow of these mortars has a plastic consistency (spread diameter from 140 to 200mm [21]) and is within the maximum workability range defined by standard UNE-EN 1015-2:1999/A1:2007 [22] (175mm±10mm, for fresh mortars with a density higher than 1200kg/m3). Although the CSW particles presented an irregular shape with sharp edges (Materials section), which hampered their accommodation and, consequently, could reduce the flow table spread results, their smooth surface and low porosity contributed to maintaining the workability of the mortars whatever the CSW content. Values were close to that previously reported for mortars prepared with ceramic tile waste to partially replace PC, where the workability values also varied within a narrow range (155±5mm, 0–50wt.% PC replacement) [17]. Pereira-de-Oliveira et al. [6] did not observe any significant workability variations, and reported reductions of 7.3% and 4.5% in mortars containing 40% red-clay ceramic bricks and tiles, respectively.
Compressive strength of mortar samplesThe compressive strength and standard deviation of mortars prepared by replacing 0–50wt.% PC with CSW, cured at 20°C for up to 365 days, are summarized in Table 3. Although mechanical properties are significantly reduced with the addition of CSW waste at short curing ages (3 and 7 days), the values came close to that of the control mortar with the curing time. So, after 180 and 365 days of curing, the strength of mortars blended with up to 25wt.% matched or even exceeded that of the control mortar.
Compressive strength results for the PC mortars blended with 0–50wt.% CSW.
| PC replacement, wt.% | Compressive strength, MPa | |||||
|---|---|---|---|---|---|---|
| 3 days | 7 days | 28 days | 90 days | 180 days | 365 days | |
| 0 | 43.31±0.76 | 44.96±1.69 | 52.86±3.12 | 57.67±1.85 | 62.07±0.98 | 61.23±3.70 |
| 15 | 35.12±1.39 | 40.15±3.06 | 49.80±3.15 | 56.20±1.70 | 64.19±1.54 | 65.42±3.95 |
| 25 | 27.70±0.85 | 34.29±2.65 | 44.59±2.39 | 52.07±0.84 | 61.30±2.30 | 62.54±1.63 |
| 35 | 21.95±0.54 | 28.85±0.78 | 40.06±1.24 | 49.03±1.17 | 55.62±1.07 | 55.69±3.14 |
| 50 | 14.62±0.32 | 19.63±0.94 | 31.23±0.59 | 38.65±0.80 | 43.10±0.80 | 44.56±1.36 |
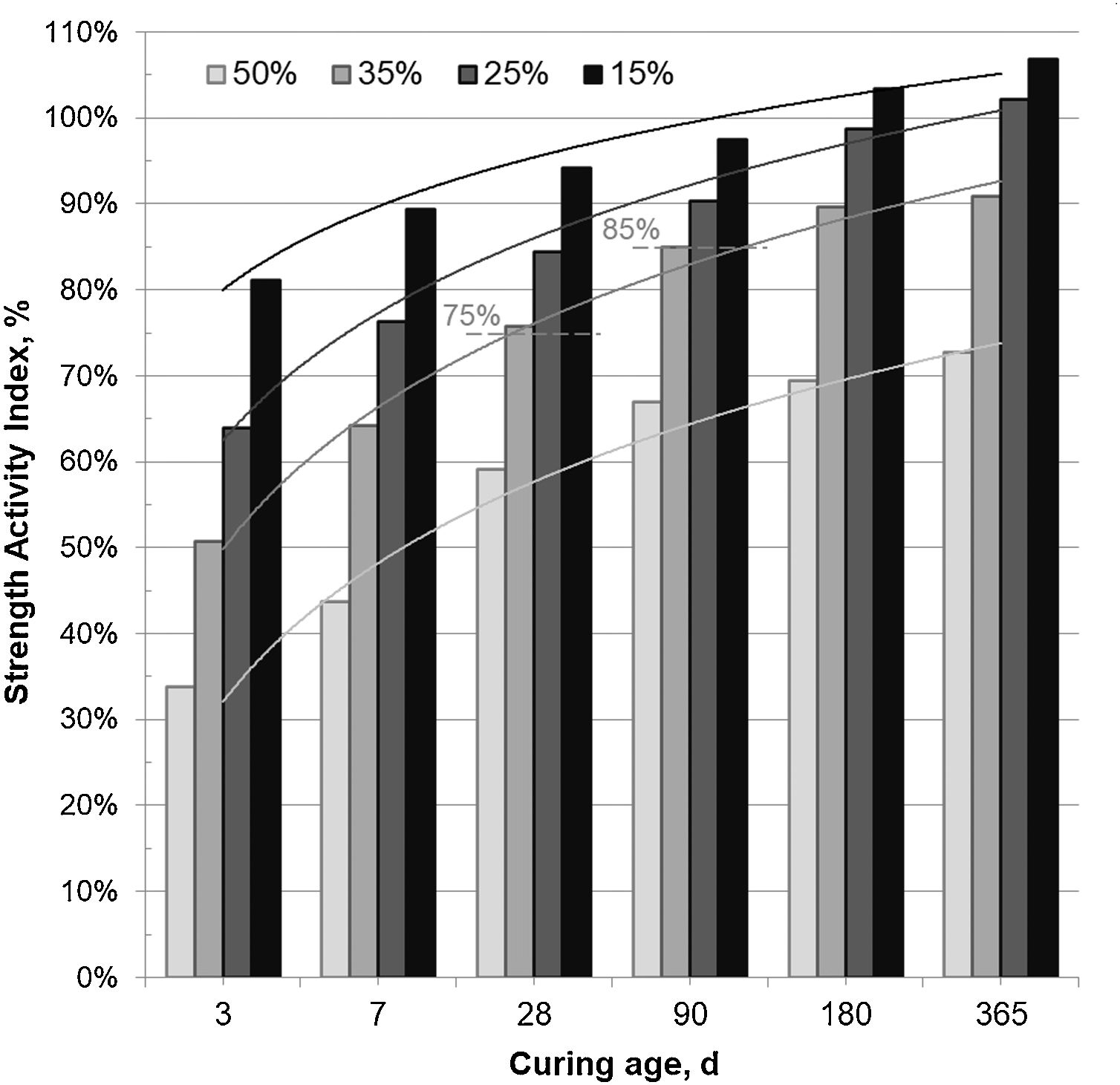
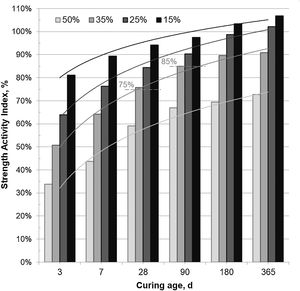
Fig. 3 shows the SAI registered in mortars prepared with 15–50wt.% CSW, cured at room temperature for up to 365 days. The SAI values of mortars prepared with up to 25wt.% CSW, cured for 28 and 90 days, met the specifications set out for fly ash [15], being higher than 75% and 85%, respectively. Although low SAI results were obtained with up to 7 days of curing, the pozzolanic reactivity of CSW improved with the curing age, and values close to 100% (same strength as the control mortar) were achieved in 15wt.% CSW mortars cured for 90 days or 25wt.% cured for longer periods (180 and 365 days). Although the strength of mortars containing 35wt.% CSW barely met the specifications established in UNE EN 450-1 [15], the mechanical properties provided were especially significant after 180 days of curing, since only a 10% reduction in the compressive strength (SAI 90%) was observed in samples developed with 65wt.% PC. This makes CSW35 samples an interesting option for uses that do not demand the acquisition of strength in relatively short times.
