This study focuses on the degradation of kaolinite clay added with Al2O3 and Ta2O5, also known as tantite, to increase its resistance to an acid solution (H2SO4) at 300°C. The samples tested for degradation were previously crushed, sieved, mixed, formed under cold isostatic pressing and sintered at 1150°C. X-ray fluorescence (XRF) and X-ray diffraction (XRD) techniques were used to determine the composition and reveal the phases present in the samples. Degradation by static immersion (98wt% H2SO4) was evaluated by the ASTM C267 mass loss technique. Predominant oxides of SiO2, Al2O3 and Fe2O3 were found within the kaolinite clay, as well as other oxides in proportions lower than 2.39wt%. The immersion of natural clay in sulfuric acid (at 300°C) caused an increase in SO3 content as well as in the mass of all samples. The X-ray diffractograms revealed the presence of quartz, cristobalite, curundum and tantite, as well as a significant reduction in their peak intensities after the interaction with H2SO4. The results showed that adding 20wt% (sample M3C) and 40wt% (sample M4C) of Ta2O5 to the kaolinite clay (sample M1C), produces a more stable structure and greater resistance to attack by H2SO4.
Se estudia la degradación de una arcilla caolinítica ante una solución ácida (H2SO4) a 300°C, la cual fue adicionada con Al2O3 y Ta2O5, también conocida como tantita, para elevar la resistencia al ataque por la solución ácida. Las muestras sometidas a degradación fueron previamente trituradas, tamizadas, mezcladas, conformadas bajo compresión isostática en frío y sinterizadas a 1150°C. Se utilizaron técnicas de fluorescencia de rayos X (XRF) y difracción de rayos X (DRX) para determinar la composición y revelar las fases presentes en las muestras. La degradación por inmersión estática (H2SO4 al 98% en peso) se evaluó a través de la técnica por pérdida de masa ASTM C267. En la arcilla caolinítica se encontraron óxidos predominantes de SiO2, Al2O3 y Fe2O3, y otros óxidos en proporciones menores al 2.39% en peso. La inmersión en ácido sulfúrico (a 300°C) ocasionó una elevación en el contenido de SO3 y un incremento en la masa de todas las muestras. Los difractogramas de rayos X revelaron la presencia de cuarzo, cristobalita, corindón y tantita, así como una reducción significativa en la intensidad de los picos de todas estas fases después de la interacción con el H2SO4. Los resultados mostraron que al adicionar 20% y 40% en peso de Ta2O5 a la arcilla caolinítica (M1C), muestras M3C y M4C, respectivamente, se obtiene una estructura más estable y con mayor resistencia al ataque por H2SO4.
Numerous publications agree that Ta2O5 is a good refractory material for some applications in furnaces, combustion equipment, etc. [1,2], even as a ceramic coating [3,4], because of its high chemical stability when immersed in acid solutions, particularly H2SO4, even in concentrations of 98wt% [5]. It is widely used in the chemical industry as a coating to protect surfaces from reducing atmospheres [6]. It is known that chemical stability of pure ceramic materials or alloys subjected to acid solutions depends on the aggressiveness of the acid medium, the alloying elements or components of the sample, and the temperature at which the events occur [7,8]. The degree of degradation suffered by Ta2O5 in acid solutions has been determined in multiple analysis [9–11]. Other studies evaluated alloys such as Ta2O5–Al2O3, which offer an optimum performance in contact with said acid solution [12]. The main objective when formulating Ta2O5–Al2O3 alloys or forming coatings with these oxides is to demonstrate their high resistance to degradation by acid solutions [13,14], without forming compound phases such as AlTaO4 that require more energy for their conformation. Corrosion results have also been reported for tantalum and titanium oxide composites, showing that the higher the Ta2O5 content, the mass gain is reduced after contact with H2SO4. There are also corrosion results for alloys of tantalum and titanium, where the higher the content of Ta2O5 the lower the mass gain after its interaction with H2SO4[8]. Evaluations carried out on corrosion resistance of Ta2O5–Al2O3 materials demonstrated their ability to develop a passivation behavior when immersed in an acid solution [9,14]. Other studies analyzed this behavior with the deposition of superficial layers of Ta2O5 on a metallic material base [15,16], but further studies are still required to determine the behavior of a ternary system such as SiO2–Al2O3–Ta2O5 in contact with solutions of H2SO4.
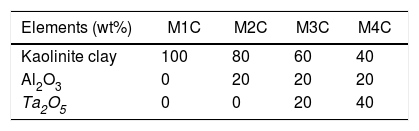
Materials and methodsThe tested samples (Table 1) were made up of Al2O3 with ≥98% purity and Ta2O5 with ≥99% purity, both commercial materials from Sigma–Aldrich. They also contained a proportion of kaolinite clay from the San Jorge mine, located in the state of Baja California, Mexico, which has been reported to be a kaolin deposit with a composition of 19.46wt% Al2O3 and 59.4wt% SiO2[17].
Ten kilograms of kaolinite clay were mined from the San Jorge Mine with less than 25.40mm in size [17], and then reduced to the test size by the ASTM-702 method using a mechanical sample separator. The clay was milled for 24h in a high energy ball mill until obtaining a particle size of ≤75μ, using a ball-to-powder weight ratio of 10 to 1, and mechanically sieved in a Ro Tap agitator model RX-29-9 following standard ASTM C-136.
