Background: Perinatal tuberculosis (TB) is acquired during birth or during the early neonatal period. Although its incidence is unknown, a search was conducted in Medline and 28 cases of perinatal TB were found from 1983 to 2011. Diagnosis of this disease is important because of the nonspecific symptoms that are mistaken for other infectious diseases. The disease has a high mortality rate (60%); therefore, it requires prompt diagnostic suspicion by the medical staff to prevent a fatal outcome.
Case report: We describe the case of a 3-month-old male whose 29-year-old mother died of septic shock at 15 days of delivery. The infant’s condition began within 30 days of age with cough and difficulty breathing with a diagnosis of multiple foci pneumonia. The infant presented respiratory impairment, meriting change of antibiotics twice, without improvement. The autopsy report of the mother revealed peritoneal TB. PCR was carried out using tracheal aspirate and pleural fluid of the patient, which were positive for Mycobacterium tuberculosis. Perinatal TB diagnosis was established. No hepatic granulomas were found.
Conclusions: Perinatal infection should be suspected in children with sepsis and/or pneumonia unresponsive to antibiotics. In this care, the history of TB in the mother should have oriented the diagnosis.
Introducción: La tuberculosis perinatal es adquirida durante el parto o durante el periodo neonatal temprano. Aunque su incidencia es desconocida, se realizó una búsqueda en Medline y se encontraron 28 casos de tuberculosis perinatal reportados de 1983 a 2011. El diagnóstico de esta enfermedad es importante debido a que los síntomas son inespecíficos y se confunden con otras enfermedades infecciosas. Además, presenta una alta tasa de mortalidad (hasta del 60%), por lo que se requiere de una pronta sospecha diagnóstica por parte del personal médico para evitar un desenlace fatal.
Caso clínico: Se describe el caso de un masculino de 3 meses de edad, hijo de madre de 29 años que falleció por choque séptico a los 15 días del parto, que inició padecimiento a los 30 días de vida con tos y dificultad respiratoria. Se realizó el diagnóstico de neumonía de focos múltiples. Presentó deterioro respiratorio, por lo que requirió cambio de antibiótico en dos ocasiones aunque no se observó mejoría. Se recabó el dictamen de la autopsia de la madre que reveló tuberculosis peritoneal. Al paciente se le realizó PCR de aspirado traqueal y de líquido pleural, los cuales fueron positivos para Mycobacterium tuberculosis. Se estableció el diagnóstico de tuberculosis perinatal. No se encontró granuloma hepático.
Conclusiones: La infección perinatal debe sospecharse en niños con sepsis o neumonía sin respuesta a antibióticos. En este caso, el antecedente de la madre con tuberculosis orientó al diagnóstico.
1. Introduction
WHO registers 8–10 million new cases of tuberculosis (TB) annually worldwide with ~3 million deaths.1 Perinatal TB is acquired during labor or during the early neonatal period.2 The incidence by itself is unknown3 because many children die before the diagnosis is established,4 which contributes to an underreporting of this disease.5 When a Medline search for information was carried out, there were 28 cases of perinatal TB reported in a period from 1983-2011.
Understanding of the pathogenesis of this disease is important because of its non-specific symptoms that are mistaken for other infectious diseases, which may delay diagnosis. In addition, the disease has a high mortality rate (up to 60%).6 Therefore, prompt diagnostic suspicion is required of the medical personnel.
2. Case report
We present the case of a 3-month-old male from the State of Mexico. The patient’s mother was a 29-year-old, apparently healthy policewoman who died of septic shock at 15 postoperative days. Immunizations included BCG, dose of influenza, hepatitis, polio and pneumococcus. The patient was the product of the first gestation with eight prenatal visits. There were no complications reported during pregnancy. A family member reported that the mother had a genital infection but did not know the type of infection or what treatment she received. Birth was at 34 weeks via cesarean section due to umbilical cord coiling and acute fetal distress. Birthweight was 2500 g; height and Apgar score were unknown. The patient remained for 6 days in the neonatology service because the mother was in the ICU. At 5 days of life the patient had conjunctivitis and was treated with an ophthalmic solution (unknown name).