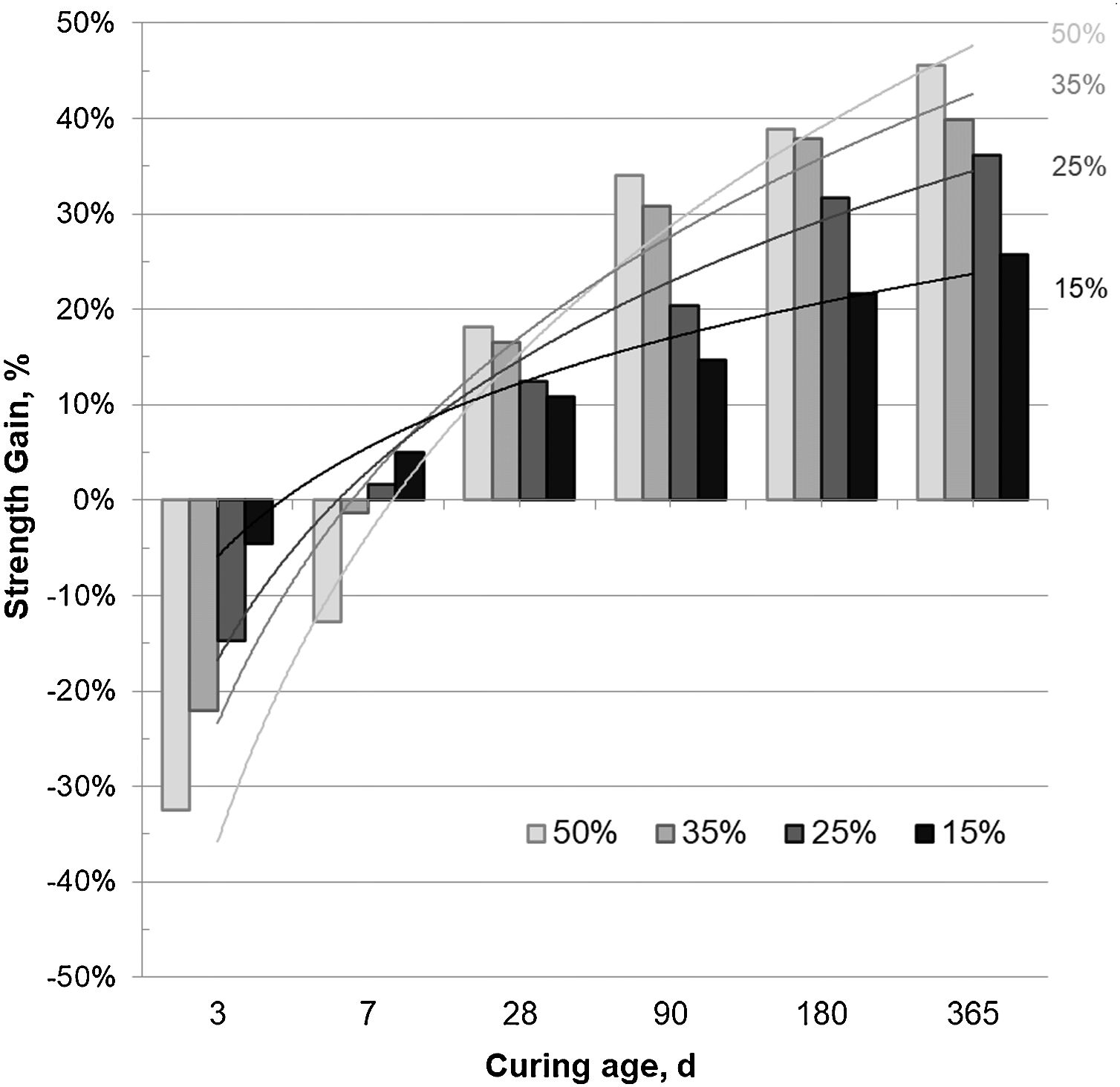
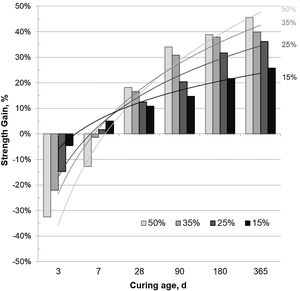
The SG values presented in Fig. 4 corroborate the low pozzolanic activity of the CSW waste at short curing ages and its improvement with the curing time. Negative SG results were obtained mainly in mortars cured for 3 and 7 days, with lower contributions to the strength with increasing ceramic waste contents. However, the pozzolanic activity of the CSW progressively increased with the curing time and the tendency reversed after 28 days of curing, so that higher ceramic waste contents originated better strength gain values.