The homogeneous mixing of the kaolinite clay, Al2O3 and Ta2O5, is a factor of high importance; therefore, a mechanical agitator was used. Also, cylindrical samples (50mm diameter×25mm height) were formed by cold isostatic pressing at 27MPa with an hydraulic press Carver series M3853, see Fig. 1[18].
The densification of the samples (kaolinite clay with and without added Al2O3 and Ta2O5), Table 2, was developed under solid-state reaction synthesis, thermally treating them at 1150°C/2h [19] in a Sentro Tech furnace, with a heating rate of 5°C/min [20].
To determine the chemical stability of the samples, a corrosion resistance test was carried out by a static immersion method [21,22]; the analysis was focused on assessing the degradation caused by sulfuric acid, through the method of mass loss established by the standard ASTM C-267 [23,24].
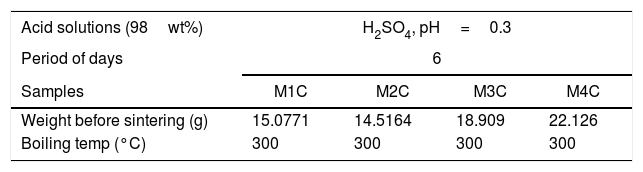
To evaluate the mass loss by immersion, each sample was submerged individually in 60ml of acid solution (98wt% H2SO4) inside a sealed Erlenmeyer flask. The experiment was carried out at 300°C in a lab muffle furnace for a period of 6 days, as shown in Table 2.
Subsequently, the samples were removed from the acid solution and exposed to a drying cycle for 24h. The mass in each sample was recorded before and after the corrosion test using a SCIENTECH ZSA 210 precision analytical scale.
The composition of the kaolinite clay and clay mixtures added with Al2O3 and Ta2O5 was determined by X-ray fluorescence (XRF) spectrometry [25,26], using a Bruker equipment Model S4 PIONNER.
The phase analysis of the kaolinite clay M1C and that of the sintered M2C, M3C and M4C mixtures were carried out by X-ray diffraction (XRD) with a Bruker AXS diffractometer model D8Advance using Cu-Kα radiation. The Rietveld refinement method was used to carry out the quantification of the phases.
All samples were observed by optical microscopy, as well as on an environmental scanning electron microscope (MEB), Philips XL30 ESEM, where they were analyzed by Energy-dispersive X-ray spectroscopy (EDS), before and after the acid attack.
The density of the sintered samples, without acid attack, was determined by the Archimedes’ principle in water.
Results and discussionsThe kaolinite clay without sintering reveals initial contents of SiO2≤74.32wt% and Al2O3≤21.83wt%, with traces of other materials in smaller proportions. According to Gazulla et al. [27], among the phase changes that occur in a kaolinite clay there are intermediate transitions, which start with formation of metakaolin at 500°C and conclude with mullite and cristobalite appearing as major phases at 1550°C [28,29]. Although it is not decisive that the formation of mullite occurs at 1120, 1150 or 1200°C [27,28], the sintering at 1150°C of the kaolinite clay of this study, is in an area where the primary mullite phase begins to form [28], and quartz theoretically begins to disappear at 800°C, which ends at 1200°C [29]. However, the x-ray diffractograms of kaolinite clay showed a higher concentration of the quartz phase when sintering at 1150°C. On the other hand, the detection of significant amounts of cristobalite phase is attributed to the high content of SiO2 in the kaolinite clay [30,31].
The XRF analysis (Table 3) revealed a high increase of SO3 content in all the samples after their immersion in H2SO4. This happened through a chemical decomposition that was activated by raising the temperature of H2SO4 and keeping it within an evaporation–condensation cycle [32,33], for fuel-free atmospheres [34]. Interactions between the acid solution and the mixtures formed with kaolinite clay and the added oxides (Al2O3 and Ta2O5), produced an average reduction of 57% in the alumina oxide content of the mixtures (Table 3). Also, the initial content of Fe2O3 registered a minimum reduction (27.55wt%) in the mixture of kaolinite clay added with 20wt% of Al2O3, as well as a maximum reduction (73.83wt%) when 40wt% of Ta2O5 was added, after subjecting it to the attack by H2SO4. It is necessary to emphasize that the reduction in the amount of the iron and alumina oxides by immersion in H2SO4 is a behavior that has been reported in previous studies. It is known that the acid solution is effective decreasing the Al2O3 and Fe2O3 concentrations [35,36]. This effect is consistent with the results shown in Table 3. In the samples analyzed by XRF, weight losses due to ignition (LOI) are observed. Ignition losses of ≤0.24wt% correspond to samples sintered at 1150°C, without interaction with the acid solution. These losses occur mainly due to the dehydroxylation of kaolinitic clay or to the oxidation of organic matter. The samples sintered at 1150°C, and with interaction with H2SO4, show high weight losses of 20.50≥31.10wt% due to ignition, which are considered to also occur by dehydroxylation, but due to a high content of water molecules that were incorporated into the sample during the decomposition of H2SO4 at 300°C [37,38].
