Introducción: La piedra angular del tratamiento de alergia alimentaria es la eliminación de los alimentos causantes. Sin embargo, los niños que restringen el consumo de alimentos básicos tienen un mayor riesgo de desnutrición.
El objetivo del estudio fue identificar el estado nutricional de pacientes con dieta de eliminación e identificar la proporción de pacientes del grupo con verdadera alergia alimentaria.
Métodos: Se realizó un estudio transversal de enero a octubre de 2014 en el Hospital Infantil de México Federico Gómez. Se incluyeron pacientes de 1 a 11 años con historia de eliminación de, al menos, uno de cinco alimentos (huevo, leche, trigo, maíz, soya) por un mínimo de 6 meses. Se realizó la valoración nutricional completa y se compararon los índices antropométricos con tablas de Z score para la edad. Se analizaron los datos por medio de estadística descriptiva, y posteriormente con prueba de Kruskal-Wallis y correlación de Spearman.
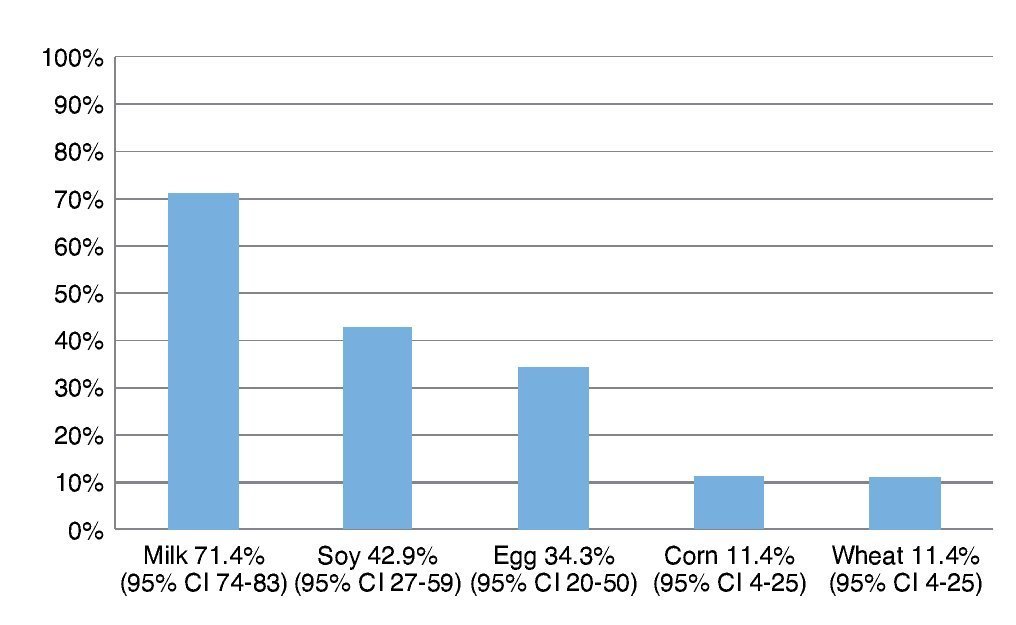
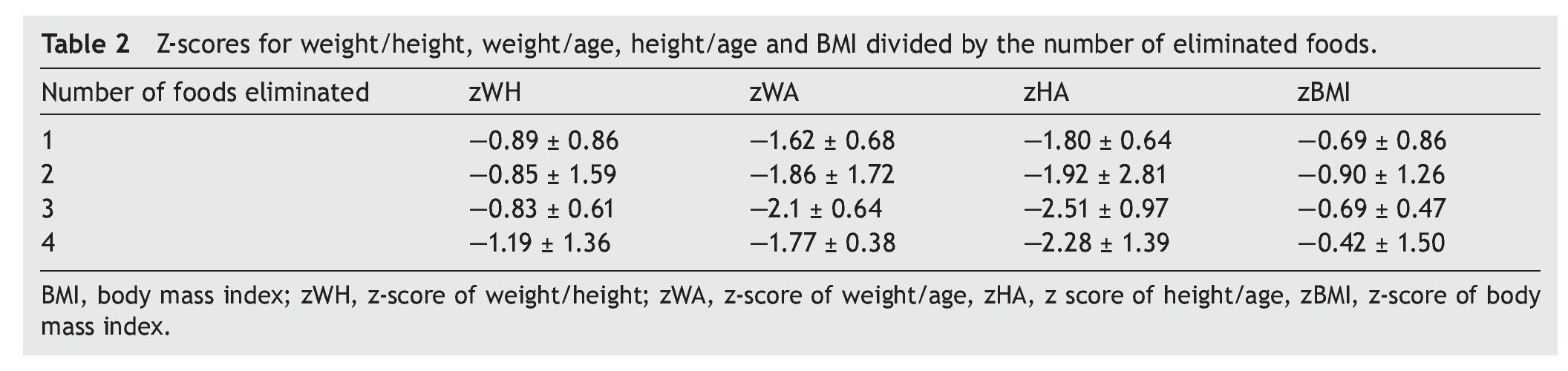
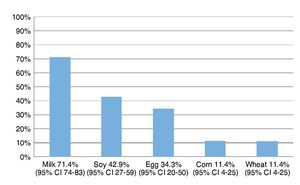
Resultados: Los alimentos más frecuentemente eliminados fueron leche, soya, huevo, maíz y trigo. Al comparar el número de alimentos eliminados de la dieta con los distintos índices antropométricos evaluados, se encontró que entre mayor cantidad de alimentos eliminados, el score Z de peso para la edad (PE) y talla para la edad (TE), así como peso para la talla (PT) fueron menores, y el más afectado fue la reserva grasa. Solamente en el 5% de los niños se corroboró alergia alimentaria.
Conclusiones: Nuestro estudio confirma la necesidad de una correcta asesoría nutricional en aquellos pacientes que cuenten con dietas de eliminación, así como el sobrediagnóstico que existe de alergia alimentaria.
Background: The cornerstone of food allergy treatment is the restriction of causative foods. These interventions have shown that children who restrict the consumption of basic foods have a higher risk of malnutrition.
The aim of the study was to identify the nutritional status of patients with elimination diet, characterizing their anthropometric indexes and identifying the percentage of patients in the group with true food allergies.
Methods: A cross-sectional study was carried out from January to October 2014 at the Hospital Infantil de Mexico Federico Gomez. Patients 1 to 11 years of age with a history of elimination of at least one of five foods (eggs, milk, wheat, corn, soybeans) for a minimum of 6 months were included. Full nutritional assessment was performed by comparing the anthropometric indexes to z score for age. Data analysis used descriptive statistics. Kruskal-Wallis and Spearman correlation were performed.