Simple correlations between the SG or SAI values and the curing age have been established according to Eq. (2):
where:SINDEX=strength index: SG(%) or SAI (%);
a and b=constants for a given percentage of PC substitution;
ln(t)=natural logarithm of the curing time, days.
Regression data for mortars containing 15–50wt.% CSW are summarized in Table 4. The high coefficient of determination obtained (R2>0.97) illustrates a good correlation between the natural logarithm of the curing time and the strength parameters (SG and SAI). The ‘a’ constant of the SAI and SG curves generally increased with higher CSW contents, which denotes greater pozzolanic activity of the ceramic waste (a one-unit increase in the ln(t) is expected to improve the SG/SAI by the value of ‘a’). In line with the lower SG values registered for up to 7 days of curing with increasing amounts of CSW, the SG ‘b’ values became progressively more negative with higher CSW waste contents. However, this tendency reversed after 28 days of curing, when increasing ceramic waste additions led to greater contributions to the mechanical properties of the mortars that were developed (corroborated by the higher SG ‘a’ constant with increasing waste contents).
Analysis regression parameters for logarithmic correlations SG (%) or SAI (%)=a·ln(t)+b.
| Strength parameter | Regression parameters | PC replacement, wt.% | |||
|---|---|---|---|---|---|
| 15 | 25 | 35 | 50 | ||
| SAI, % | a | 0.140 | 0.215 | 0.239 | 0.233 |
| b | 0.801 | 0.624 | 0.498 | 0.321 | |
| R2 | 0.977 | 0.986 | 0.989 | 0.972 | |
| SG, % | a | 0.165 | 0.286 | 0.368 | 0.466 |
| b | −0.059 | −0.167 | −0.234 | −0.358 | |
| R2 | 0.977 | 0.986 | 0.989 | 0.972 | |
The results obtained are close to those previously reported by Mas et al. [17], who, after analyzing the pozzolanic activity of ceramic tile waste observed that the strength gain provided by this material was significant after 28 days of curing at room temperature. In their study [17], mortars prepared with up to 35wt.% CSW exhibited SAI values higher than 75% and 85% after 28 and 90 days, respectively. Lavat et al. [7] investigated the use of three different types of roof tiles as pozzolanic admixtures, and also observed a slow pozzolanic activity, with SAI values ranging from 74.9% to 88.3% in mortars prepared with 30wt.% ceramic waste, cured for 7 and 28 days.
