Introducción: La neumonía adquirida en la comunidad (NAC) es una de las causas infecciosas más frecuentes de morbi-mortalidad a escala mundial en niños menores de 5 años. El objetivo del estudio fue precisar el diagnóstico bacteriano etiológico en lactantes con NAC.
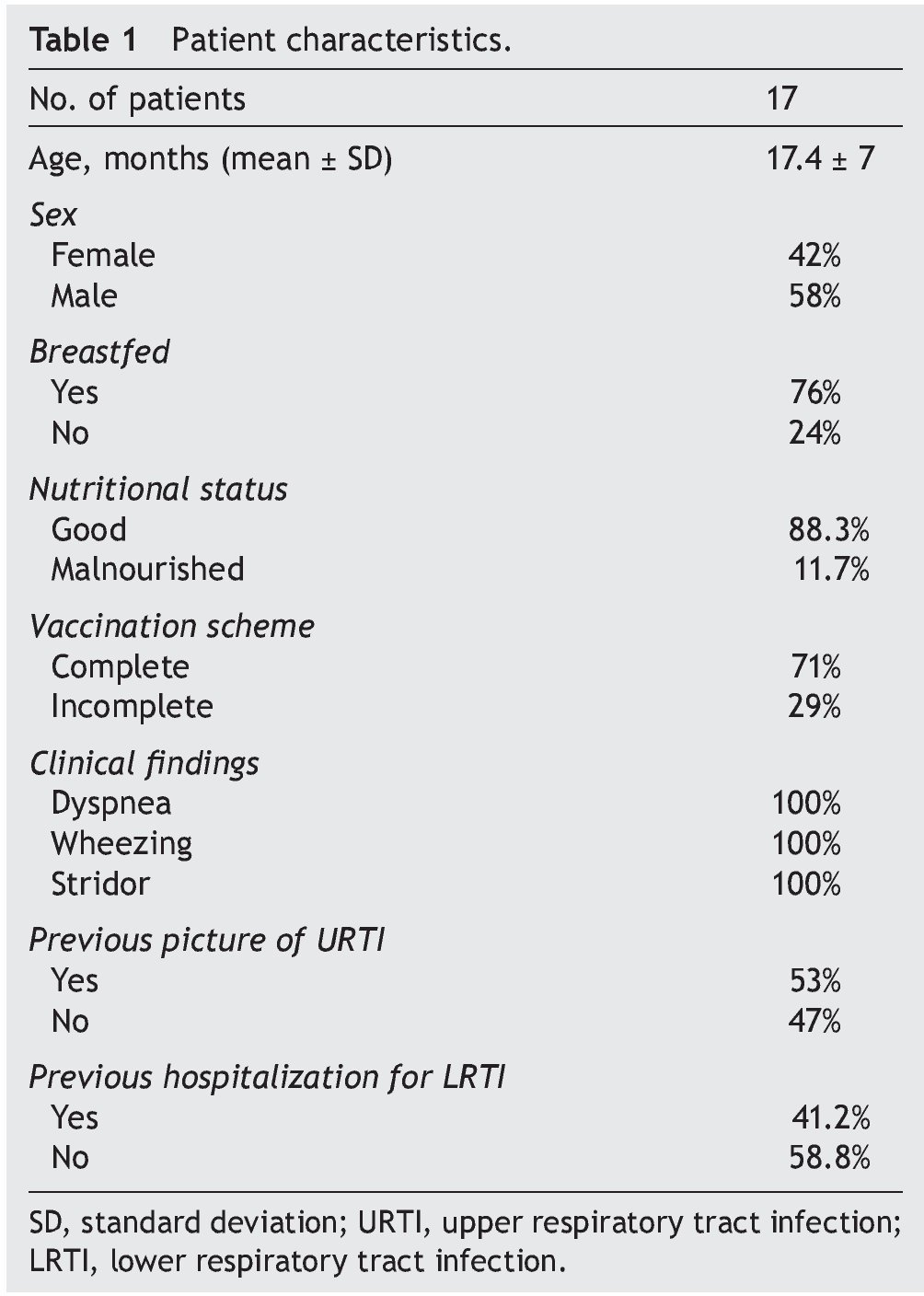
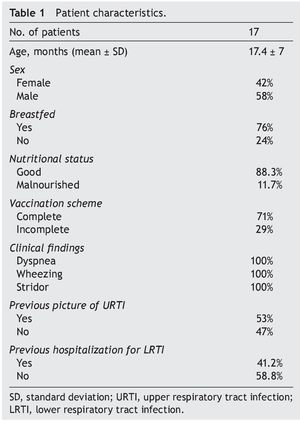
Métodos: Se condujo un estudio prospectivo, transversal y descriptivo en 17 pacientes de 6 meses a 2 años 11 meses de edad con NAC de mala evolución, que ingresaron al servicio de Neumología pediátrica. A los pacientes se les realizó broncoscopia con lavado broncoalveolar (LBA) con las medidas pertinentes durante el procedimiento para limitar el riesgo de contaminación.
