Herein, mesoporous Li2Si2O5 hydrate dumbbell-like structures were for the first time fabricated by a fluorine-induced in situ crystallization route. The obtained dumbbells assembled by nanowire-nanoparticles featured highly porous structures with mesoporous pores below 13nm, enabling a remarkably large surface area of 76.71m2·g−1 which was 4.09 times that of the structures in NH4F-free solution and represented one of highest values reported to date on Li2Si2O5 structures. The novel mesoporous structures revealed a new strategy to enhance the methylene blue adsorbance to 66.09mgg−1, allowing for their promising functional application in effluent treatment.
Aquí, un hidrato de Li2Si2O5 mesoporoso en forma de mancuernas fue obtenido por primera vez usando una ruta de cristalización in situ inducida por flúor. Las mancuernas obtenidas, formadas por el ensamblaje de nanopartículas en forma de nanohilos, presentaron una porosidad elevada, con mesoporos por debajo de los 13nm, habilitando un área superficial particularmente grande, de 76,71m2·g−1, siendo este valor 4,09 veces el de las estructuras obtenidas sin el empleo de NH4F, lo que representa uno de los valores más altos descritos hasta la fecha en estructuras de Li2Si2O5. Las nuevas estructuras mesoporosas revelaron una estrategia novedosa para mejorar la adsorción de azul de metileno a 66,09mg·g−1, lo que supone una prometedora aplicación en el tratamiento de efluentes.
Li2Si2O5 is considered to be one of the most promising functional materials in many areas, such as dentistry, CO2 adsorption, tritium breeders and so on [1–3]. Research in these areas is closely related to the synthetically controlled morphologies that represent the key modulation in the properties of final products that largely determine the field of material capability [4–6]. Conventionally, direct solid-state reaction or chemical precipitation processes are the most common methods for obtaining granular Li2Si2O5 powders [7,8]. However, these strategies are solely effective for relative dense Li2Si2O5 bulk crystals without pores, because the rapidly short-length diffusion of lithium arouses compact surface layers formed on pristine particles. An alternative route is to deposit 2D sheet-like Li2Si2O5 nanostructures through a mild hydrothermal process, which endows a useful function [9,10]. Despite the success in functional enhancements, it still faces problems of fairly low surface area, resulting in heavy loss of other functions. Up to now, little success has been achieved in constructing functional Li2Si2O5 hierarchical structures owing to the difficulty in simultaneously controlling the growth of Li2Si2O5 and their properties. Herein, we reported a fluorine-induced in situ crystallization route to synthesize a novel type of mesoporous dumbbell-like Li2Si2O5 hydrate structures with large surface area and potential methylene blue (denoted as MB) adsorption function.
ExperimentalTypically, a certain weight of NH4F (0, 0.074, 0.148, 0.296, 0.592g) was dissolved in 75mL of deionized water and magnetically stirred, followed by addition of 0.2M LiOH·H2O and TEOS with Li/Si=1. After vigorously stirring for 30min, the solution was transferred into a 100mL Teflon-lined autoclave and maintained at 180°C for 48h. The as-obtained white products were collected by washing several times with alcohol, and then dried at 80°C for 24h.