Chemical composition of clay and mixtures determined by XRF.
| Composition (wt%) | M1 | M2 | M3 | M4 | ||||
|---|---|---|---|---|---|---|---|---|
| Before H2SO4 | After H2SO4 | Before H2SO4 | After H2SO4 | Before H2SO4 | After H2SO4 | Before H2SO4 | After H2SO4 | |
| Ta2O5 | N/R | N/R | N/R | N/R | 44.04 | 25.59 | 48.08 | 43.09 |
| SiO2 | 68.34 | 44.49 | 48.81 | 25.31 | 32.44 | 14.80 | 27.70 | 13.25 |
| SO3 | 0.474 | 16.22 | 0.156 | 19.02 | 0.215 | 18.01 | 0.19 | 9.95 |
| Al2O3 | 22.88 | 12.56 | 42.42 | 21.57 | 20.88 | 14.29 | 22.42 | 12.16 |
| Fe2O3 | 4.97 | 2.89 | 5.833 | 1.607 | 1.548 | 0.925 | 0.837 | 0.618 |
| Others | 3.239 | 2.329 | 2.642 | 1.182 | 0.780 | 0.736 | 0.499 | 0.391 |
| LOI | 0.075 | 21.50 | 0.120 | 31.10 | 0.070 | 25.60 | 0.240 | 20.50 |
The measured apparent densities were 2.65, 2.82, 3.28 and 3.91g/cm3, for sintered samples M1, M2, M3 and M4, respectively, without acid attack. This reflects the increment in the content of Ta2O5 and the diminution in the content of SiO2, which occur simultaneously in the materials following the same order. Since all samples were formed using cold isostatic pressing, it was estimated that in all cases the porosity level was very small (maximum ∼1%).
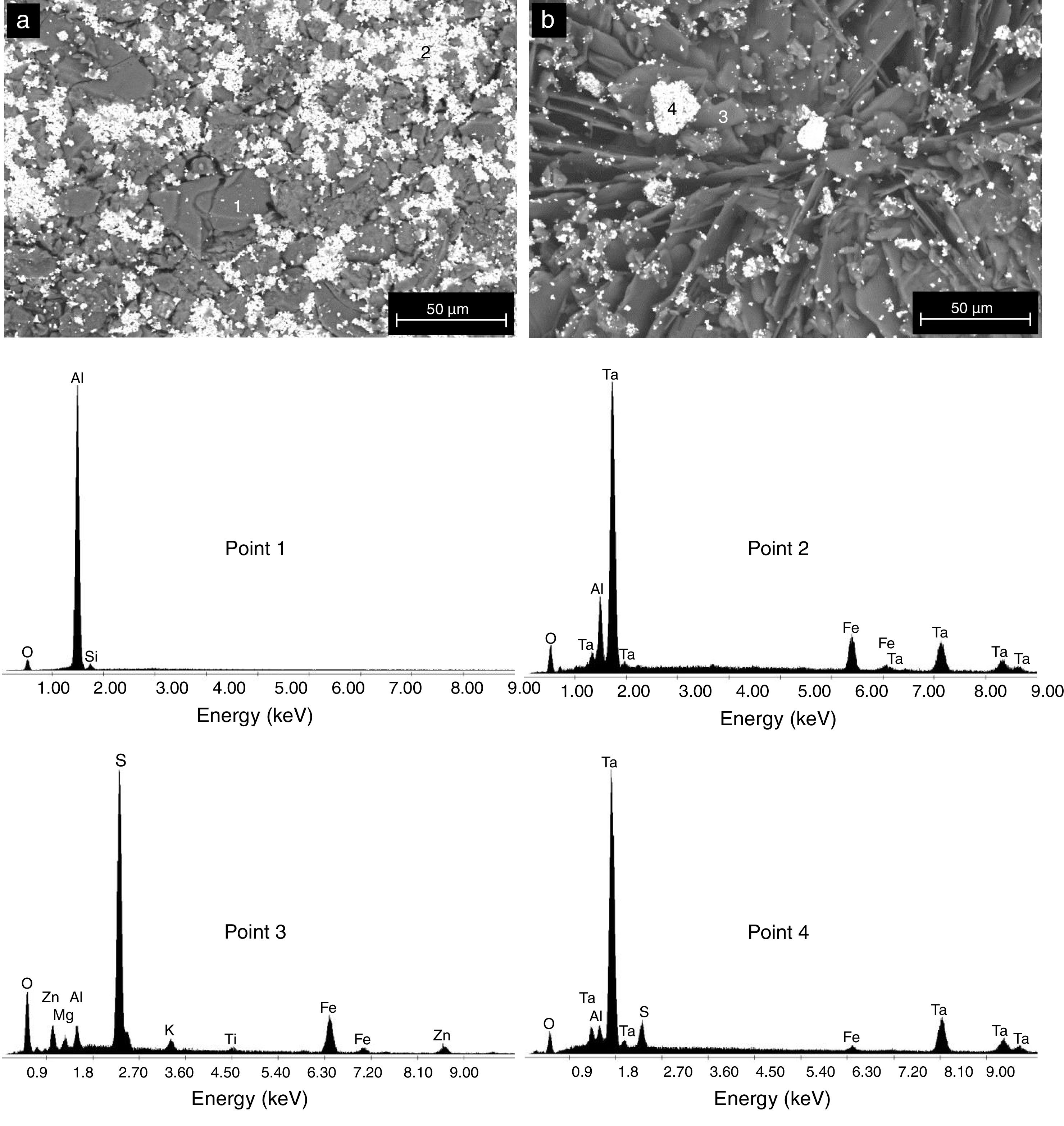
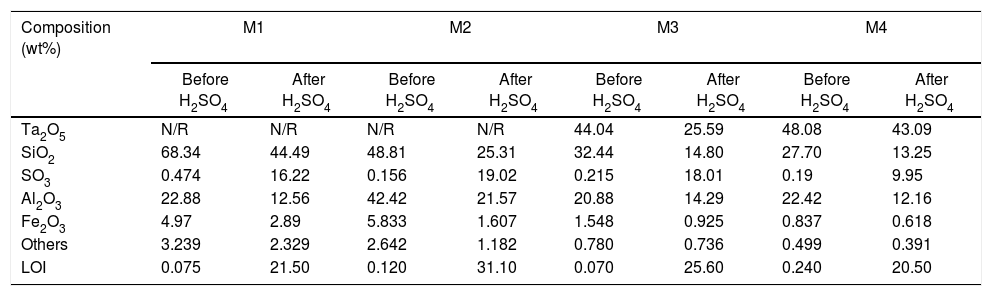
All samples subjected to H2SO4 increased their mass by forming passive compound layers [10,39], which are visible on the surfaces of the samples as small spots of similar distribution, see Fig. 2[40]. It is considered that the spots of pink color revealed in the samples correspond in greater proportion to the formation of sulfates of Fe, Al and Si. The relative amount of the formed sulfates decreased in this order, since the Fe ions are altered to a greater extent than the Al ions [41,42].