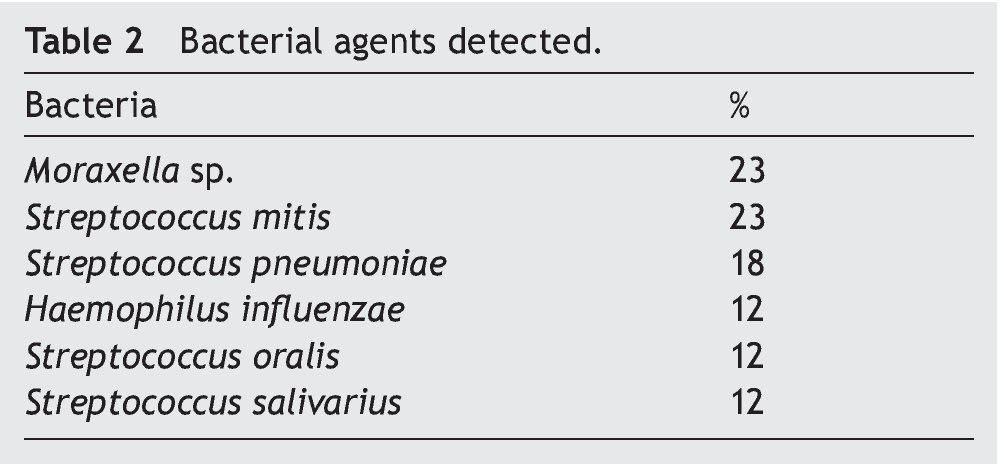
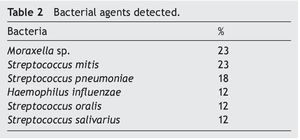
Resultados: Las bacterias aerobias aisladas fueron Moraxella sp. (23%), Streptococcus mitis (23%), Streptococcus pneumoniae (18%), Haemophilus influenzae (12%), Streptococcus oralis (12%), y Streptococcus salivarius (12%).
Conclusiones: En contraste con otros informes se observó que Moraxella sp. es un importante patógeno potencial bacteriano, posiblemente debido a la mejora en la detección con broncoscopia más LBA.
Background: Community-acquired pneumonia (CAP) is one of the most common infectious causes of morbidity and mortality in children <5 years of age. The aim of the study was to clarify the bacterial etiologic diagnosis in infants with CAP.
Methods: A prospective, cross-sectional and descriptive study in patients 6 months to 2 years 11 months of age with CAP with poor outcome was conducted. Patients were admitted to the Pediatric Pneumology Service and underwent bronchoscopy with bronchoalveolar lavage (BAL), taking appropriate measures during the procedure to limit the risk of contamination.
Results: Aerobic bacteria isolated were Moraxella sp. 23%, Streptococcus mitis 23%, Streptococcus pneumoniae 18%, Haemophilus influenzae 12%, Streptococcus oralis 12%, and Streptococcus salivarius 12%.
Conclusions: In contrast to other reports, we found Moraxella sp. to be a major bacterial pathogen, possibly because of improved detection with bronchoscopy plus BAL.
1. Introduction
Pneumonia is a major cause of morbimortality in childhood. Its treatment consumes significant health and economic resources within hospitals as well as outside the hospital. Peak incidence occurs in children aged 1-5 years and is especially prevalent during the winter season. Pneumonia is defined as inflammation of the lung tissue due to an infectious agent that stimulates the inflammatory response, resulting in lung injury. This response causes migration of neutrophils, release of inflammatory mediators and oxidative enzymes, with plasma leakage and surfactant loss, which result in the absence of air and freezing of the organ known as consolidation. The causative organisms may be bacteria, viruses, fungi or parasites. However, the high mortality rate of pneumonia is attributed, in most part, to bacterial etiological agents as well as to the lack of prompt and appropriate access to treatment.
Community-acquired pneumonia (CAP) is an acute infection of the pulmonary parenchyma that affects an immuno-competent patient exposed to a microorganism outside the hospital. Classically it is considered as a condition in which the patient has not been hospitalized 7-14 days before symptom onset or that symptoms begin in the first 48 h since the patient’s hospitalization.