At 1 month of age the patient initiated with symptoms characterized by hyaline nasal secretion and dry cough. He was dyspneic and there was no cyanosis. There was vomiting on occasions of short duration without predominance as to the time and without fever. He was taken to the clinic on three occasions and treated with mucolytics, antibiotics and nebulization with salbutamol, although without symptom improvement. Due to the respiratory pattern and subcostal retraction, the patient was admitted to a second-level care hospital with diagnosis of atypical pneumonia. He was treated with ceftriaxone and clarithromycin for 15 days along with a nasal oxygen mask. Because of symptom persistence, chest computed tomography (CT) was done and showed a bilateral alveolar pattern with pneumonia of multiple foci. The drug scheme was switched to meropenem and vancomycin for 14 days. No organisms were isolated in the blood cultures.
The patient was referred to a tertiary care hospital for evaluation by a pulmonologist. On admission he presented respiratory insufficiency, desaturation to 75%, and nasal flaring. The chest was noted to be symmetrical with marked subcostal retraction, coarse rales and bilateral expiratory wheezing. Abdominal examination showed hepatomegaly of 4 cm below the right costal margin. Arterial blood gas showed the following values: pH 7.30, pCO2 50 mmHg, pO2 40.1 mmHg, HCO3 23.9 mmol/l, lactate 3 mmol/l. He was admitted to the pediatric intensive care unit (PICU) due to decompensated respiratory acidosis and placed on three phase ventilation. Diagnosis was made of multiple foci pneumonia and suspected TB. The mother’s autopsy reported peritoneal TB.