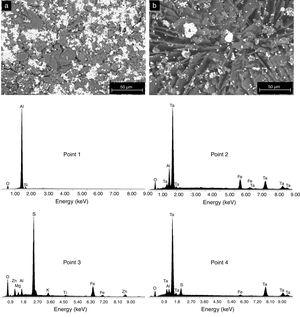
The formation of Al2(SO4)3, Ta2(SO4)5, FeSO4, and possibly also Si(SO4)2, during the attack with H2SO4 given to the kaolinite clay added with Al2O3 and Ta2O5, was confirmed by the analyzes of SEM/EDS performed, see Fig. 3. Probably these phases were not detected by XRD due to their relatively small amount. The SEM micrographs (Fig. 3a and b) showed that the surface of the samples changed completely as a result of the acid attack, from a polycrystalline microstructure typical of a sintered material, in which the presence of a large quantity of agglomerates of very fine Al2O3 and Ta2O5 particles are clearly seen, to a microstructure formed by a mixture of sulphates with plate-like morphology, with large hollow spaces formed between them due to the partial dissolution of the matrix, and with the presence in the attacked sample of a relatively small amount of unreacted Al2O3 and Ta2O5 remaining.
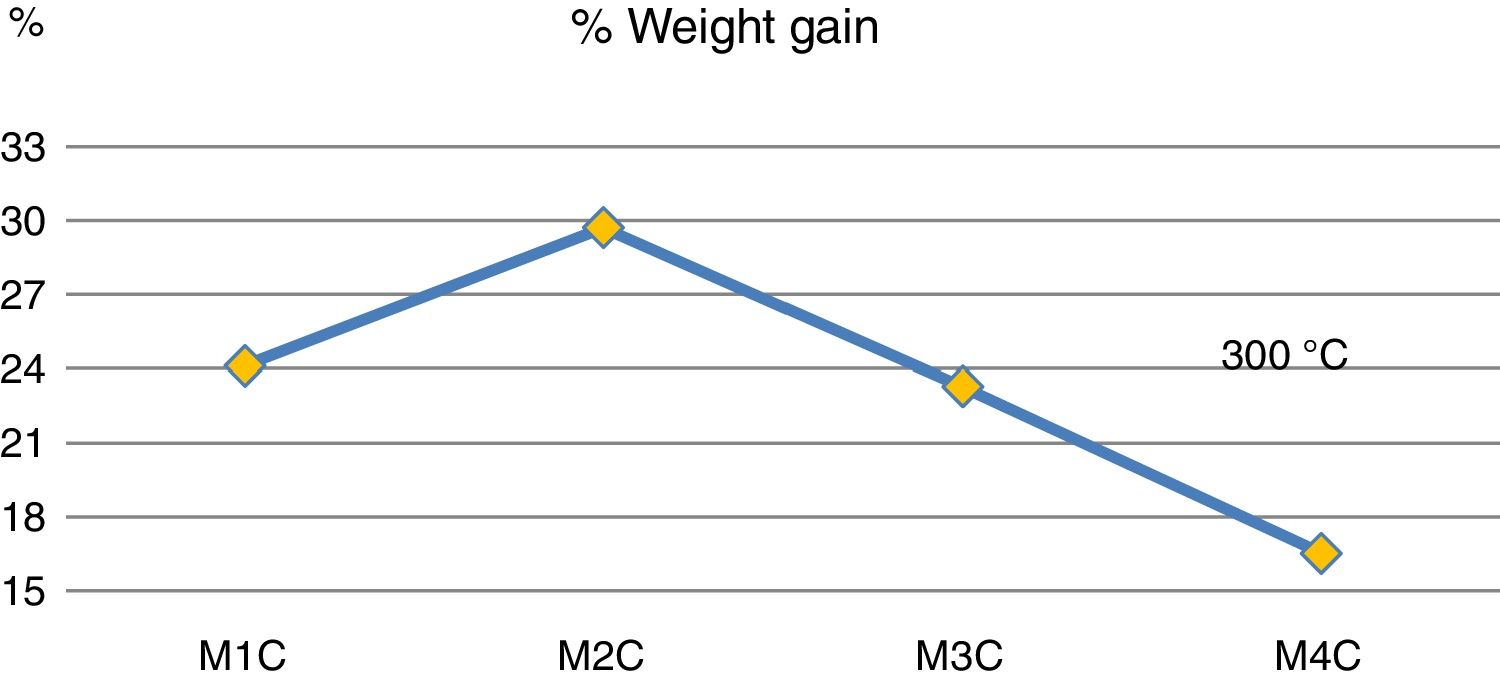
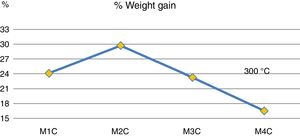
Fig. 4 shows mass increases after 6 days of immersion in H2SO4. It is observed that the addition of 20wt% (sample M3C) and 40wt% (sample M4C) of Ta2O5 to the kaolinite clay (sample M1C), which is composed basically of SiO2 and Al2O3, produced the lowest mass gain due to the interaction with the acid solution. This behavior indicates that the passive layers produced by the addition of Ta2O5 had a greater resistance to H2SO4 than kaolinite clay. And when 20wt% of Al2O3 was added to the kaolinite clay (sample M1C), it produced the highest mass increase compared to the rest of the samples. Other studies have recorded favorable yields combining Al2O3–Ta2O5 and subjecting them to acid solutions [14], as well as by increasing the content of Ta2O5 in the mixtures [8].