Effective antimicrobial therapy depends on the identification of the etiologic agent. It is therefore necessary to obtain the appropriate material for bacteriological diagnosis, including Gram stain and culture. In children <5 years of age, it is difficult to obtain sputum samples. Use of bronchoscopy and bronchoalveolar lavage (BAL) reduces contamination in obtaining samples. BAL is the method used to obtain a representative sample of fluid and secretions from the lower respiratory tract (LRT), which will be useful for cytological and microbiological diagnosis of lung diseases, particularly in pediatric patients. Bronchoscopy with BAL is a noninvasive sampling option of the LRT. Enriched culture media will also be used for the nutritional requirements demanded by bacterial growth.
Most CAP in children is of viral etiology. However, the possible existence of bacterial infections often conditions the prescription of antibiotic treatment. It should be noted that the radiologic patterns described in different etiological forms do not have absolute validity. Although the existence of alveolar infiltration is characteristic of bacterial pneumonia, it can also be observed in those cases caused by viruses.
In Mexico there are few studies of the bacterial etiology of CAP in children. During the first 3 years of life the virus is clearly highlighted. Streptococcus pneumoniae and Haemophilus influenza should also be considered.
The greatest diagnostic difficulty lies in identifying the etiological agent. This fact is related to the problems that present in obtaining suitable samples for biological cultivation.14 The pediatric patient usually does not cough up blood and blood cultures are of little value for the etiologic diagnosis of pneumonias.15,16 Fever is a characteristic of pneumonia and can be present in 88-96% of radiologically confirmed cases17 but should be evaluated in the context of all the information. In children of any age with persistent or recurrent fever >38.5ºC and increased output and respiratory rate, bacterial pneumonia should be considered.18
BAL allows microbiological diagnosis in 50% of cases with a minimum complication rate. This study was performed to appropriately identify the bacterial etiology in these patients.
2. Patients and methods
2.1. Study design
A cross-sectional prospective study was performed with infants (6 months-2 years and 11 months old) who were admitted with a diagnosis of poorly evolved CAP confirmed by chest x-ray according to WHO guidelines19 at the Pediatric Pneumology Service of the Hospital Gaudencio González Garza (HGGG) from September 1–November 30, 2009.
Patients who underwent BAL for culture and who met the following inclusion criteria were included: tachypnea [>50 respirations/min (rpm) in children <1 year of age and >40 rpm in older children], suprasternal subcostal or inter-costal retraction, rales and wheezing, radio-opaque homogeneous opacities with segmental or lobar location with bronchogram or bilateral diffuse heterogeneous radiopacities, multiple foci, leukocyte count >15,000/mm3, C-reactive protein (CRP) and increased sedimentation velocity with or without antimicrobial therapy.
Written informed consent of parents or guardians was obtained prior to participation in the study. Study procedures were in compliance with the standards of the Institutional Committee for Responsible Human Experimentation and according to the World Medical Association and Declaration of Helsinki. The study was previously accepted by the Research and Ethics Committee of the Medical Unit of High Specialty Gaudencio González Garza, Centro Medico Nacional (CMN) La Raza, IMSS.
Operational definition of "poor outcome”
– Moderate respiratory distress
– O2 sat <92%
– Dehydration
– Hemodynamic compromise
– Persistence of symptoms and radiographic abnormalities
– Lack of response to empirical treatment
2.2. Sampling procedure
Bronchoscopy and BAL were performed using a standardized protocol. A pediatric bronchoscope was inserted orally to avoid nasal contamination. Breathing aliquots of saline solution (0.9%) were instilled in the lobar bronchus or diseased segment (maximum volume, 3 ml/kg of body weight). BAL first fraction was discarded. All patients were sedated with i.v. midazolam, atropine sulfate and tramadol hydrochloride. Intracutaneous monitoring was conducted to assess oxygen saturation and heart rate, and supplemental oxygen was administered as necessary during the procedure.6
2.3. Microbiology
BAL samples were processed using standard techniques. Briefly, samples were sent immediately to the laboratory, centrifuged for Gram stain of sediment and injected into BacT/ALERT PF plus bottles (bioMérieux) with activated carbon, which serves to sequester the antibiotic used and allows growth of the organism. Infants admitted with diagnosis of CAP were managed with antibiotic schemes. Developing microorganisms were cultivated in sheep blood agar with 5% chocolate agar in an aerobic atmosphere with CO2 to 5% at 37ºC for 2 days for the development of aerobic and facultative bacteria. After pure isolation, identification by Gram stain was done using manual tests (catalase, oxidase, optochin sensitivity, and satellite test according to the isolated microorganism (VITEK® and API® 20 Strep system, bioMérieux).