ThermogravimetryThe main quantitative data obtained after thermogravimetric analyses are summarized in Table 5. The total weight loss (TWL), water loss due to Ca(OH)2 dehydration (CH), amount of hydrates formed during the paste hydration (H, calculated as the difference between TWL and CH) have been determined. The fixed Ca(OH)2 (FL) due to pozzolanic reactions in the Portland cement/CSW systems developed was calculated according to Eq. (3):
where:TGA results: Total weight loss (TWL), Hydrates (H), and Fixed Lime (FL).
| PC replacement, wt.% | Curing time, days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 90 | 180 | 365 | |||||||||
| TG parameters, wt.% | ||||||||||||
| TWL | H | FL | TWL | H | FL | TWL | H | FL | TWL | H | FL | |
| 0 | 20.45 | 16.81 | 20.79 | 18.23 | 21.20 | 17.40 | 23.70 | 19.86 | ||||
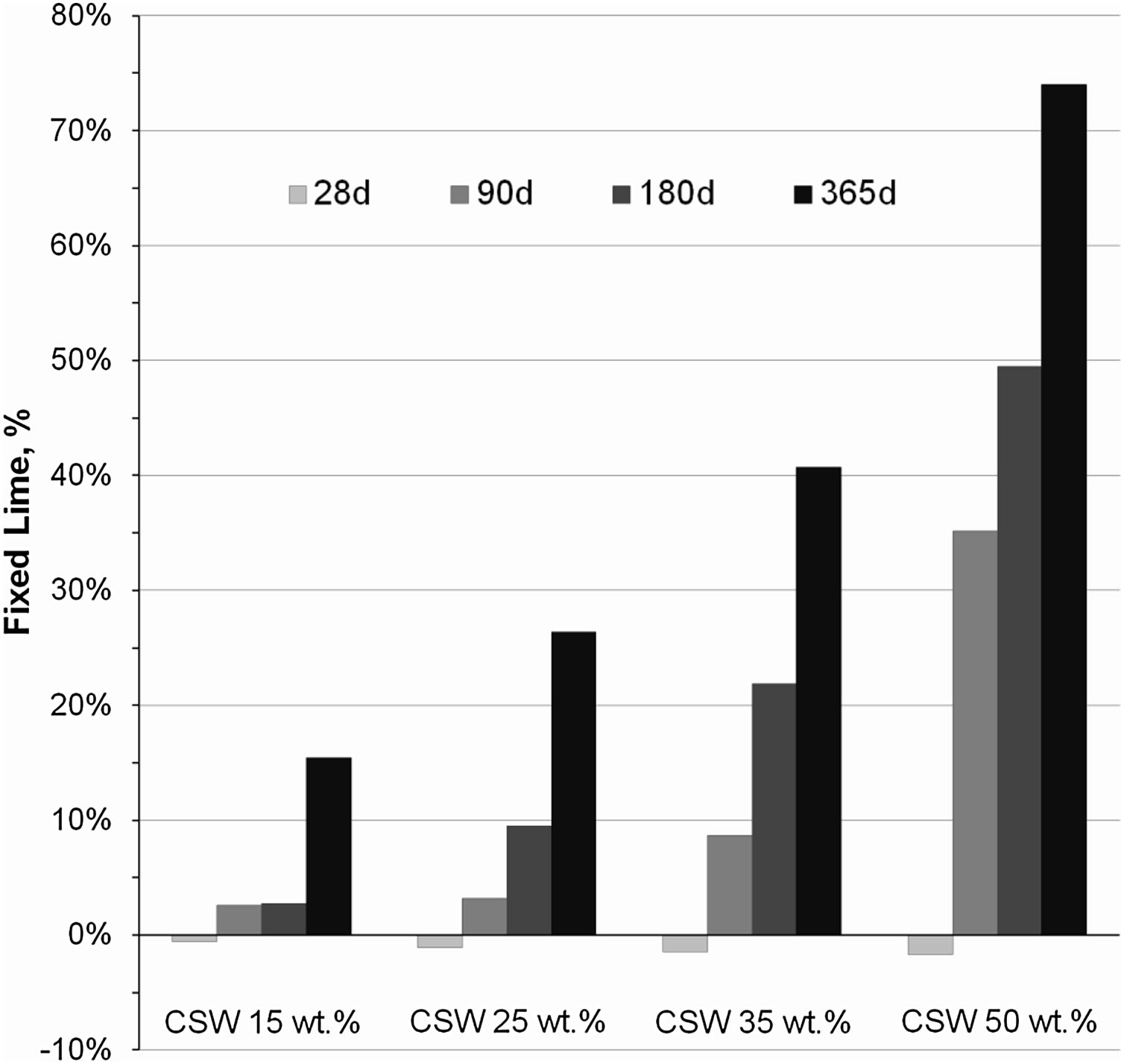
| 15 | 19.56 | 16.45 | −0.52 | 19.12 | 17.00 | 2.57 | 19.64 | 16.50 | 2.79 | 22.31 | 19.55 | 15.44 |
| 25 | 18.34 | 15.58 | −1.10 | 16.89 | 15.03 | 3.21 | 18.03 | 15.45 | 9.47 | 21.59 | 19.47 | 26.39 |
| 35 | 15.64 | 13.24 | −1.44 | 16.44 | 14.92 | 8.65 | 15.95 | 14.02 | 21.86 | 20.96 | 19.48 | 40.71 |
| 50 | 13.45 | 11.6 | −1.65 | 13.56 | 12.73 | 35.16 | 14.15 | 13.19 | 49.47 | 18.62 | 18.12 | 73.96 |
CHC: amount of Ca(OH)2 in the control paste;
CHCSW: amount of Ca(OH)2 in the PC/CSW blended paste (same curing age as the control paste);
%Cem: proportion of PC in the blended paste (per unit).
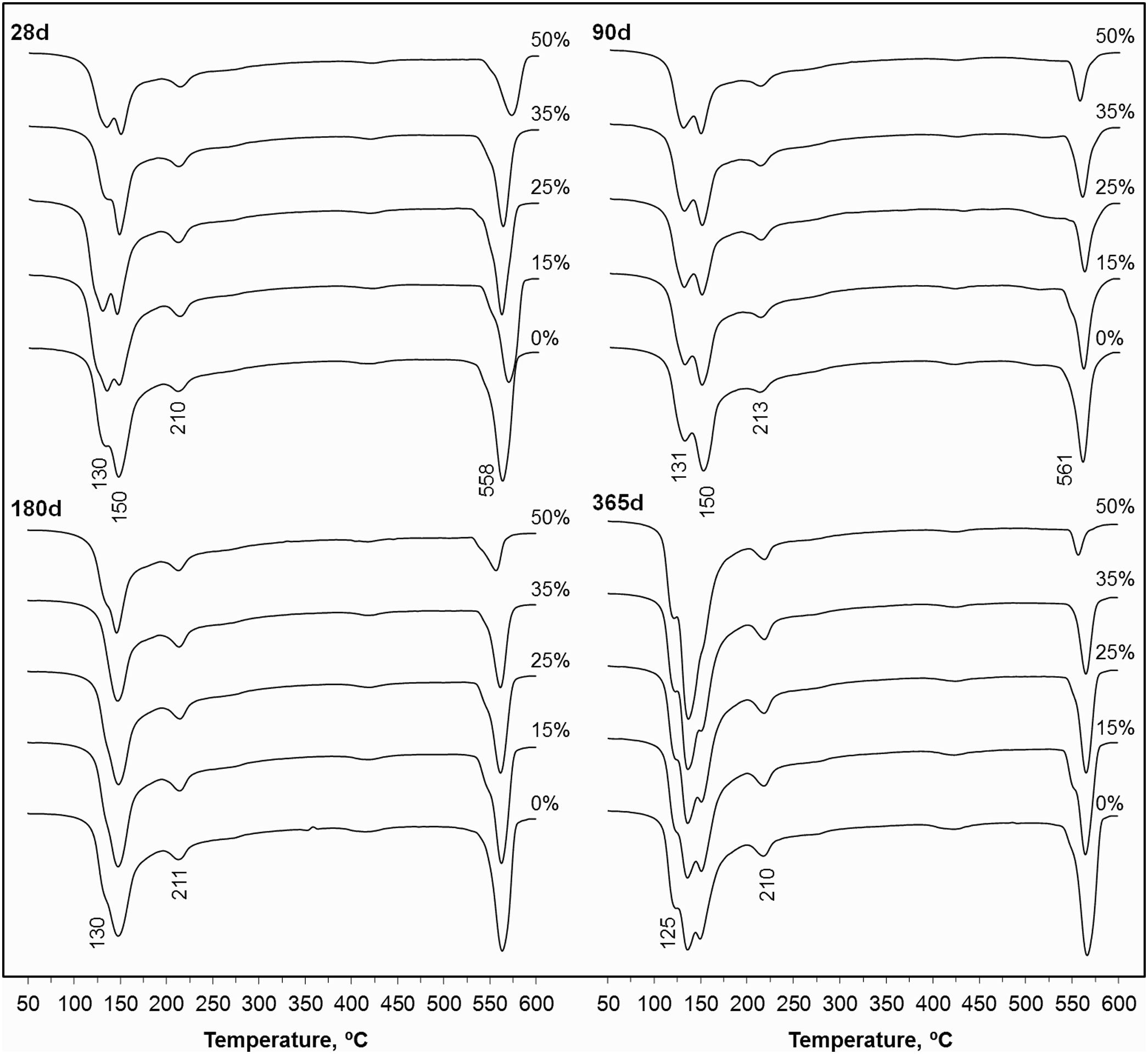
In agreement with the compressive strength results, the TWL and percentage of hydrates formed diminished with increasing CSW contents. However, the differences between the control paste and that containing 50wt.% ceramic waste were reduced with the curing time, which denotes an improvement in the pozzolanic activity of the CSW.
