Introducción: La diabetes tipo 1 (DT1) es una enfermedad autoinmune que cada vez es más frecuente y se presenta a edades más tempranas. Su desarrollo requiere de predisposición genética y factores ambientales que influyen sobre la microbiota intestinal en la infancia temprana que pudieran aumentar el riesgo de autoinmunidad asociada con DT1. En México no existen publicaciones de estudios de microbiota en niños menores de 6 años con DT1.
Casos clínicos: Se reportan dos casos de DT1 contrastantes de niños mexicanos menores de 6 años de edad y un tercer caso de un niño sano, previo al desarrollo de autoinmunidad y DT1. Se discuten los factores perinatales, los regímenes de alimentación en el primer año de vida y la microbiota intestinal en relación con el desarrollo de DT1. Los tres casos presentaron una microbiota particular con disminución de la diversidad bacteriana en comparación con los niños sanos, lo cual pudiera estar relacionado con factores ambientales previos al desarrollo de la enfermedad y con el control de la DT1.
Conclusiones: Los niños con DT1 presentaron una diversidad bacteriana disminuida que aparece antes de la autoinmunidad y DT1. El control glucémico podría corregir la disbiosis intestinal en DT1. Faltan estudios prospectivos que den seguimiento a niños sanos con alto riesgo genético y evalúen factores relacionados con la estructura de la microbiota.
Background: Type 1 diabetes (T1D) is currently an autoimmune disease occurring more frequently and early in life. T1D development requires genetic predisposition and environmental factors, which influence the gut microbiota in early infancy and could increase the risk for T1D-associated autoimmunity. In Mexico there are no published microbiota studies in children <6 years old with T1D.
Case reports: We report two contrasting Mexican T1D cases of children <6 years of age and a third case of a healthy child prior to autoimmunity and T1D onset. Perinatal factors, feeding regimes in the first year of life and gut microbiota composition are discussed and related to the T1D onset. The three cases show a particular microbiota profile with decreased bacterial diversity as compared with healthy children, which could be related to environmental factors prior to the development of T1D and disease control.
Conclusions: T1D infant cases presented a decreased bacterial diversity, which appeared before autoimmunity and T1D onset. Glycemic control could tend to correct the gut dysbiosis in T1D children. Prospective studies are needed to follow-up healthy children at high genetic risk to assess factors related to the microbiota structure.
1. Introduction
Type 1 diabetes (T1D) is one of the most frequent autoimmune diseases in children with a prevalence of 1:3001. Advances in the study of predisposition factors during the last decade increase the evidence of an intestinal origin for T1D which, in turn, reveals an unexplored area for understanding the diabetes etiology. The interplay between early feeding patterns, infections and use of antibiotics during the first years of life has taken a central role because all influence the microbiota composition and gut permeability. Associations have been found in animal models of the interactions of these factors in relation to the development of T1D2. Although the first studies in children at high genetic risk have shown gut dysbiosis at the T1D onset, these changes are affected by diet, geography3 and other factors such as age4. Furthermore, it is unknown whether these changes in microbiota are a cause or an effect of the characteristic metabolic disturbance of T1D.
We report a case series of two contrasting Mexican T1D patients (6 years old and younger) and a third case of a healthy child prior to autoimmunity and T1D onset, all recruited from the Children’s Hospital of Sonora (HIES), Mexico. Elements of their diet in the first year of life and perinatal factors are discussed and related to the T1D diagnosis and the gut microbiota composition, assessed by pyrosequencing of the V4 region of the 16S rRNA gene following the methods described previously5. Microbiota data of the cases were processed and analyzed in the Quantitative Insights Into Microbial Ecology (QIIME) pipeline6.
2. Case reports
2.1. Case 1
We present the case of a 6-year-old female with 3 days evolution of T1D diagnosed when she was hospitalized for diabetic ketoacidosis (glucose: 538 mg/dl). The patient was the product of a fifth pregnancy complicated with oligo-hydramnios and fetal distress. The mother was a passive smoker during pregnancy. The patient was born vaginally at 38 weeks of gestation and breastfed until 2 months of age. She had complementary milk formula since birth. At 6 months of age, wheat and other cereals were introduced into her diet. Since the first year, the child presented an average of six infections per year (respiratory and gastrointestinal) treated with antibiotics (amoxicillin, trimethoprim/sulfamethoxazole, and cephalexin). She suffered from acute tonsillitis 2 weeks prior to the diagnosis of T1D.
T1D-associated autoantibodies were as follows: positive to anti-glutamic acid decarboxylase (Anti-GAD) and anti-tyrosine phosphatase-like protein (Anti-IA2).