2.4. Statistical analysis
Fisher’s exact test was used for comparison of proportions under the low number of observed frequencies.
3. Results
3.1. Study population
Seventeen infants who met the inclusion criteria were included. The highest prevalence was observed in the 12- to 24-month-old group; 24% were fed with formula and 76% were breastfed. In the breastfed group, feeding time was <6 months in 69.2% and >6 months in 30.8%. Of patients with CAP, 29% presented with diarrhea and 59% presented previous otitis media. CAP diagnosis was based on clinical criteria. At the time of admission, cough was a finding in 94% of patients, of which 82% demonstrated wet cough and 12% with dry cough. Duration was 5-20 days. Fever occurred in 88.2%; hyperemic pharynx in 82%; adenomegaly in 76%; runny nose in 53% (green or yellow discharge in 33.4% and hyaline presentation in 66.6%). Previous clinical pictures of upper respiratory tract (URT) were shown in 2-5 patients <6 months of age. Hospitalizations for LRT were reported as 1-8 occasions (Table 1). It is worth mentioning that BAL complications were not presented.
3.2. Microbiology
Samples studied were appropriate. According to the criteria described by Murray and Washington, there were >25 polymorphonuclear leukocytes and <10 epithelial cells per field at low magnification (100X) in a Gram-stained smear.20
All bacterial isolates were pure cultures. Moraxella sp. and S. mitis were the predominant bacterial agents and both were isolated in 23% of samples followed by S. pneumoniae, accounting for 18% of cases (Table 2).
Unfortunately, accurate identification of M. catarrhalis by VITEK® system was not satisfactory, only with Moraxella sp.
The presence of acute otitis media (AOM) was associated with Moraxella sp. in 23.5% of the cases, a difference in the S. viridans group of 11.7% (p = 0.03). The age group affected by Moraxella sp. was 12–28 months in comparison with the rest of the isolated bacteria (6–35 months) (p = 0.03).
4. Discussion
Pneumonia is a leading cause of mortality in children <5 years of age. This study addressed a group of 17 newborns with CAP who required hospitalization and determined the bacterial etiology of infection.
Isolated aerobic bacteria mainly were Moraxella sp. and S. mitis, 23% for both agents followed by S. pneumoniae in 18%. Risk of contamination in BAL samples was limited significantly because various technical measures were taken to avoid contamination by secretions from the URT.3,6,21
When comparing the results with samples of pharyngeal swabs on a review of statistics from the Microbiology Section of the General Hospital Gaudencio González Garza (January 1, 2009-November 26, 2009) of patients diagnosed with pneumonia from the Pediatric Pneumology Services, it was observed that the report of bacteria present in the flora of the URT was 74% for Streptococcusanginosus, S. mitis and S. sanguis, and 26% in S. pyogenes, without the presence of Moraxella sp. or S. pneumoniae.
Despite the low number of patients, the results of this study are consistent with studies conducted by PAHO/WHO with regard to males who are more likely to contract pneumonia than females.22 In accordance with previous studies, the majority of children with CAP were well nourished.10,23
The problem of poorly immunized children is still present in a country where the vaccine is compulsory and free. It was found that 29% of the population did not have its full vaccination scheme. This failure at the primary level of care determines the need to maintain an active surveillance of the occurrence of preventable diseases such as those caused by type B H. influenzae. The presence of AOM was found associated with Moraxella sp. and not with H. influenzae, diverging from a previous study.24 AOM is a frequent infection in children <1 year of age and ~50% of children have experienced at least one incidence of AOM. This proportion increases to 70% at the age of 3 years. Undoubtedly, it is the most frequent and severe infection caused by M. catarrhalis in children and, as such, causes high morbidity and requires widespread use of antibiotics.