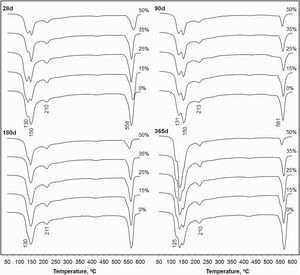
Fig. 5 shows the thermogravimetric curves (DTG) for the PC pastes blended with 0–50wt.% CSW waste, cured from 28 to 365 days at room temperature. Bands appearing in the 100–180°C range are attributed to the dehydration of calcium silicate hydrates (CSH) and ettringite (AfT), while those arising from 180–240°C are associated with calcium aluminate hydrates (CAH) and calcium aluminosilicate hydrates (CASH) [16,17]. The shape of these bands was modified and their intensity increased with the curing time, which, in good agreement with the data reported in Table 5 and the evolution of the compressive strength (see Compressive strength of mortar samples section), denotes the progressive formation of hydrates that make the system stronger. The intensity of the signals arising in the 520–600°C range, associated with the dehydroxylation of portlandite [16,17], was reduced with increasing CSW contents, due to the dilution effect and the consumption of portlandite during pozzolanic reactions.
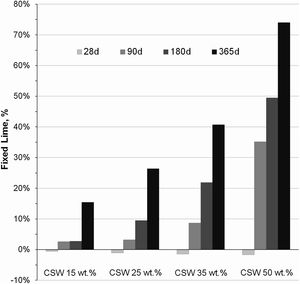
Data in Fig. 6 show the percentages of fixed Ca(OH)2 in PC/CSW blended pastes cured from 28 to 365 days at room temperature. The negative fixed lime values registered in the CSW blended samples cured for 28 days indicate that higher amounts of portlandite formed in the pozzolanic paste in comparison to that expected in a theoretical binder that contained the same amount of PC but no CSW. These results denote that the CSW waste pozzolanic reaction was kinetically slow, and the particle effect prevails over the pozzolanic reaction up to 28 days of curing, which facilitates the hydration of Portland cement and leads to higher portlandite formation. As explained by Payá et al. [16] and Cyr et al. [23], CSW particles provide extra surface where the first hydration products formed may accumulate, thus extending the free surfaces available for cement hydration to progress. This also justifies the slightly more negative values obtained with increasing CSW waste contents in the samples cured for 28 days, since a higher number of nucleation sites are provided. Fixed Ca(OH)2 rates became positive and progressively increased with the curing time and addition of CSW, which corroborates the partial consumption of portlandite during the pozzolanic reaction of the ceramic waste.
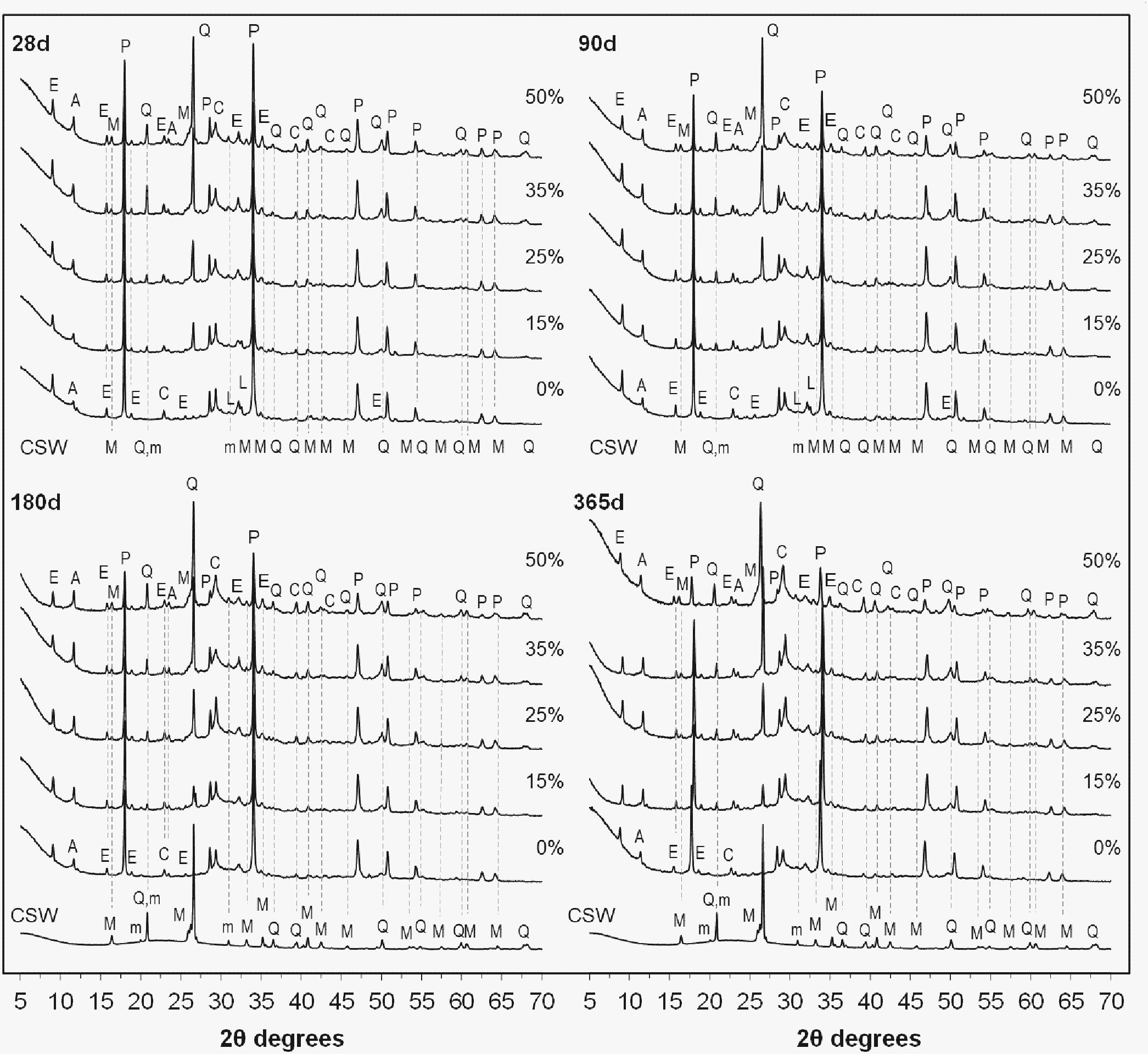
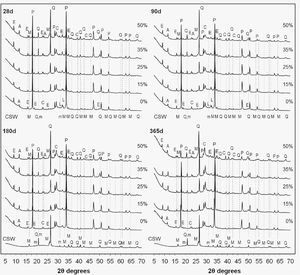