Results: The most frequent eliminated foods were milk, soy, eggs, corn, and wheat. Comparing the number of foods eliminated with different anthropometric indexes, with a greater amount of eliminated food, the z-score of weight/age (W/A), height/age (H/A) and weight/height (W/H) were lower and the most affected index was fat reserve. Only in 5% of children was food allergy confirmed.
Conclusions: The study confirms the need for nutrition counseling for patients who have elimination diets and overdiagnosis of food allergy.
Pagina nueva 1 1. Introduction
A wide spectrum of adverse reactions may occur following the ingestion of a food and are typically classified based on the underlying pathogenesis.1 Food allergy is defined as the adverse effect caused by a specific immune response that occurs after exposure to a food and is reproducible.2 Its prevalence has been reported to be 5-6% in children <3 years old3 and can even falsely be increased to 17% if the diagnosis is made based on only self-reports and without an adequate approach.4
The first step in diagnosing food allergy is to perform a complete medical history,5 which will suggest the underlying immunological reaction that will try to be demonstrated by skin tests (in vivo) and laboratory tests (in vitro). Finally, a provocation test must be performed, which is considered the gold standard for diagnosis.
To a comprehensive and systematic approach performed in patients with adverse effects associated with food, it is found that many of them have been diagnosed with food intolerance rather than allergy. The cornerstone of treatment for food allergy is strict elimination of causative foods2 which, in any case, must be accompanied by substitutes to maintain the nutritional balance of the individual.6 Elimination diets in times of rapid growth may involve serious nutritional and psychological consequences,7 which is particularly true in pediatric patients with high metabolic demands and who more commonly display this type of pathology.8
When anthropometric indexes of children with restrictive diets are evaluated, it has been found that they have z-score of weight for age, height for age and weight for height lower than healthy controls in children their own age.6 These differences are more evident when more foods are involved.6.9 It has also been observed that the decline in their growth coincides with both the onset of symptoms of allergy and implementation of the elimination diet.10
Alteration of “nutritional status” in children with food allergy goes hand in hand with lower intake of total kcal, fat, protein, riboflavin, niacin and calcium,7,8,11 vitamin D7,12 and E, compared with similarly aged children. Although changes in nutrition are true for the majority of patients with elimination diets, weight and height are fortunately recoverable with proper diet.13 In Mexico, data are unavailable evaluating the effect on weight and height in children with elimination diets.