With regard of the identification of M. catarrhalis, the VITEK® automated system was unsatisfactory because it could not identify the species. Other authors have reported the same dificulty.25M. catarrhalis is an oxidase-positive aerobic accepted as a pathogen, although until recently it was considered a relatively harmless commensal of the URT. M. catarrhalis has been recognized as a specific pathogen in AOM for almost 70 years.26 Although infections of the LRT in children are a common cause of morbidity and even mortality worldwide, obtaining a microbiological diagnosis is difficult. Most studies use combinations of conventional serological and microbiological methods (e.g., culture or PCR). Many of these methods have only been used in research applications and are not always reliable or readily available to clinicians. Consequently, data on the role of M. catarrhalis infections of the LRT are not conclusive.26
LRT infections caused by M. catarrhalis are rare in childhood. The majority of infections occur in children <1 year of age.27M. catarrhalis has been isolated first from pure culture of secretions obtained by tracheal aspiration in neonates, nursing infants and children with pneumonia.28,29 Considering that the prevalence rate due to Moraxella sp. is greater than S. pneumoniae and H. influenzae, results obtained here differ from those reported by other authors.30 LRT infections caused by this agent should be suspected, perhaps when there is therapeutic failure to treatment with β-lactams. Therefore, involvement of Moraxella sp. should be investigated as a causative agent of acute and chronic respiratory infections in parallel with S. pneumoniae and H. influenzae. Moraxella sp. was one of the main agents found, possibly due to the improved detection by bronchoscopy with BAL. This suggests that pediatric CAP could be reduced by considering Moraxella sp. as an important causal agent.
Pneumonia causes more deaths worldwide than any other infectious disease. Although it can be cured with antibiotics, patients can die if timely, appropriate and adequate treatment is not initiated.31 The present study coincided with other authors24 who detected Moraxella sp. and S. pneumoniae in 28.9 %and 13.3% of cases, respectively. M. catarrhalis usually overinfects children <2 years old with viral infection.5 The results obtained here are consistent with current guidelines that recommend antibiotic treatment for all cases of CAP because an established viral or atypical etiology does not exclude a concomitant bacterial infection.32,33 Early identification of pathogens and appropriate antimicrobial treatment are essential to treat patients with pneumonia. Therefore, pulmonologists have long tried to find a sensitive, specific and safe method for obtaining from patients the pathogens causing LRT infections. BAL allows half of the microbiological diagnoses in cases with minimal complication rates. It is important to keep in mind that in experienced hands this technique provides much clinical information.
S. mitis and S. oralis are part of the streptococcus group mitis.34S. mitis is usually an etiologic agent in odontogenic infections and endocarditis, and only in some cases has it been recognized as a respiratory pathogen and pneumonia agent in immunocompromised subjects. It is a commensal microorganism closely related to the S. pneumonia pathogen, the causative agent of AOM, pneumonia, sepsis and meningitis. Homologous recombination has been observed among these species. Transfer of the genetic determinants of S. mitis to S. pneumoniae contributes to the resistance to penicillin in nasopharyngeal pathogen.35 Nasopharyngeal microflora have been the main reservoir of potential respiratory pathogens. S. mitis has been found in the oral cavity in all infants during each sampling time36 probably due to vaccination against S. pneumoniae and H. influenzae, explaining the lower percentage obtained in their insulation.
The limitations of this study include the small number of patients and the strains identified as Moraxella sp. It is very likely to be M. catarrhalis, which could not be identified with the VITEK® system. In addition, selection bias should be mentioned because we are a tertiary care hospital and this may factor in our results. However, the contribution of this study is to determine the circulating strains to improve empirical treatment and to help define the epidemiology in future studies.
In conclusion, as in previous studies, we found a variety of microorganisms involved in CAP in the pediatric population. In contrast to other reports, we found that Moraxella sp. is an important potential bacterial pathogen, possibly due to improved detection during bronchoscopy with BAL. Additional research is required to clarify the pathogenic mechanisms of this agent in pediatric CAP. However, the findings suggest that this infection could be reduced, considering Moraxella sp. as a major causative agent in terms of treatment and prevention strategies. Although the current literature does not provide a definitive answer, available data suggest that M. catarrhalis may be involved in the LRT in children. Therefore, the question remains open to new research. Fortunately, pneumonia caused by M. catarrhalis tends to be a relatively mild disease.
Ethical disclosure
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Funding
Medical Unit of High Specialty's own resources.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 13 August 2015;
accepted 11 September 2015
☆ Please cite this article as: García-Elorriaga G, Palma-Alaniz L, García-Bolaños C, Ruelas-Vargas C, Méndez-Tovar S, del Rey-Pineda G. Microbiología de lavado broncoalveolar en lactantes con neumonía bacteriana adquirida en la comunidad de mala evolución. Bol Med Hosp Infant Mex. 2015. http://dx.doi.org/10.1016/j.bmhimx.2015.09.004
* Corresponding author.
E-mail:gelorriaga@webtelmex.net.mx (G. García-Elorriaga).