X-ray diffraction (XRD)The results of the XRD analyses obtained for the pastes containing 0–50wt.% CSW, cured at 20°C from 28 to 365 days are presented in Fig. 7. A diffractogram of the milled CSW waste has also been plotted as a reference. The peaks associated with mullite (M, Al6Si2O13, PDF #150776), quartz (Q, SiO2, PDF #331161), and microcline (m, KAlSi3O8, PDF#190926), identified in the spectral pattern of the CSW, were identified in the pozzolanic pastes.
XRD patterns of the PC pastes blended with 0–50wt.% CSW, cured from 28 to 365 days at 20°C: M, mullite (Al6Si2O13); Q, quartz (SiO2); m, microcline (KAlSi3O8); P, portlandite (Ca(OH)2); E, ettringite (Ca6Al2(SO4)3(OH)12·26H2O); L, larnite (β-Ca2SiO4); C, calcite (CaCO3); A, carboaluminate (Ca4Al2O6CO3·11H2O).
In agreement with the thermogravimetric results, the peaks attributed to portlandite (P, Ca(OH)2, PDF #040733) exhibited a similar intensity in the pastes cured for 28 days, whatever the ceramic waste content. This is explained by the particle effect, which facilitates the hydration of Portland cement and, consequently, balances lower cement contents with its faster hydration. As expected, consumption of Ca(OH)2 increased with the curing time, leading to lower intensities of the corresponding peaks with higher amounts of CSW waste. In line with the thermogravimetric and compressive strength results, this corroborates the consumption of portlandite during the pozzolanic reactions to provide new hydration products.
Signals due to larnite (L, β-Ca2SiO4, PDF #330302), also known as belite, appeared in the pastes cured for up to 90 days, which denoted the presence of unreacted Portland cement particles. The XRD spectra also revealed minor amounts of ettringite (E, Ca6Al2(SO4)3(OH)12·26H2O, PDF #411451) in all the samples prepared. Calcite (C, CaCO3, PDF #050586) and carboaluminate Ca4Al2O6CO3·11H2O (A, PDF #410219) were also identified in all the pastes that were prepared. Up to 90 days of curing, the signals due to calcite exhibited similar intensities whatever the CSW waste content. This is explained by the dilution effect, since calcite is mainly associated with the limestone filler in the PC composition. Signals of both calcite and carboaluminate became more significant after 180 and 365 days of curing, and intensified with the CSW waste content, which denotes a slight increase in the carbonation with higher PC substitutions. This behavior is linked with the lower strength values exhibited by the PC/CSW blended mortars compared to the control sample, especially up to 28 days of curing (Table 3). It can be attributed to a certain extent to a higher porosity produced with increasing waste contents, which would facilitate CO2 penetration, especially until pozzolanic reactions take place to provide new reaction products.