The aim of the study was to identify the nutritional status of patients with elimination diet for suspected food allergy, characterize the main anthropometric indexes and identify the proportion of patients in the group with true food allergy.
2. Methods
A cross-sectional study was conducted in the Department of Allergy and Clinical Immunology at the Hospital Infantil de México Federico Gómez (HIMFG) and Nephrology Research and Bone Mineral Metabolism Laboratory from January to October 2014. Patients 1- to 11-years of age were included who initially were seen for possible renal tubular acidosis; however, this condition was ruled out. All had history of removing at least one of five foods (egg, milk, wheat, corn or soy) for at least 6 months. Elimination diets were imposed outside the HIMFG. All parents of patients provided informed consent. Assent was requested in patients >8 years of age. This study was approved by the Ethics, Research and Biosafety Committees from HIMFG (protocol number HIM/2012/036) following the rules of the institution.
On admission, demographic information and history of allergic diseases, GI symptoms, duration of food removal and indication for the same were recorded. In all cases the complete medical history was recorded, with special emphasis on the risk factors for developing malnutrition such as characteristics of the evacuations, reflow data, phenotypic alterations compatible with syndromic complex, among others. All cases were evaluated by certified pediatricians and, when necessary, patients were sent to the subspecialist indicated. Patients suspected of any additional pathology were excluded from this protocol.
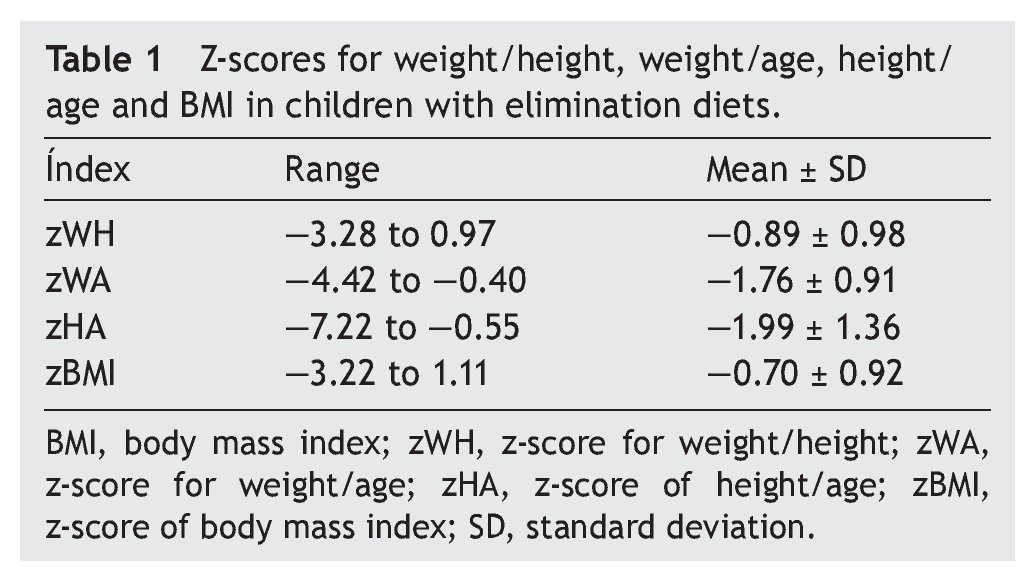
A complete nutritional assessment was made gathering information such as weight, height, arm circumference and triceps skinfold, calculating BMI and fat and lean reserve percentage. All measurements were compared with z-value tables for age indexes with reference values of the WHO.14
Two observers measured the length in children <2 years old using an infantometer (SECA model 2101721009). Height of children older than this age was evaluated using a stadiometer in Frankfurt plane. For children <36 months of age, weight was assessed using an electronic scale (Seca model 354) and for older children using an electronic health scale with stadiometer (ADE). For all cases, three indicators were calculated: weight for age (W/A), height for age (H/A) and weight for length (or weight for height) (W/H). Regarding weight for age, mild acute malnutrition data was considered when the z-score was from —1 to —2, moderate acute malnutrition data when z-score was from —2 to —3, and severe when z-score was < —3.