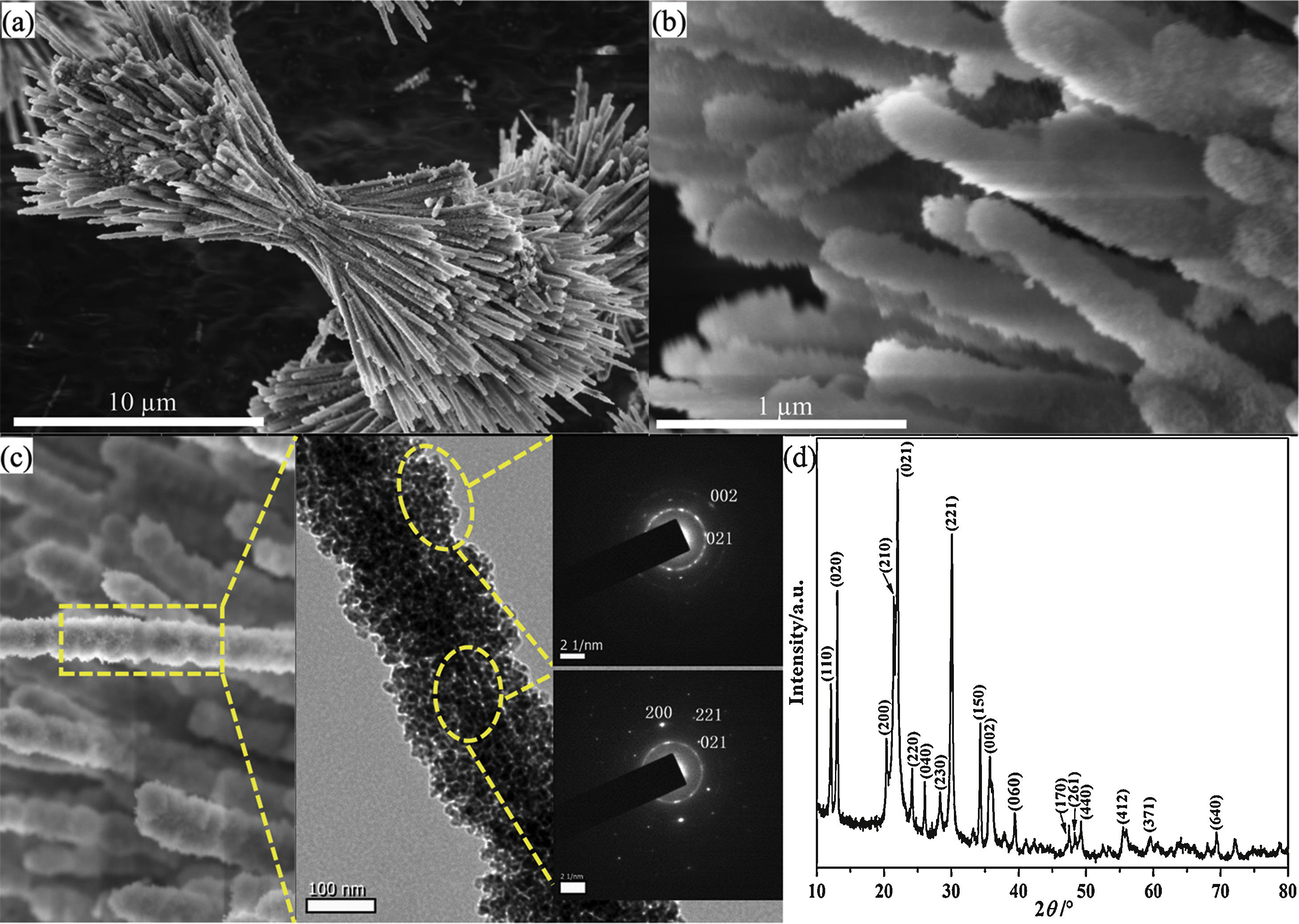
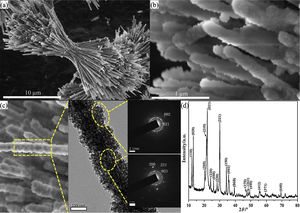
Results and discussionFig. 1 shows the morphological details and phase composition of the product prepared at 0.148g NH4F. The product presented dumbbell-like structure from the panoramic image (Fig. 1a). The two lobe brushes of the dumbbell radiated symmetrically from the center with diameter of about 2.5μm, resulting in a total size of about 25μm. It could be found that the brushes were constructed by flocky nanowires of about 200nm (Fig. 1b). From the magnified TEM image and SAED patterns (Fig. 1c), it could be seen that the single flocky nanowire exhibited composite structure consisting of highly crystallized single-crystalline gracile nanowire with agglomerated polycrystalline nanoparticles coated on. Notably, the plenty of agglomerated nanoparticles formed a mesoporous structure. The XRD peaks in Fig. 1(d) could be well indexed to the pure orthorhombic structure of Li2Si2O5 hydrate crystals (Li2Si2O5·2H2O) (JCPDS No. 33-0816), indicating a high crystallinity in consistent with the TEM result.
(a) The integral and (b) the high-magnification FE-SEM images, (c) the single nanowire and its corresponding TEM image, (d) XRD pattern of Li2Si2O5 hydrate dumbbells prepared at 0.148g NH4F. Insets in (c) are the SAED patterns of two counterparts showing polycrystalline nanoparticles and single-crystal nanowire in the dumbbell, respectively.