Risk phenotype was DRB1*04/DQA1*0301.
2.2. Case 2
This is the case of a 2-year-old female with T1D diagnosed 2.5 months before the study. She has had excellent adherence to treatment with insulin and diet. She was born by eutocic vaginal delivery without perinatal complications. She had a mixed feeding regimen with breast milk and formula from birth to 2 months old, continuing only with cow’s milk formula. Wheat and other cereals were introduced into her diet when she was 2 months old. She suffered two respiratory and four gastrointestinal infections of unknown etiology during her first year of life. All diseases were treated with antibiotics (amoxicillin, trimethoprim/sulfamethoxazole). Current fasting glucose was reported as 105 mg/dl. T1D-associated autoantibodies were positive to Anti-IA2.
Risk phenotype was DRB1*03/DR4-DQ8.
2.3. Case 3
We present the case of a 6-year-old male who was healthy at the time of the study. His brother is a T1D patient. The patient is the term product of a second pregnancy born by cesarean due to cephalo-pelvic disproportion, without peri-natal complications. He was breastfed for 3 months, continuing with cow’s milk formula since that age until he was 1 year old. Cereals such as wheat were introduced to his diet at 2 months old. He presented an average of three infectious episodes per year treated with antibiotics (mainly amoxicillin). T1D-associated autoantibodies were negative to Anti-GAD and Anti-IA2.
Risk phenotype was DR3-DQ2/DQB1*0302.
2.4. Investigation and follow-up
A stool sample was collected from each case from which gDNA was extracted to study the microbiota composition by pyrosequencing. Analysis of the intestinal microbiota identified a total of 8,758 bacterial sequences in the fecal sample of case 1, 8,698 sequences in case 2 and 6,905 in case 3.
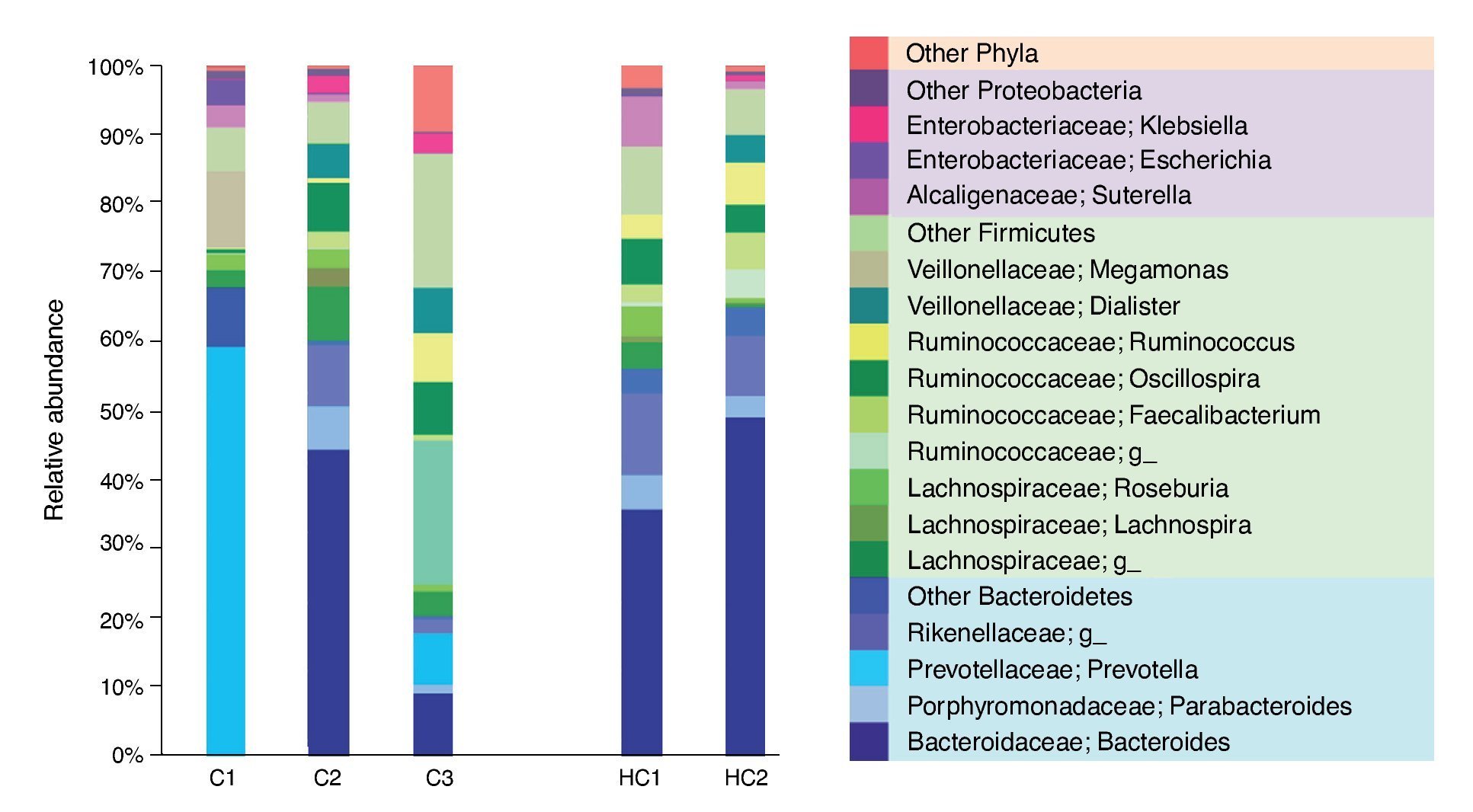
The most dominant phylum in case 1 and 2 was Bacteroidetes followed by Firmicutes with a ratio of 2:1. However, at the genus level the differences arose. Thus, 58.9% of the gut microbiota from case 1 corresponded to the Prevotella genus (Bacteroidetes), whereas in case 2 the most prevalent was Bacteroides (Bacteroidetes) with 44.5%. These genres were followed in abundance by 11.1% Megamonas (Firmicutes), 3.6% Escherichia and 3.1% Suterella (Proteobacteria). Meanwhile, in case 2 the predominant Firmicutes groups were the Lachnospiraceae family with 13% and the genus Oscillospira with 7% (Figure 1).