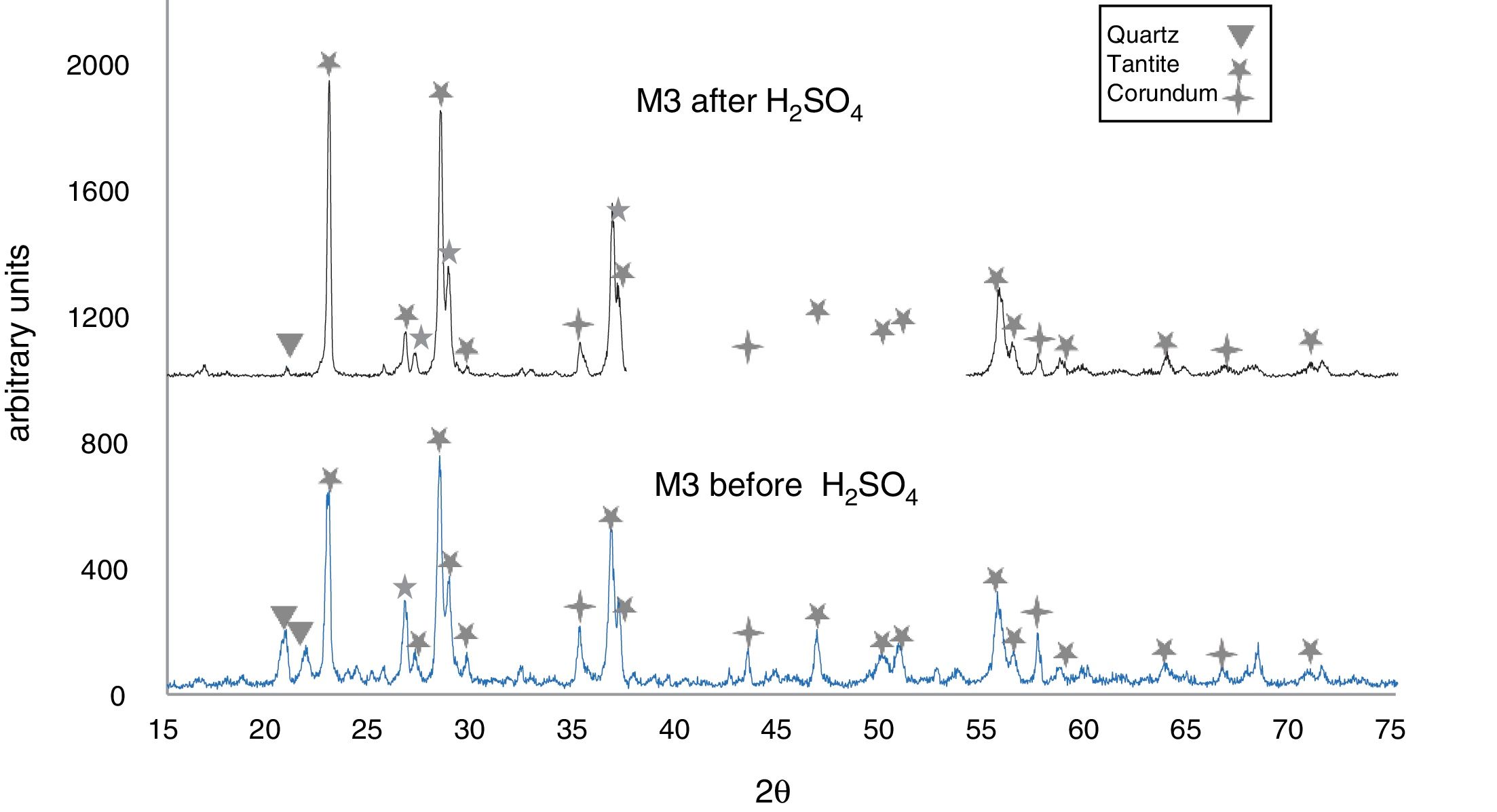
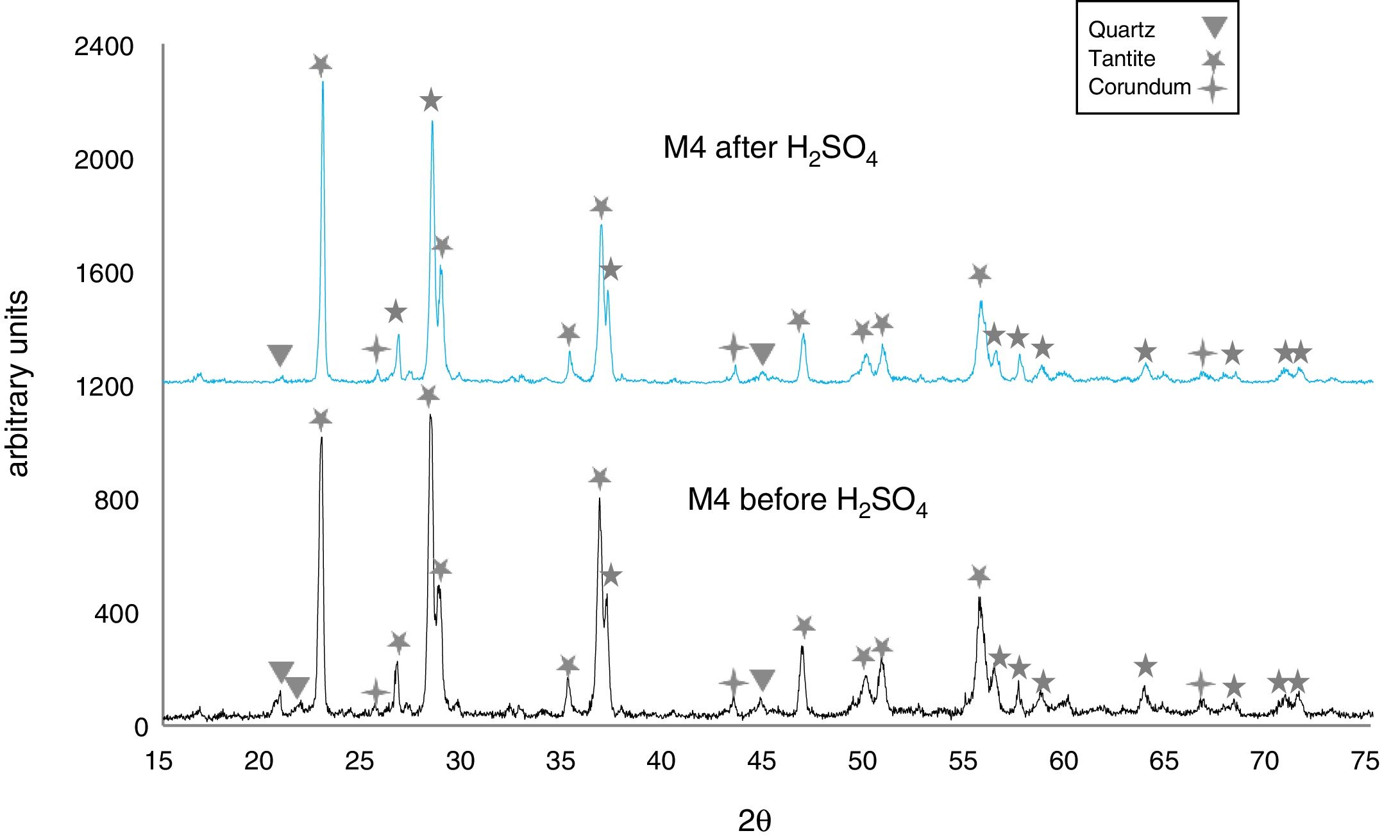
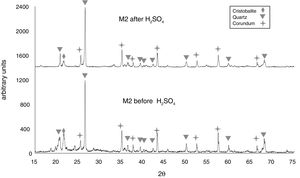
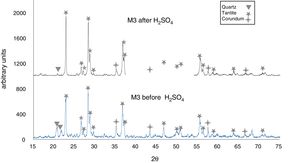
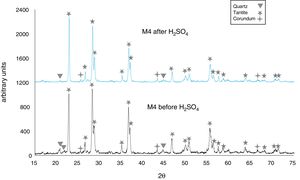
The X-ray diffractograms of Figs. 5–8 revealed a significant decrease in the intensity of the diffraction peaks after subjecting the samples to the acid solution. This indicates that the crystalline structure of quartz, cristobalite, corundum and tantite [43] partially collapsed or became amorphous after immersion in H2SO4, or that the crystalline structure of kaolinite clay added with alumina and tantalum oxide partially collapsed or became amorphous after immersion in H2SO4[9].