For all ages, arm circumference was evaluated in the middle of the left arm, half the distance from the acromion to the olecranon, using a fiberglass tape measure 6 mm wide. Calculation of the reserve of fat mass (FM) and lean body mass (LBM) was recorded first. Tricipital skinfold was measured at the back of the left arm with a Harpenden caliper at midpoint between the acromion and the radial head with the arm bent at 90º at the elbow, and palm of the hand facing toward the front of the body. After this, FM and LBM were calculated by the Frisancho formula.15
In all patients, double-blind placebo-controlled challenges were made for suspected foods in order to clarify the diagnosis of food allergy. The methodology used for this procedure was based on the guidelines issued by the American Academy of Allergy, Asthma and Immunology (2009).16
The challenge for each food was randomly administered on 2 separate days; one day placebo and the other day Verum® (betahistine) every 30 min for 4 h to subsequently remain under monitoring for 2 h. Upon completion of 2 days without symptoms, we proceeded to daily administer the food in question. Over the next 7 days the presence or absence of abdominal, respiratory and skin symptoms was reassessed and, if they were not present, it was concluded as a negative challenge. Data were analyzed using descriptive statistics and later with the Kruskal-Wallis and Spearman correlation.
3. Results
A total of 35 patients were included with a history of eliminating some of the five foods included in the protocol for at least 6 months. Ages were between 1 and 9.9 years, with a median of 31 months; 37% were male (95% CI 23-53) and 63% female (95% CI 46-76). Of these patients, 31.4% (95% CI 18-47) reported symptoms of allergic rhinitis and 14.3% (95% CI 6-29) wheezing at some point in their lives; 82.9% of subjects (95% CI 67-91) reported suffering some type of abdominal symptoms more than four times a week for at least 4 weeks. The most frequently reported symptom was abdominal pain with 51.4% of subjects affected (95% CI 35-67). As for the report of foods eliminated, 60% eliminated only one food (95% CI 43-74) and 22.9% eliminated at least four foods (95% CI 12-39).
The most frequently eliminated foods were milk, soy, egg, corn and wheat (Fig. 1). When questioned about the reason for the elimination diet, 58.5% (95% CI 40-72) reported that they carried it out for food allergy diagnoses although the total number of patients avoided at least one of the foods included in the study.
Figure 1 Foods most frequently eliminated in a group of 35 patients with suspicion of food allergy.
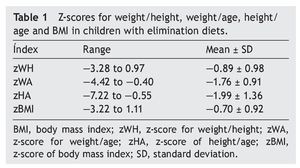
Regarding anthropometric indexes, results of the z-scores of W/A, H/A, W/H and BMI are shown in Table 1. It was identified that 42.9% (95% CI 27-59) of patients had a z-score between —1 and —2 (mild malnutrition); 20% (95% CI 10-35) presented z-score from —2 to —3 (moderate malnutrition) and, finally, 5.7% (95% CI 1-18%) presented z-score <—3 (severe malnutrition).
When comparing the number of foods eliminated from the diet using assessment of different anthropometric indexes, the greater number of foods removed demonstrated lower z-score for W/A, H/A and W/L were lower. This was true when comparing the group that eliminated a single food vs. the group that removed three foods. However, no statistically significant differences in these parameters were observed. Table 2 specifies the mean from the z-score of the anthropometric indexes evaluated by separating them by number of eliminated foods.
Mean arm circumference was 14.56 ± 1.79 cm and skinfold 6.5 ± 1.05 cm. When performing cluster analysis, a statistically significant relationship between the number of eliminated foods and these indexes was not found. For FM and LBM, it was found that the greater the number of eliminated foods, the lower the FM and LBM, observing a negative correlation —0.50 (p = 0.002) for the first. For the second, although an equally negative correlation was found, it was not significant. A statistically significant difference was also observed in assessing the FM using Kruskal-Wallis (p = 0.021), identifying a lower FM in the group with the most foods eliminated.