Figure 1 Relative abundance of fecal bacterial genera in each subject. Only phyla and genera with relative abundance >1% were included. All operational taxonomic units (OTUs) with lower abundance were grouped as “other”. C1: case 1, C2: case 2, C3: case 3, HC1: Healthy children 1, HC2: Healthy children 2.
In contrast, case 3 presented an inverse Bacteroidetes/ Firmicutes ratio of 1:3. The Ruminococcaceae (Firmicutes) family is highlighted with a relative abundance of 44% including the genus Oscillospira and Ruminococcus. Regarding Bacteroidetes, 9.4% of Bacteroides and 7.6% of Prevotella genus were found (Figure 1).
Case 3 developed T1D 1 year later, with fasting glucose of 372 mg/dl at onset and classic diabetes symptoms (polyphagia, polydipsia and polyuria).
3. Discussion
The perinatal period is critical for the development of the individual microbiota. The shape of the newborn first micro-biota depends directly on the delivery mode. Children born vaginally have a vertical transfer of maternal vaginal bacteria such as Lactobacillus and Prevotella spp. On the other hand, those born by cesarean acquire microorganism from the skin and operating room such as Staphylococcus, Corynebacterium and Propionibacterium spp.7. After delivery, the feeding regimes are crucial to promote the development of a diverse and healthy microbiota. Current recommendations based on evidence are focused on the maintenance of breastfeeding for at least the first 6 months, ideally continuing it throughout the first year of life and the introduction of cereals into the infant’s diet between 4 and 6 months of age. These actions promote the decrease in the risk for developing autoimmune diseases and allergies in childhood. Thus, exclusive breastfeeding longer than 5 months or total breastfeeding longer than 7 months are independent protective factors against T1D development8.
Here we presented two cases of T1D patients that share risk factors such as the inadequacy of their feeding regimes during the first year of life, even though they were born by vaginal delivery. None received an exclusively breastfed regimen and they were fed with cow milk formula as newborns. Furthermore, case 2 and case 3 have in common the introduction of wheat to their feeding regimen at 2 months old without gradually scaling portions.