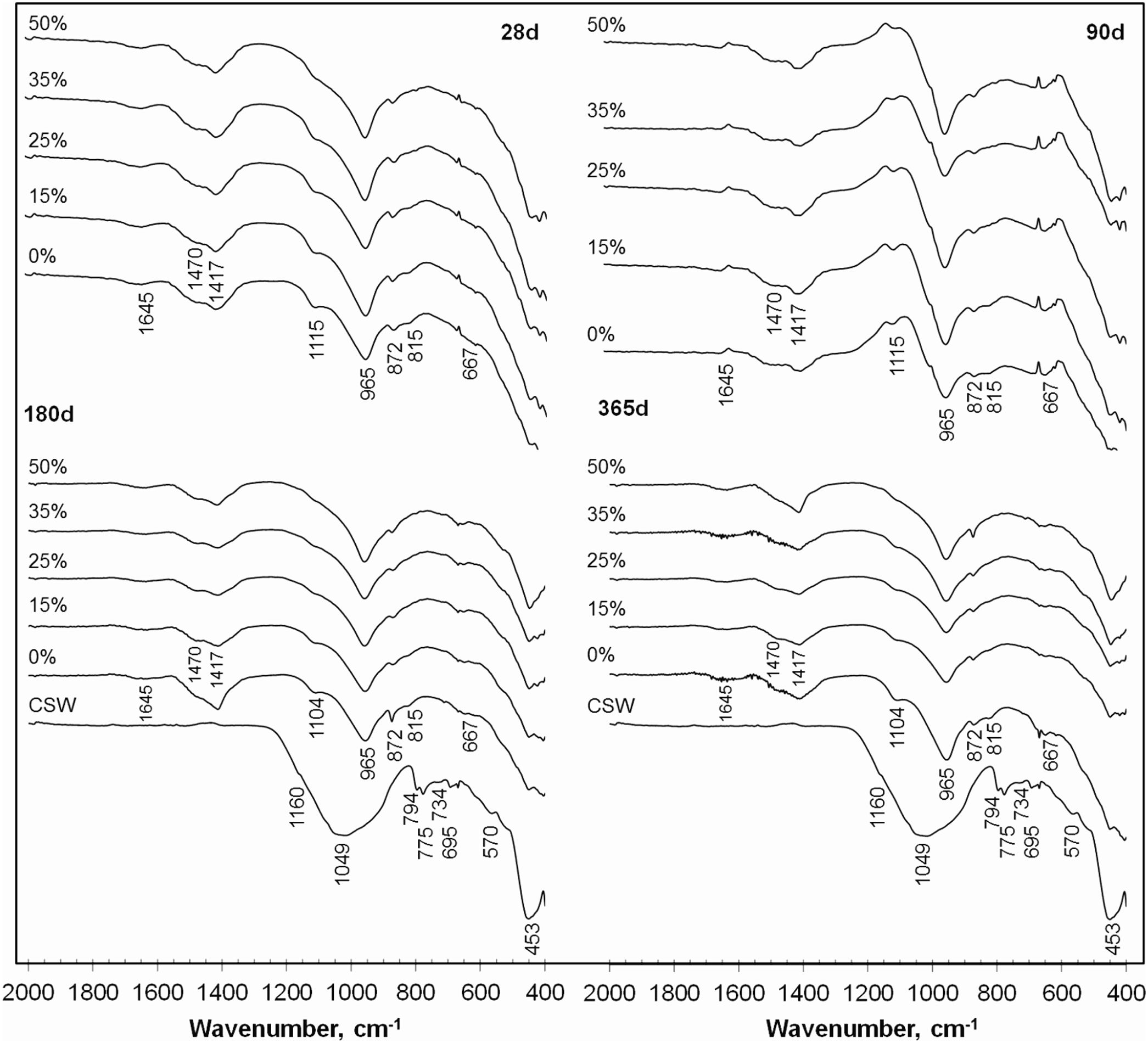
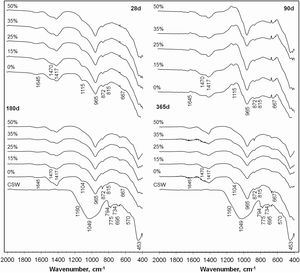
Fourier transform infrared (FTIR) spectroscopyThe FTIR spectra of the pastes containing 0–50wt.% CSW, cured at 20°C from 28 to 365 days are shown in Fig. 8. The spectral pattern of the milled CSW has also been plotted as a reference. Similarly to previously reported spectra for other ceramic materials, such as porcelain stoneware tiles [24] or ceramic tiles [7], it presents a main band centered at 1049cm−1 (associated to amorphous phases), signals attributed to quartz (1145, 1084, 794–775, 695, 522 and 453cm−1) [25,26] and bands produced by the presence of mullite (1185cm−1[26] and low intensity bands at 570 and 734cm−1[7]). As previously observed by XRD, these signals also arose in the pozzolanic pastes, with higher intensities with increasing CSW contents.
As reported earlier by García-Lodeiro et al. [25,27], the bands arising at 965cm−1, 815cm−1 and in the 650–450cm−1 range in the PC/CSW blended pastes are characteristic of the C–S–H gel. Si–O asymmetric stretching vibrations generate the band at 965cm−1, the signal at 815cm−1 is attributed to Si–O symmetric stretching vibrations, and the series of bands arising between 650 and 450cm−1 occur due to Si–O–Si deformation vibrations (bending band). Formation of ettringite, previously identified by XRD, was corroborated by the single band at 1104cm−1[7], the intensity of which decreased with the addition of CSW waste. According to Hidalgo et al. [28] and Lavat et al. [7], signals at 1641cm−1 are attributed to bending vibrations of –OH in the hydroxyl groups (H2O) of hydrated products. The presence of portlandite, the evolution of which was assessed by TGA and XRD analyses, could not be confirmed by FTIR tests, since it is associated with a sharp absorption peak at 3635cm−1, which is a characteristic feature of the stretching vibrations generated by the O–H bonds in Ca(OH)2[7,25].
The presence of carbonates and carboaluminates, which were previously identified in the XRD spectra of the PC/CSW blended pastes, was confirmed by the signals arising within the 1400–1500cm−1 range, which is typical of C–O stretching vibrations. While asymmetric stretching vibrations in calcite give rise to a band at 1420cm−1[28], carbonate impurity species, such as natrite (Na2CO3) or vaterite (CaCO3), slightly displace this signal to higher wavenumbers (1455cm−1 and 1484cm−1, respectively) [28,29]. As described by Pacewska et al. [30] and Lavat et al. [7], signals at 712cm−1 and 875cm−1, when appearing simultaneously with those around 1450cm−1, are also attributed to carbonate salts.