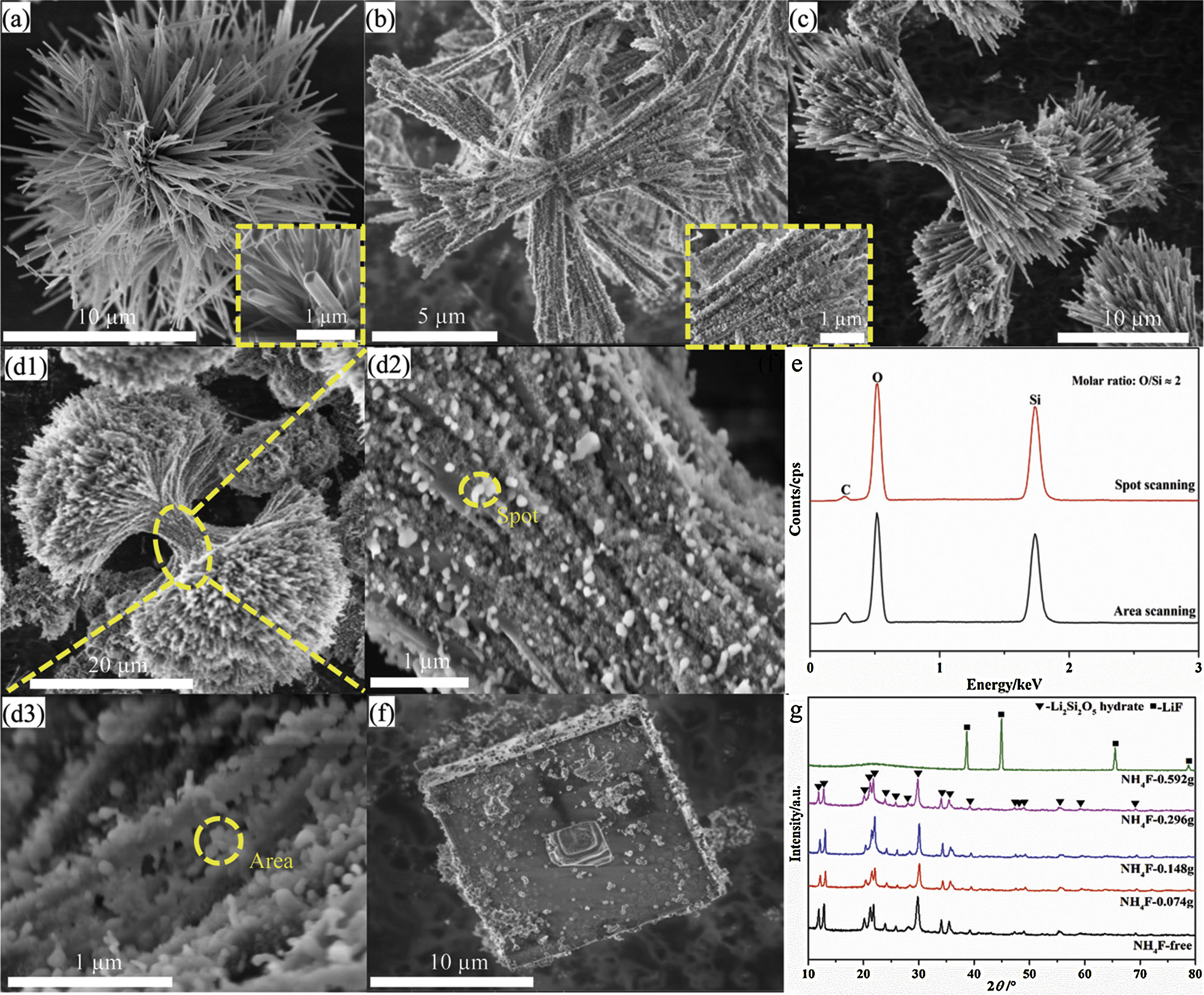
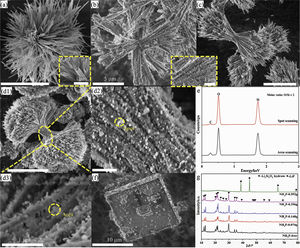
Fig. 2 shows the morphological evolution and phase composition of at different NH4F content. In the absence of NH4F, only Li2Si2O5 hydrate clusters assembled by multidirectional prismatic rods radiating from the center were observed (Fig. 2a). Increasing the NH4F content to 0.074g, Li2Si2O5 hydrate rod-nanoparticle composites were derived and packed together to form delicate brushes that were radially but symmetrically arranged and bundled at one center (Fig. 2b). Adjusting the content to 0.148g, dumbbell-like Li2Si2O5 hydrate structures that composed of two symmetrical lobes were formed (Fig. 2c). When 0.296g NH4F was introduced, the two lobes of the dumbbell derived into two hemispherical brushes with high-density nanowire-nanoparticles as shown in Fig. 2(d1). From the enlarged views (Fig. 2(d2–d3)), it could be visibly observed that numerous bigger white nanoparticles were adhered on the pristine Li2Si2O5 hydrate nanoparticles. This might be ascribed to amorphous SiO2 by the EDS results in Fig. 2(e), in agreement with the amorphous characteristics in the XRD domain peaks from 20 to 25° (Fig. 2(g)) that differed from the product at 0.148g NH4F. However, when 0.592g NH4F was introduced, only cubic LiF (JCPDS No. 04-0857, Fig. 2(g)) blocks were formed accompanied with a spot of amorphous SiO2 (Fig. 2(f)).