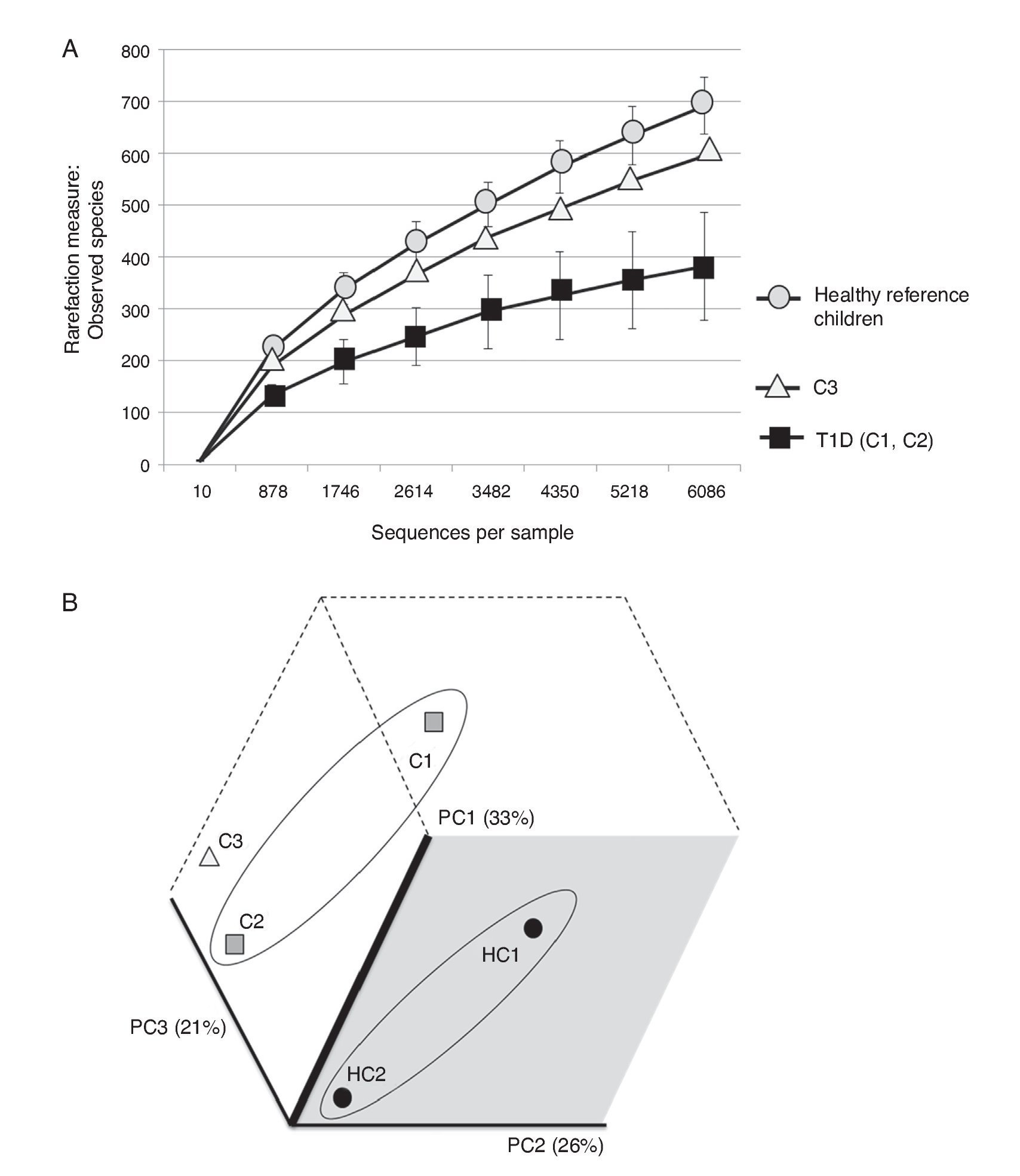
The three cases are compared with two healthy Mexican children (3 and 5 years old) recruited from the same population and who were negative for HLA haplotypes DR3-DQ2 and DR4-DQ8 (Figure 2). Both healthy children were born vaginally, breastfed for more than 6 months and cereals, including wheat, were introduced into their diet when they were about 5-6 months old.
Figure 2 (A) Rarefaction curve of observed species in fecal samples from cases and two healthy reference children. (B) Communities’ clustered using Principal Coordinates analysis of the unweighted UniFrac distance matrix. The percentage of variation explained by the plotted principal coordinates (PC) is indicated in parenthesis on the axes. T1D: Type 1 diabetes, C1: case 1, C2: case 2, C3: case 3, HC1: Healthy children 1, HC2: Healthy children 2.
Therefore, as they were close to the current recommendations, for purposes of this analysis, their microbiota was considered as a reference of what might be the normal gut bacterial proportions under optimal conditions in children of this age range in the study region (Sonora, Mexico).
This is an important consideration to contextualize the presented cases because the microbiome of T1D high-risk infants is associated with geographic location. Kemppainen et al.3 found great variability in the structure and diversity of gut microbiomes among countries and continents, even in populations with homogeneous HLA class II genotypes.