As for the diagnosis of food allergy, it was confirmed by double-blind placebo-controlled challenge in three patients, i.e., 5%.
4. Discussion
In this study we evaluated patients carrying out an elimination diet of at least one of five foods (milk, soy, eggs, corn and wheat). The first point to be identified was that >40% (95% CI 25-56) of the individuals did not have a food allergy diagnosed by a physician; however, they eliminated some of these foods. When asked about the reason for such action, the responses were mixed: 1) some eliminated them because of positive studies of specific IgG in blood; 2) others eliminated them due to suspicion without arriving at a diagnosis; and others due to 3) unsupported medical recommendation without symptoms.
Of the total number of patients included, food allergy was confirmed in only 5%, which is consistent with reports of global prevalence and demonstrates the high incidence of diagnosis of this condition and, therefore, unnecessary elimination diets. Nowadays, over-diagnosis of food allergy is a problem causing significant risks to the health of children and even more in those who do not receive proper supplementation. Association of the restriction of dairy products with serious conditions such as marasmus and kwashiorkor has been previously described.17 This study found that only a third of the children had normal weight and 5.7% had severe malnutrition. Although these results cannot assume the absolute certainty of the cause-and-effect relationship between elimination diets and long-term growth in this group, results point to an association between the lowest anthropometric indexes and the highest quantity of food eliminated. In addition to being an emotional burden on both the child and the family, unsubstantiated suspicion of food allergy involves an important economic flow caused by the unjustified use of health services, school absenteeism, lost work days, introduction of expensive supplements, etc.18 The total estimated time for the completion of controlled double-blind placebo challenges was nearly 1,000 h. This figure takes into account both the direct and indirect costs, which demonstrates the high economic investment of a child in whom these studies are not warranted.
Notably, there were patients with elimination diets secondary to positive studies of specific IgG in blood. Currently, the European Academy of Allergy and Clinical Immunology Task Force Report and the National Institute of Allergy and Infectious Diseases Sponsored Expert Panel2,19 oppose the use of IgG levels as indicators of food allergy. This behavior is not unique to this medium because there are similar cases reported in the literature.17
Among the evaluated anthropometric indexes, greater involvement in LBM was found, which was lower among those who eliminated the most foods from the diet. This allows suspecting an acute imbalance between the necessary requirements and intake, resulting in an acute more than chronic malnutrition.20
The results obtained are consistent with those reported by several authors. In 2014, Meyer et al. also showed the relationship between the lowest weight and height with the greatest number of eliminated food.21 Moreover, Zeiger et al. reported that children who have allergies to soy and milk had lower anthropometric indexes than those with allergies to milk.22
Whereas it can be inferred with these results that the higher food restriction, weight and size are more affected, a limitation of this study is the number of patients included and absence of biomarkers to assume that one results from the other. Additional long-term studies are required that follow growth curves for early detection of the damage. Without proper nutritional counseling, this is being done with elimination diets with an impact on child development.
The double-blind placebo-controlled challenge is considered the gold standard for diagnosing food allergy. However, in daily practice it is laborious, making the food diary and a correlation with the signs and symptoms more convenient than carrying out open challenges prior to eliminating foods that will affect further development.
For individuals with an elimination diet of one or more foods, only a small percentage of food allergy diagnosis (5%) was confirmed. Most subjects do not require the restriction. Furthermore, a significant fraction of these subjects met the criteria for malnutrition (68%). Although this study cannot completely attribute the cause of malnutrition to the restrictive diet, it does indicate that perhaps clinicians prescribe these diets without solid foundations and without considering the impact on the nutritional status of patients.
It is the first responsibility of every pediatrician and allergist to ensure proper nutritional intake despite the need to eliminate foods from the diet and to only do so when necessary.
Ethical responsibilities
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Funding
This study was funded by Federal Funds, Protocol HIM/2012/036, and Funds from the Ministry of Health (Secretaría de Salud), Protocol HIM/2012/036/SSA.1063.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 11 February 2015;
accepted 27 April 2015
http://dx.doi.org/10.1016/j.bmhimx.2015.04.002
* Corresponding author.
E-mail:blancadelrionavarro@gmail.com (B.E. Del Río-Navarro).