Apparently, NH4F played a decisive role in directing the Li2Si2O5 hydrate structures. On the basis of previous studies [11–13], the growth of dumbbell-like structures was highly related to an in situ crystallization process that occurred solely along the crystal facet direction adsorbing fluorine due to the lower surface energy. In this process, NH4F hydrolyzed into ammonia acting as a pH buffer to slowly release OH−[14,15]. The surfaces of early formed Li2Si2O5 hydrate nanowires were unsaturated charged and surface Li+ could interact with fluorine to form Si-O-Li-F complexes as confirmed by the XPS spectra of surface composition containing O, Si, F and Li (Fig. S1). The hydrogen bonding between Si-O-Li-F complexes and ammonia in the solution made the complexes act as anchor sites to capture OH−. OH− exchanged with fluorine resulting in chemical defects that benefited heterogeneous nucleation on the nanowires [16–18]. The more the NH4F content was, the more nucleation sites on the surfaces formed, accelerating the nuclei deposition and leading to further quantities of nanowires derived (0.296g) and vice versa (0.074g). Importantly, excess consumption of OH− by ammonia led to the surplus of TEOS due to Li+/OH−/Si=1 for Li2Si2O5·2H2O, thereby SiO2 nanoparticles formed. When using 0.592g NH4F (Li/F=1), the reaction between NH4F and LiOH dominated because the supersaturation toward LiF was satisfied, resulting in a mixture of amorphous SiO2 nanoparticles and highly crystallized LiF.
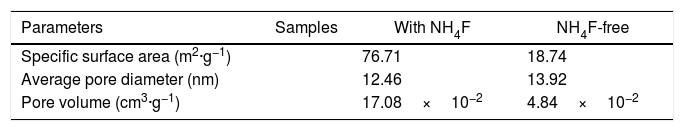
Table 1 compares the BET parameters of the dumbbells (0.148g NH4F) with the product of NH4F-free. The specific surface area of the dumbbells was 76.61m2·g−1, 4.09 times that of NH4F-free product and higher than that of other related report [19]. The average pore diameter and pore volume were 12.46nm and 17.08×10−2cm3·g−1, respectively, indicating that the product was mesoporous material [20] in agreement with the TEM result.
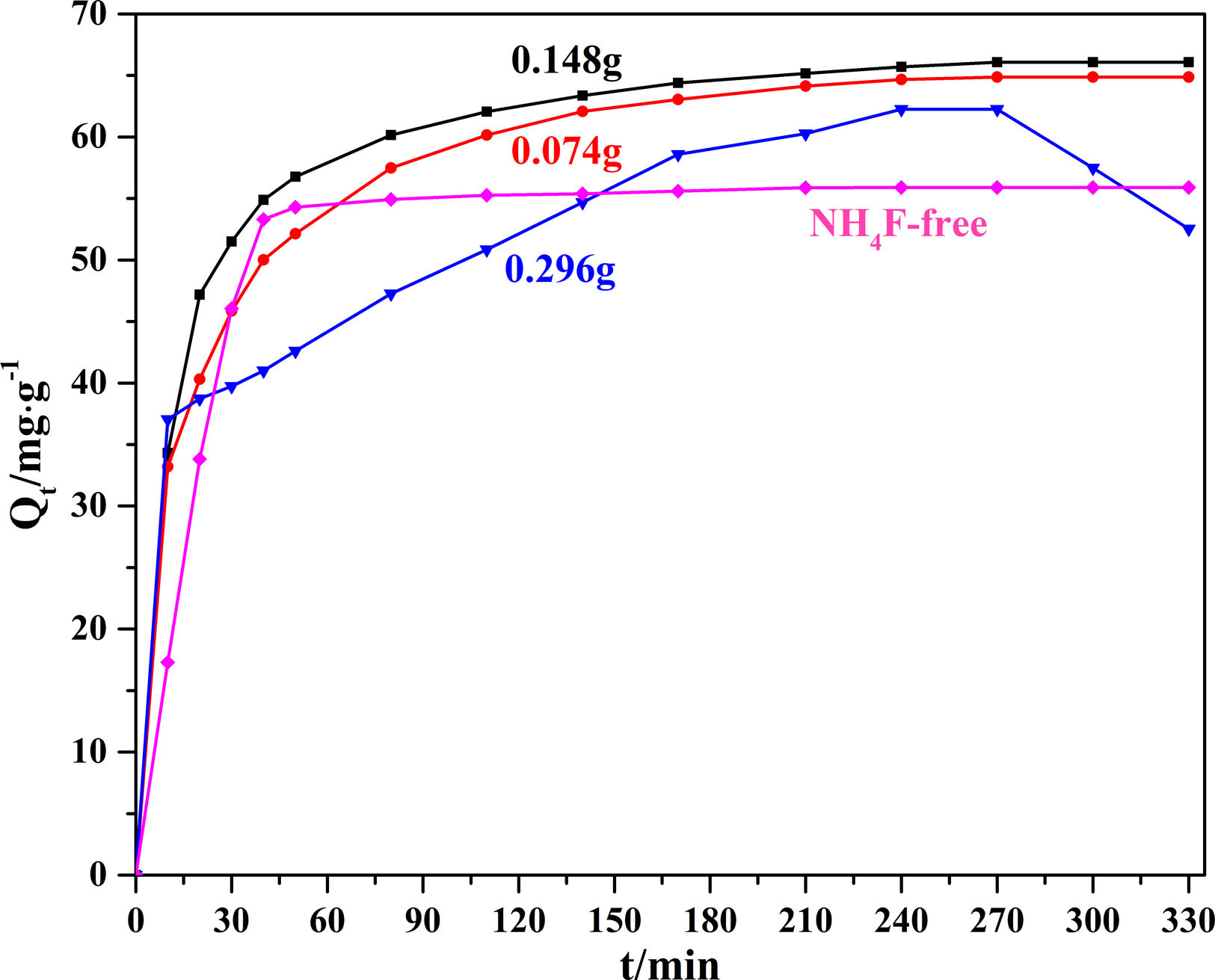
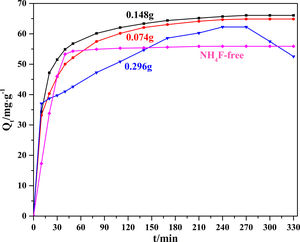