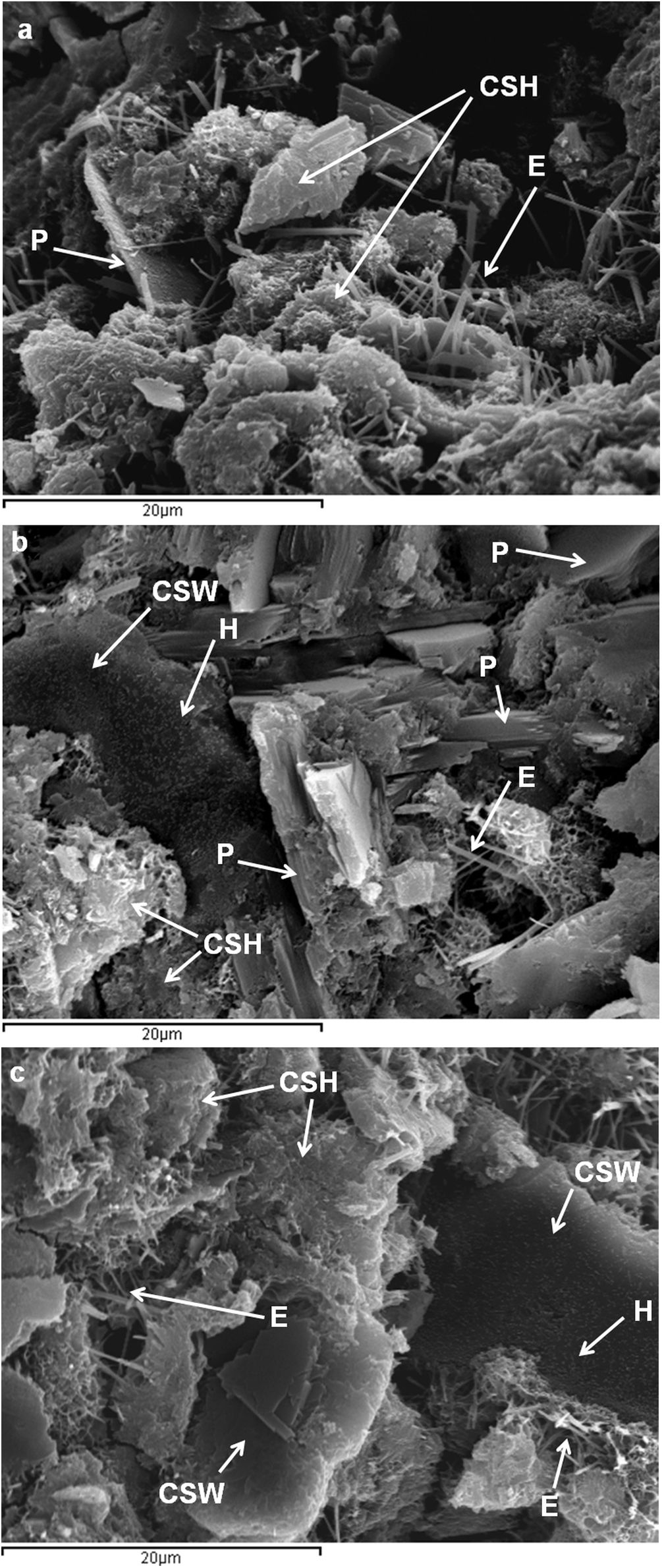
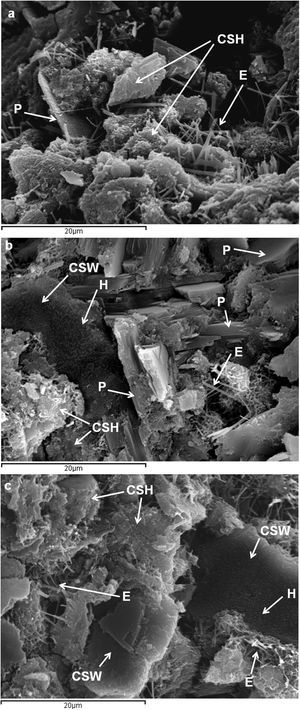
Scanning electron microscopyFig. 9 shows the microstructure of the control paste and those blended with 25wt.% and 50wt.% CSW waste, after 28 days of curing at room temperature. SEM analyses corroborated the existence of ettringite needles, Ca(OH)2 hexagonal plates, unreacted CSW particles, and amorphous hydration products, all of which had previously been identified by TGA, XRD or FTIR tests. Hydration products were also identified on the surface of CSW particles.
ConclusionsThis research has proven that ceramic sanitary ware (CSW) waste can be successfully used as a pozzolanic admixture in Portland cement blended systems, leading to more sustainable construction materials with environmental benefits. According to the results obtained:
- -
Similar workability values were registered whatever the CSW content, which would facilitate the placement of mortars and concrete on the building site.
- -
The pozzolanic activity of CSW was slow: up to 28 days of curing CSW facilitated PC hydration due to the particle effect, and the pozzolanic reaction significantly improved after 90 days of curing.
- -
The strength gain attributed to CSW waste started to be significant after 28 days of curing. From then on, higher contributions to the strength were observed with increasing CSW contents and longer curing times.
- -
PC mortars blended with up to 25wt.% CSW satisfied the compressive strength requirements established for other pozzolanic materials such as fly ash.
- -
35wt.% CSW mortars could be considered for applications where the kinetics of the process is not a decisive parameter since, although their strength barely satisfied the specifications for fly ash, it improved significantly with the curing time.
The authors are grateful to the Spanish Ministry of Science, Innovation and Universities for supporting this research through Project ECOSOST RTI-2018-097612-B-C21, and to FEDER funding. They also thank the Institute for Science and Technology of Concrete (ICITECH) and Universitat Jaume I for providing the means to perform this study. Thanks are extended to the Scientific Instrumentation Centre of the Universitat Jaume I and the Electron Microscopy Service of the Universitat Politècnica de València for their help with the microstructural characterization analyses.