Results of blood analyses upon admission were as follow: white cells 13,500, segments 24%, bands 1%, lymphocytes 47%, monocytes 9%, eosinophils 17%, platelets 355,000; VSG 5 mm/h; C-reactive protein (CRP) 2.09; total bilirubin (TB) 0.1 mg/dl; direct bilirubin (DB) 0.07 mg/dl; indirect bilirubin (IB) 0.03 mg/dl; albumin 2.8 g/dl; globulin 3.6 g/dl; aspartate aminotransferase (AST) 36 U/l; alanine aminotransferase (ALT) 38 U/l; uric acid (UA) 3.4 mg/dl; creatinine 0.3 mg/dl; glucose 72 mg/dl.
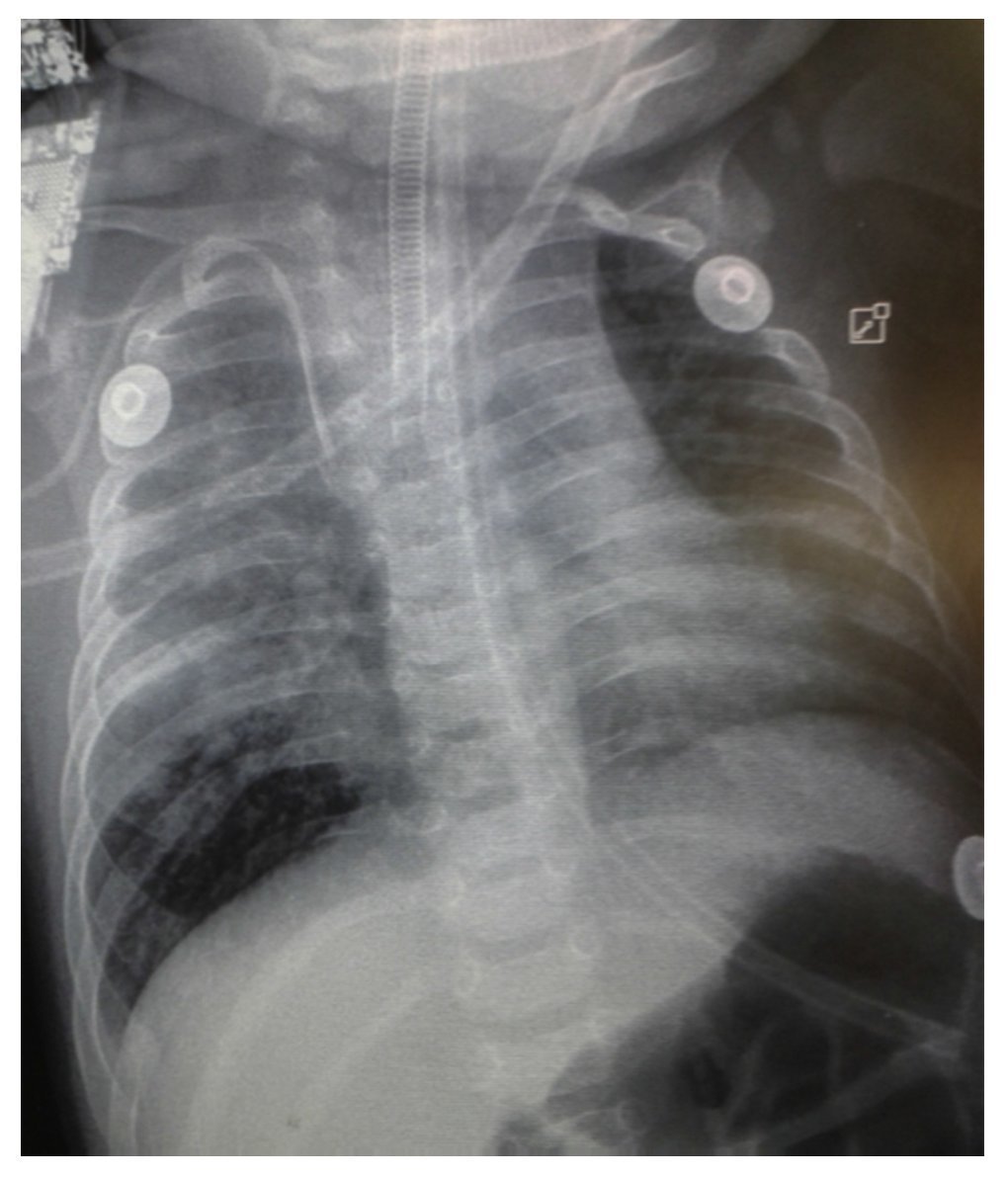
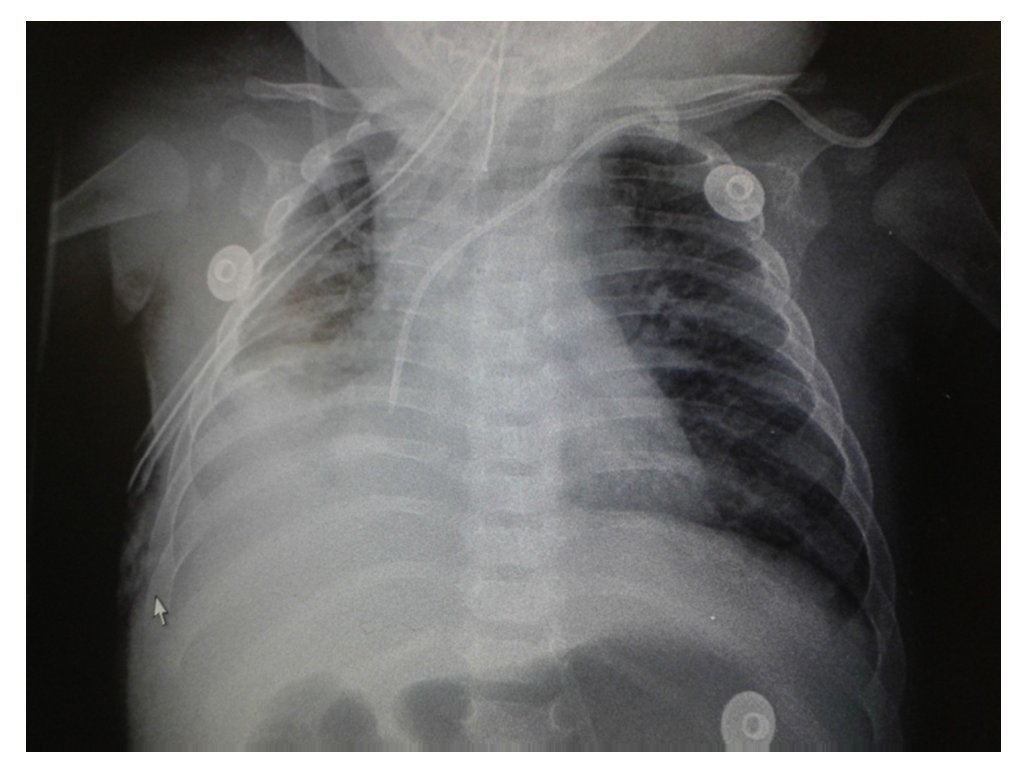
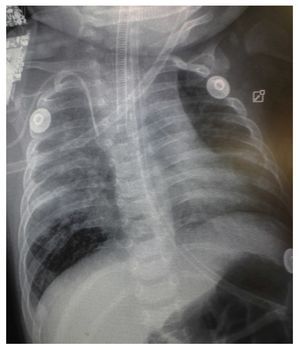
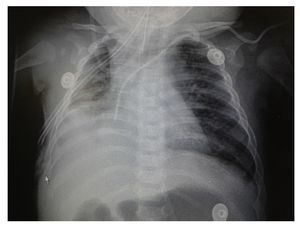
Chest x-ray demonstrated a bilateral nodular-reticular pattern in the lung parenchyma with a tendency towards consolidation in the right lung (Figure 1). At 3 days of hospitalization he presented right pleural effusion (Figure 2). Thoracentesis was done. A sample was taken from the pleural fluid obtained for polymerase chain reaction (PCR), which was positive for TB. A tracheal aspirate was also taken for Xpert MTB/Rif®, which was positive. There was no resistance to rifampicin, AARB (alcohol acid-resistant bacteria) in gastric fluids were negative in three samples. Gastric aspirate culture looking for M. tuberculosis was negative. Purified protein derivative test (PPD) and Mantoux test were negative. Enzyme-linked immunosorbent assay (ELISA) for HIV was negative. Blood culture and urine culture results were negative.