Fig. 3 compares the MB adsorption kinetics of Li2Si2O5 hydrate products at different NH4F content. The MB adsorbance of all the products rose rapidly at the very beginning (0–20min) due to the strong electrostatic interaction between chromophoric groups of MB and surfaces of Li2Si2O5 hydrate and a large number of unoccupied vacant surface. Then the adsorption rate gradually slowed down until equilibrium (4h) due to the remaining vacant surface sites were difficult to be occupied resulted from the steric barrier between MB molecules on the surface [21]. Distinctly, the adsorption rate of products at 0.296g NH4F was slower than that of other products and the MB desorption occurred after equilibrium. It might be explained that numerous amorphous SiO2 strong adhesion on dumbbells might occupy the active sites and reduce the electrostatic interaction between MB molecules and Li2Si2O5 hydrate surfaces. The MB equilibrium adsorbance of these products were 55.89 (NH4F-free), 64.87 (0.074g NH4F), 66.09 (0.148g NH4F) and 62.26mg/g−1 (0.296g NH4F), respectively. It could also be found that Li2Si2O5 hydrate dumbbells (0.148g NH4F) showed the largest adsorbance attributed to the enlarged specific surface area owing to the cavities between nanoparticles and nanowires resulted porous structure, implying a potential application in effluent treatment.
ConclusionNovel mesoporous Li2Si2O5 hydrate dumbbell-like structures were conveniently synthesized by a facile fluorine-induced in situ crystallization route, which featured a largest specific surface area of 76.71m2·g−1 ever reported to date on Li2Si2O5 structures. Benefiting from its unique mesoporous structure, Li2Si2O5 hydrate dumbbells presented a strong methylene blue adsorbance to 66.09mgg−1, offering attractive benefits for promising functional application in effluent treatment.
We thank the National Key R&D Program of China (Grant No. 2017YFB0310300), the National Natural Science Foundation of China (Grant No. 51672209) for the support of this work.