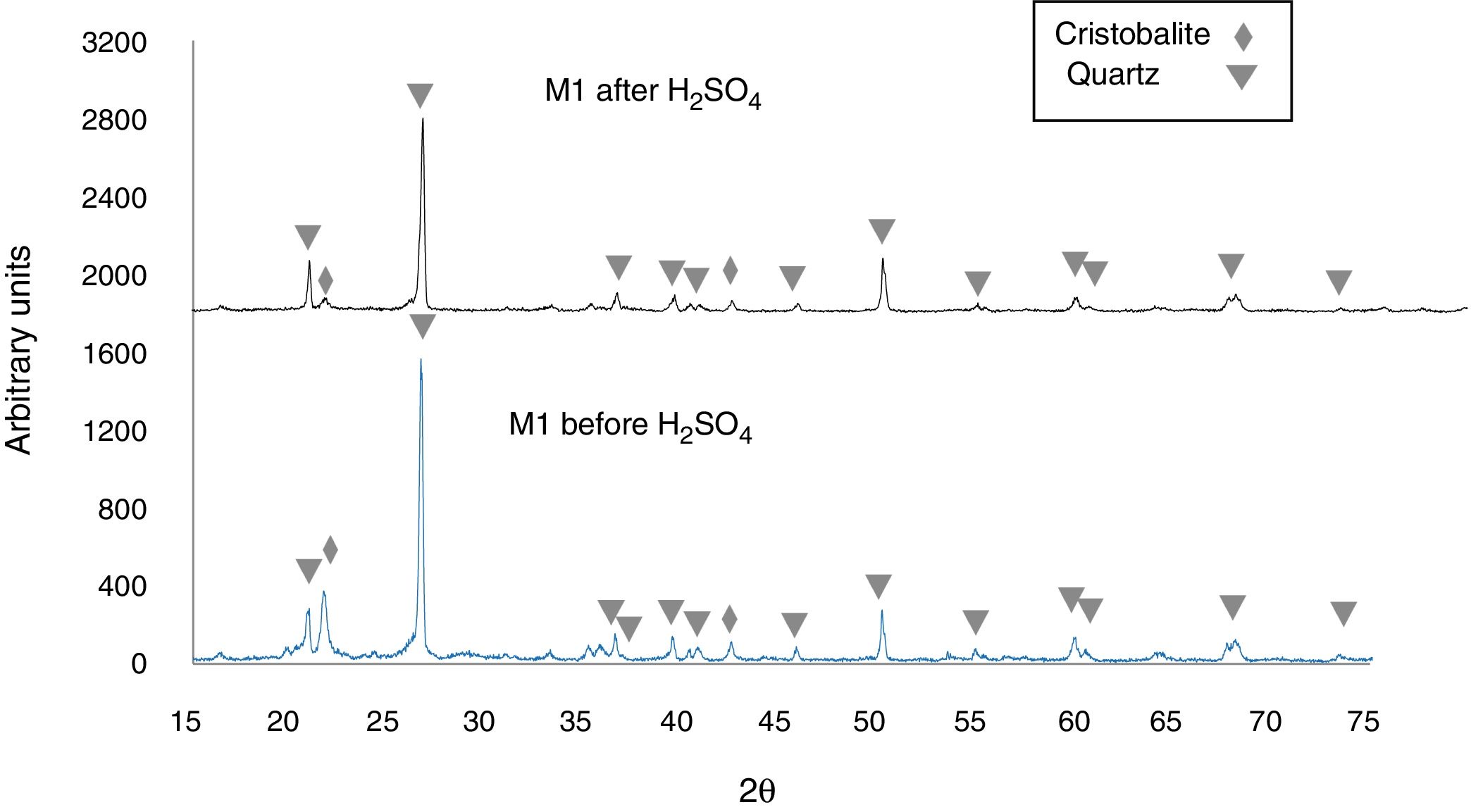
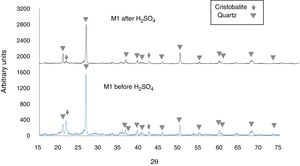
Figure 5 shows the X-ray diffractogram of sample M1C, for which 92.2wt% quartz (predominant phase) and 6.9wt% cristobalite (minor phase) was recorded before immersion of the sample in H2SO4. After immersion in H2SO4, the peak intensity was reduced for quartz, but cristobalite reacted to a greater extent, which almost caused its complete disappearance in the samples [43,44]. Lastly, the X-ray diffractograms of Fig. 5 show an amorphous “hump” overlapped with a cristobalite peak located at 2θ=21.8° [45,46], before immersion in the H2SO4, which indicates that an amorphous phase was formed during heat treatment of the sample. After the acid attack, this hump disappears, which was likely due to dissolution of the amorphous phase.