Figure 1 Anteroposterior (AP) x-ray of the chest. A bilateral reticulo-nodular pattern is observed with tendency towards consolidation in the right lung.
Figure 2 AP x-ray of the chest. A bilateral reticulo-nodular pattern is observed with right basal consolidation that effaces costophrenic and costodiaphragmatic angles.
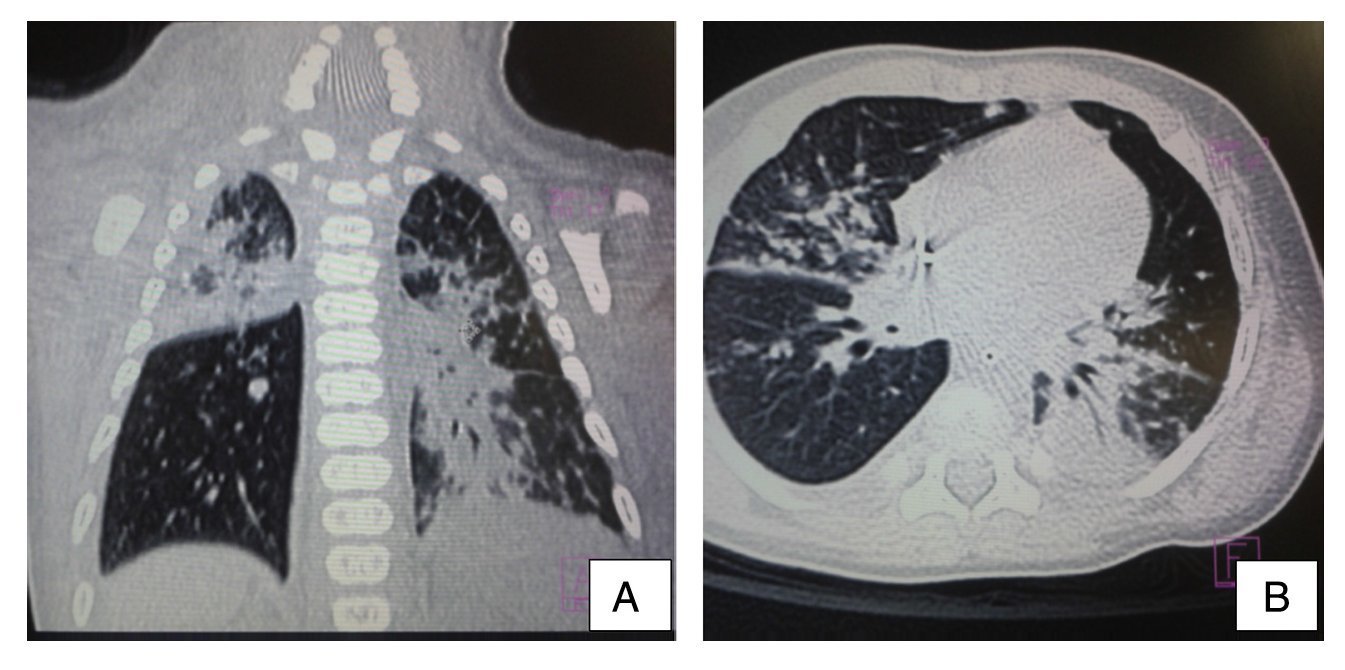
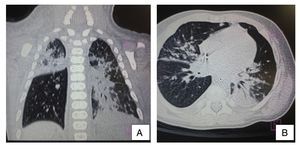
Ultrasound of the abdomen showed an enlarged liver with homogeneous echogenicity. On computed tomography (CT), axial cut of the pulmonary window showed alveolar occupation with a tendency towards consolidation in the right lung and with air bronchogram in posterior segment of the right upper lobe as well as the median lobe and both left lobes. There were hyperdense nodular images and diffuse bilateral calcifications (Figure 3). There were no liver granulomas seen in the abdomen. Diagnosis of perinatal TB was established and anti-TB treatment was initiated with isoniazid (10 mg/kg/day), rifampin (15 mg/kg/day), pyrazinamide (30 mg/kg/day) and ethambutol (15 mg/kg/day) with adequate response. The patient was discharged on day 62 without oxygen. Currently the patient has completed a 2-month phase of isoniazid and rifampin at the doses already described. He continues to be followed by the pulmonary service.
Figure 3 Computed tomography (CT) of the chest in coronal cut (A) and axial cut (B) of the pulmonary window. Alveolar occupation with tendency towards consolidation, with air bronchogram in its interior, localized in the posterior segment of the right upper lobe and both left lobes with hyperdense nodular images and bilateral diffuse calcifications.
3. Discussion
Perinatal TB is the acquired tubercular disease during labor or during the early neonatal period.1,2 In the pathogenesis of this disease the immature immune system of the child plays an important role, especially in premature infants as shown by the decrease of the specific response of Th1 helper lymphocytes, which reduces the responsive capacity of an effective response to the M. tuberculosis infection. Transmission may be due to intrapartum aspiration of amniotic fluid or by direct contact with the maternal genital tract during labor and inhalation of the bacilli from an infectious source.5
Once Mycobacterium reaches the alveoli, lung function can change. The primary pulmonary focus can remain in a latent state up to the time of birth when oxygenation improves and the increase in pulmonary circulation could stimulate growth of the Mycobacterium tuberculosis.5
Aspiration or ingestion of infected amniotic fluid results in the formation of the primary complex in the lungs or gastrointestinal tract, respectively.7,8 The organs most commonly affected are the liver, lung and the lymph nodes.11
It is important to investigate the route of transmission because it allows differentiating perinatal TB from congenital TB, for which Cantwell et al. in 1994 established that the child should show the tuberculous lesions and at least one of the following criteria:
Lesions present in the first week of life.
A primary hepatic complex or hepatic caseous granulomas.
Tuberculous infection of the placenta or genital tract of the mother.