It is evident that the intestinal microbiota of healthy Mexican infants where Bacteroides genus is the most abundant is very homogeneous (intragroup diversity) and different from the one dominated by Prevotella of case 1. Additionally, it is also different from that found in healthy children >7 years old from the same region5. It is necessary to highlight that case 2 has a composition pattern closer to the healthy children than the other T1D patient (Figures 1 and 2). The contrast found between case 1 and case 2 is probably because case 1 is a decompensated patient in the stage of diagnosis, metabolically unbalanced and coursed with uncontrolled glucose levels. Meanwhile, case 2 is a patient in a stable phase of the disease with glucose levels closer to the normal range.
Previous studies linked uncontrolled glucose levels with the microbiota imbalance associated with T1D and dysbiosis tends to be restored after control of the disease with insulin treatment for >2 years5. This would indicate that case 2 was able to partially restore the balance of her microbiota in <3 months when she achieved the control of T1D with insulin and nutritional management. Further prospective studies are needed to prove this statement.
One of the similarities between case 1 and case 2 is that they have higher levels of the Enterobacteriaceae genus Klebsiella and Escherichia. Both are pathobionts, i.e., despite being commensal microorganisms they have the potential of causing disease and therefore could activate or maintain metabolic pathways that promote inflammation. Elevated levels of Escherichia have been associated in other studies with the consumption of infant formula in bottle-fed children9.
Case 3 presented high risk factors for the development of autoimmunity associated with T1D, including a very high-risk phenotype. His microbiota structure appears to be different from both T1D cases and from the one of the reference children. It also showed an inverse ratio for Firmicutes/Bacteroidetes when compared to healthy Mexican children, but also to those already diagnosed with T1D. This alteration apparently occurs prior to the development of autoimmunity and T1D and is reflected as a decrease in microbial diversity (Figure 2A) without being as limited as in T1D. These results are in part different from those reported by Kostic et al.4 where the changes were displayed after development of autoimmunity, prior to T1D diagnosis. However, we only evaluated Anti-GAD and Anti-IA2 autoantibodies and not IAA, ZNT8A and ICA as done by Kostic et al.4
Based on these observations, in this process Oscillospira and Ruminococcus could be involved. Case 3 presented higher levels of these bacterial genera with respect to the healthy children. These findings are consistent with results in NOD mice10 where levels of Ruminococcus and Oscillospira had an inverse relationship with T1D age of onset. Related to this, de Goffau et al.11 found that in young European children between 3 and 5 years old, Ruminococcus obeum was positively correlated with the recent development of T1D. However, the remaining microbiota composition of these children differs from that found in the Mexican cases.
Case 3 could be an example of how diet and environmental factors in early life could establish an adverse environment, which promotes the development of autoimmunity in children with genetic susceptibility. During the first three years of life, the shaping of the immature microbiota into an adult-like composition could be directed in an aberrant direction11 if these factors are not taken into account in the infants. This idea is supported by the fact that although the intestinal microbiota of infants is very unstable, micro-organisms acquired in the first year of life are preserved throughout childhood, at least up to 3 years when the T1D-associated autoimmunity mainly begins to appear. Likewise, despite abundance of variations, the metabolic pathways associated with the microbiota remain over time4. These early findings complement our results from another study with a larger sample of older T1D children5. Together they may provide the basis for the design and future implementation of primary prevention strategies for T1D, customized according to the geographic origins and characteristics of the groups at risk.
In conclusion, fecal microbiota of the presented cases appears to be different from the one of healthy infants considered as reference. This could be related to the feeding patterns and environmental factors prior to the development of autoimmunity and T1D. Prospective studies are needed to follow-up on healthy children at high genetic risk, to assess their perinatal history, diet and fecal microbiota in relation to their place of origin.
Ethical disclosure
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Ethical considerations
Informed consent was obtained from legal guardians of all the cases and controls. All procedures performed were approved by the Ethics Committee of the Centro de Investigación en Alimentación y Desarrollo (CIAD) and by HIES Learning and Research Board.
Funding
Financial support was obtained from the Mexican Council for Science and Technology (CONACYT), grant S0008-2009-01-115212.
Author contributions
M.E.M. and A.M.C. conducted the study, collected samples and clinical records, extracted DNA, typed haplotypes, analyzed data and wrote the main manuscript text.
Conflict of interests
The authors declare that they have no conflict of interest.
Received 15 June 2015;
accepted 17 August 2015
☆ Please cite this article as: Mejía-León ME, Calderón de la Barca AM. Perinatal factors and type 1 diabetes-associated dysbiosis in Mexican infants. Bol Med Hosp Infant Mex. 2015. http://dx.doi.org/10.1016/j.bmhimx.2015.08.001
* Corresponding author.
E-mail:amc@ciad.mx (A.M. Calderón de la Barca).