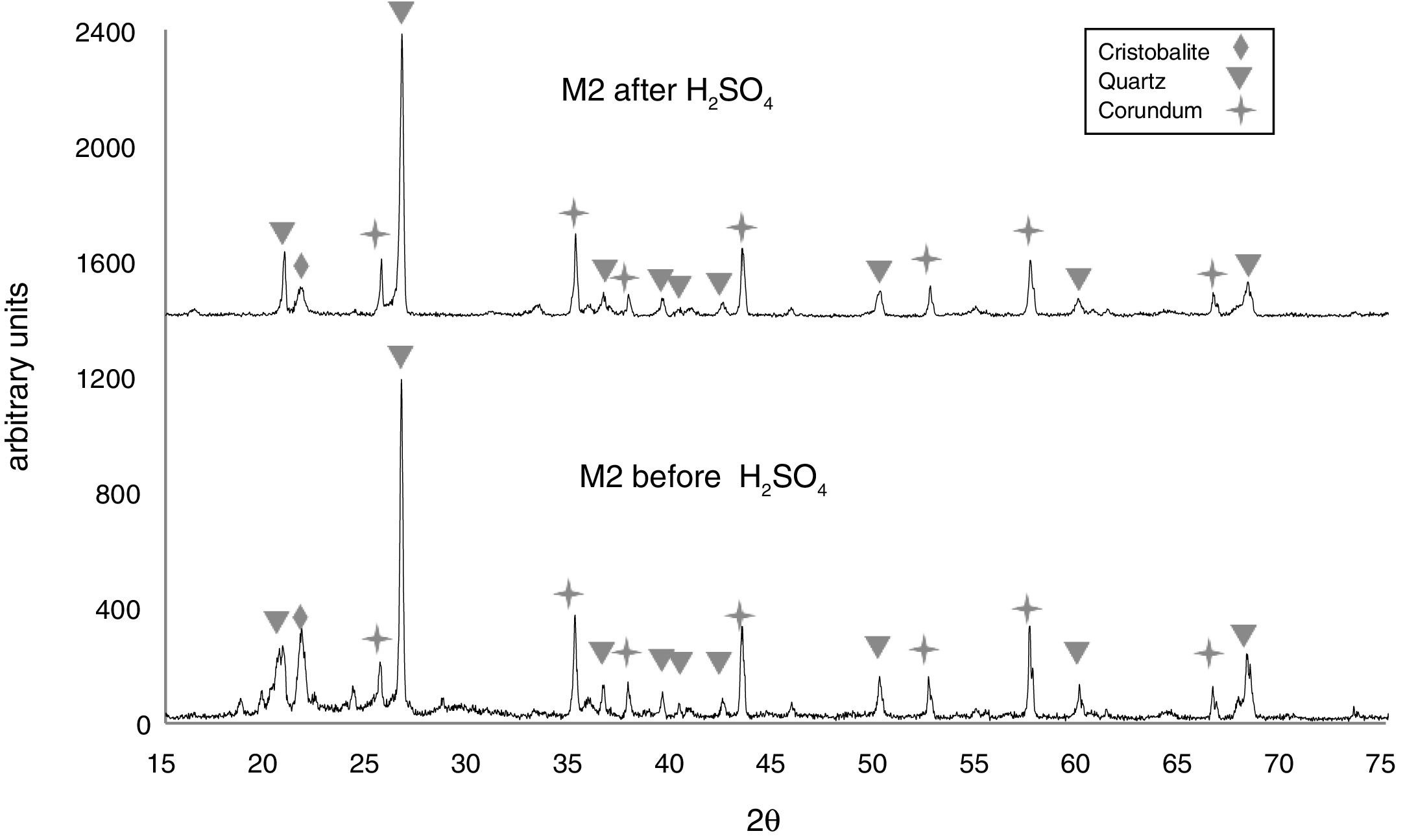
In Fig. 6, the X-ray diffractogram shows the presence of quartz (48wt%), corundum (43.4wt%), and cristobalite (4.2wt%) phases in sample M2C, before its immersion in the acid solution. The peak intensity of the cristobalite phase was again drastically reduced, after interacting with sulfuric acid.
The X-ray diffractograms of Figs. 7 and 8 of materials of the SiO2–Al2O3–Ta2O5 system did not reveal the formation of new phases, considering that the reactions occurred in the sub-solid region of Ta2O5 at 1150°C [47]. It is also likely that the solubility limit between Ta2O5 and Al2O3 was not exceeded in the solid-solid region, due to the absence of the AlTaO4 phase [12,48]. The interactions of the acid solution with the tantite (Ta2O5) [49] and corundum phases resulted in significant reductions in their peak intensities after their immersion in H2SO4. However, the interaction between the acid medium and the quartz phase, reduced its peak intensities almost completely. This may mean that the proportion of these phases decreases in the samples as a consequence of the attack by the acid solution, either because they react with the acid to form sulfates, or because they become dissolved into the solution.
ConclusionsThe present study was focused on the degradation of kaolinite clay with added Al2O3 and Ta2O5 to increase its resistance to an acid solution (H2SO4) at 300°C. Based on the reported results and their implications, the following conclusions can be drawn:
- •
Exposure to 98wt% H2SO4 at 300°C produced a high increase in the SO3 content of kaolinite clay samples with added alumina and tantalum oxide, due to the chemical decomposition of H2SO4 activated by increasing the temperature above its boiling point.
- •
Addition of alumina and tantalum oxide to kaolinite clay, in proportions of 20wt% and 20–40wt%, respectively, develops passive layers with better resistance to the acid solution.
- •
In all X-ray diffractograms of Figs. 5–8, a severe decrease in the intensity of the peaks of the cristobalite phase was detected as a result of the interaction with H2SO4, indicating that this is the least stable phase in the mixture.
- •
None of the X-ray diffractograms obtained in this work revealed the formation of new crystalline phases when the kaolinitic clay added with Al2O3 and Ta2O5 was sintered at 1150°C.
A. Valenzuela-Gutiérrez wrote the draft paper, got the raw materials and synthesized the studied samples.
A. González-Ángeles ensures that all authors are included in the author list, its order has been agreed by all authors, and that all authors are aware that the paper was submitted.
J. López-Cuevas made the characterization of samples and supervised the final manuscript.
Conflict of interest statementThe authors declare that they have no conflict of interest.
Authors are greatly grateful to the Universidad Autónoma de Baja California and CINVESTAV-Saltillo for facilitating access and use of their facilities and equipment to carry out this research.