Exclusion of the possibility of postnatal transmission by a thorough investigation of contacts.2,8,9
The usefulness of differentiating congenital TB from perinatal TB is only epidemiological as it helps to identify the latent infectious source for treatment and avoids transmission.8,10 Clinical manifestations tend to be nonspecific and may be present at birth, but the open expression of the disease is more often produced in the second or third week of life with fever and without obvious foci accompanied by hepatosplenomegaly, adenopathy, respiratory distress, abdominal distention, lethargy and irritability. Other clinical characteristics may include skin lesions, seizures, icterus, otorrhea, hematological abnormalities and ascites which, due to mimicking an infectious process, delays the diagnosis. However, due to the poor response to the antibiotics and support therapy, TB should be considered.2,12,13
Because of the rapid advance of perinatal TB, investigation and treatment are urgent.9 Diagnosis of perinatal TB is without a doubt difficult unless there is a suggestive maternal history.14,15 A study of household contacts should immediately be initiated when TB is suspected in the infant because it helps to establish the diagnosis. However, the inability to identify a source of contagion should not exclude the suspicion for TB.9,13
PPD test is negative in most cases of perinatal TB (in 75% of HIV-negative children14) taking into consideration that the activity of the specific response of the Th1 helper T lymphocytes is altered in the newborn and could take up to 3 months for it to be reactive. The test should be repeated after 3 months.5
Identification of the AARB in gastric fluid aspirate is negative in all cases because children are paucibacillary with few bacilli.1,4 Gastric aspirate or sputum culture looking for M. tuberculosis should be examined although the proportion of positive cultures is low. If the child is intubated, tracheal aspiration should be performed.11
Xpert MTB/Rif® is an automated cartridge-based nucleic amplification assay for the simultaneous detection of TB and resistance to rifampin directly from sputum, which is done in 90 min.12,16,17 Value of the PCR has been demonstrated in gastric aspirate, pleural fluid, cerebrospinal fluid (CSF), lymph nodes and blood.5
The release of gamma interferon assays are less sensitive in children than in adults, but when positive are useful for the diagnosis, especially if the PPD is negative. There is the advantage of results being available in 48 h;16 however, these assays are still not recommended by the WHO for the diagnosis of the disease.17
Chest x-ray is generally nonspecific. It is abnormal in up to 50% of the cases. Reticulo-nodular infiltrates can be seen (miliary pattern), but even this finding is insufficient for the definitive diagnosis. When changes in the chest x-ray are typical, CT of the chest generally adds little to the diagnosis.5
With a bone marrow aspirate, ascitic, CSF and pleural fluid in search for tuberculous bacilli can be vital for the diagnosis.4 If the diagnosis is not able to be established, liver biopsy should be done in order to find granulomas and, less frequently, bacilli.5 The fundus of the eye should be examined, looking for chorioretinitis and granulomas.1,5
Ideally, samples of the placenta, vagina or endometrium of the mother should be taken for culture and histopatho-logical study with the purpose of finding granulomas. However, this is frequently difficult due to the late presentations and because up to 75% of pregnant females with TB remain asymptomatic. Detection of the infection in the child commonly precedes the maternal diagnosis.16
Once perinatal TB is diagnosed, risk of airborne spread to healthcare workers and other children is very low because children are paucibacillary.7 Co-infection with HIV in a child with TB should be investigated because they are interrelated.12
Perinatal TB tends to be fatal if left untreated.14 Prenatal identification and treatment of the active disease or latent maternal infection could prevent perinatal TB or may allow for an early diagnosis.9
After appropriate investigation, children should begin empiric therapy according to national guidelines.1,9 Treatment of perinatal TB consists of the administration of anti-tuberculosis drugs for 6 months. During the first phase (intensive), four drugs are given: isoniazid (10-15 mg/kg/ day), rifampin (15-20 mg/kg/day), pyrazinamide (25-40 mg/ kg/day) and ethambutol (15-30 mg/kg/day) or streptomycin (15-30 mg/kg/day) for a 2-month period. Subsequently, in the supportive phase, isoniazid and rifampin are given (at doses already described) for 4 months1,18 in contrast to neonatal TB, whose treatment has a 9-month duration.1
Therapeutic response is determined by regression of clinical symptoms: increase in weight, improvement in appetite, and in this case, radiological resolution.2 Patients should receive a regular monthly examination after discharge until treatment is completed. Follow-up should continue up to 2 years after anti-tuberculosis treatment has ended.1
In countries where there is a high prevalence of TB, vaccination with bacillus Calmette-Guérin is carried out a few days after birth. It has a protective effect against development of TB in the newborn and prevents more severe forms (meningeal and miliary).1
Prognosis depends on the diagnosis and timely treatment. Severe complications may occur such as respiratory failure, miliary TB and meningeal TB that cause seizures, deafness and death.13 Mortality is high and varies between 2 and 60% depending on the diagnostic delay and other factors such as prematurity or HIV co-infection.7,13,
Perinatal TB infection should be suspected in all patients with sepsis and pneumonia who do not respond to normal therapy. It is important to always keep in mind the maternal history as a key point to establish a timely diagnosis and to provide effective treatment for patient cure.
Conflict of interest
The authors declare no conflicts of interest.
Funding
No external sources of funding are declared.
Received for publication: 10-4-14;
Accepted for publication: 1-27-15
http://dx.doi.org/10.1016/j.bmhimx.2015.01.009
Correspondence:
Dra. Jessica Sáenz Gómez
E-mail:jessmary04@hotmail.com