Cancer in children has characteristics that differentiate it from other types reported in later ages. Overall survival at 3 years is up to 70% depending on the tumor studied. Major organs and systems affected are the hematopoietic system, central nervous system and sympathetic and mesenchymal tissues.
The increased incidence of neonatal tumors observed in this and other studies is based on the increasing number of solid tumors (teratomas and neuroblastomas) because cases of central nervous system tumors and leukemias have remained constant.
Ultrasonography is the first line of approach and can detect up to 70% of fetal anomalies. The physiology of the newborn causes the necessary multidisciplinary treatment in neoplastic disease to be modified substantially in this age group to avoid toxicity and sequelae. The most common treatment is surgery.
Achieving timely diagnostic treatment options are effective in improving the survival of these patients.
El cáncer en la edad pediátrica presenta características que lo diferencian de otrostipos reportados en edades posteriores. La supervivencia global a 3 años es de hasta el 70%,dependiendo de la neoplasia estudiada. Los principales aparatos y sistemas afectados son el sistema hematopoyético, el sistema nervioso central y simpático, así como tejidos mesenquimatosos.
El incremento en la incidencia de tumores neonatales observado en este y otros estudios se basa en el aumento del número de tumores sólidos (teratomas y neuroblastomas), ya que los casos de tumores en el sistema nervioso central y leucemias han permanecido constantes.
La ultrasonografía es la primera línea de abordaje y puede detectar hasta el 70% de las anomalías fetales. La fisiología del neonato hace que el tratamiento multidisciplinario necesario en las enfermedades neoplásicas sea modificado sustancialmente en este grupo de edad, para evitar toxicidady secuelas. El tratamiento más utilizado es la cirugía.
Logrando el diagnóstico oportuno existen opciones terapéuticas efectivas para mejorar la supervivencia de estos pacientes.
1. Introduction
Cancer that develops in the pediatric age has some characteristics that differentiate it from those presented at later ages in life, even according to the prognosis. Overall survival rate at 3 years up to 70% is reported, which varies according to the type of neoplasm studied. The main apparatus and systems affected are hematopoietic, central nervous system (CNS) and sympathetic systems, as well as mesenchymatous tissues.1,2
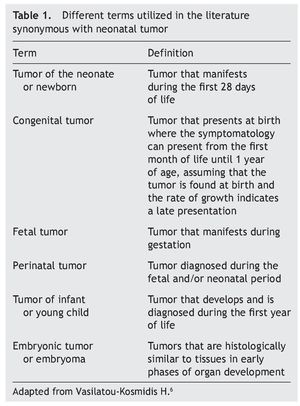
Among the group of pediatric neoplasms there is a subgroup that warrants special mention. These are the neonatal tumors that present themselves during the first 4 weeks of extrauterine life. Different terms used in the world literature that although they define different subgroups, they are frequently used synonymously (Table 1).3
Although neonatal tumors are a group of rare neoplasms, they represent a large target of attention for investigators because they represent an unknown with respect to their etiology, prognosis and treatment. It should be mentioned that they differ in incidence, degree of histological differentiation, therapeutic response (increase in toxicity with chemotherapy and increase in number of surgical complications) and prognosis which, in addition to the anatomic and physiology immaturity of the patient appropriate to the patient’s age and characteristic of the neonatal period, require special therapies and diagnostic methods.4
2. Epidemiology
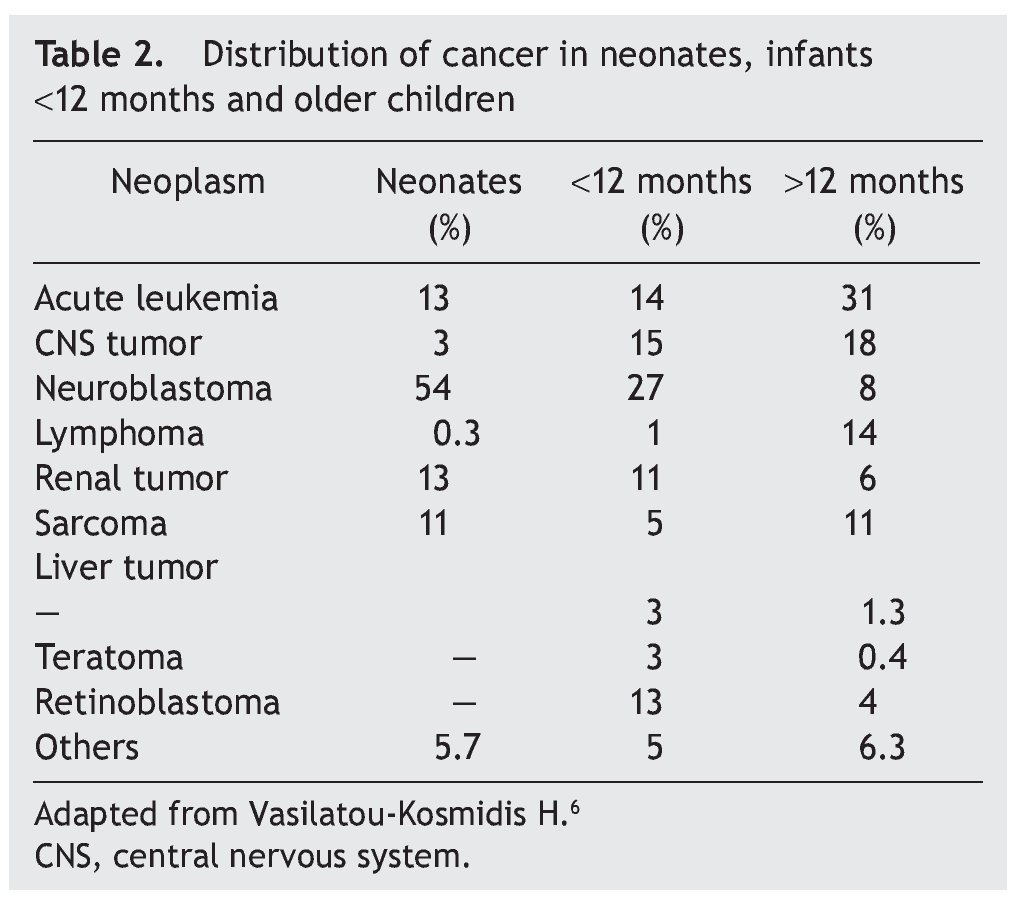
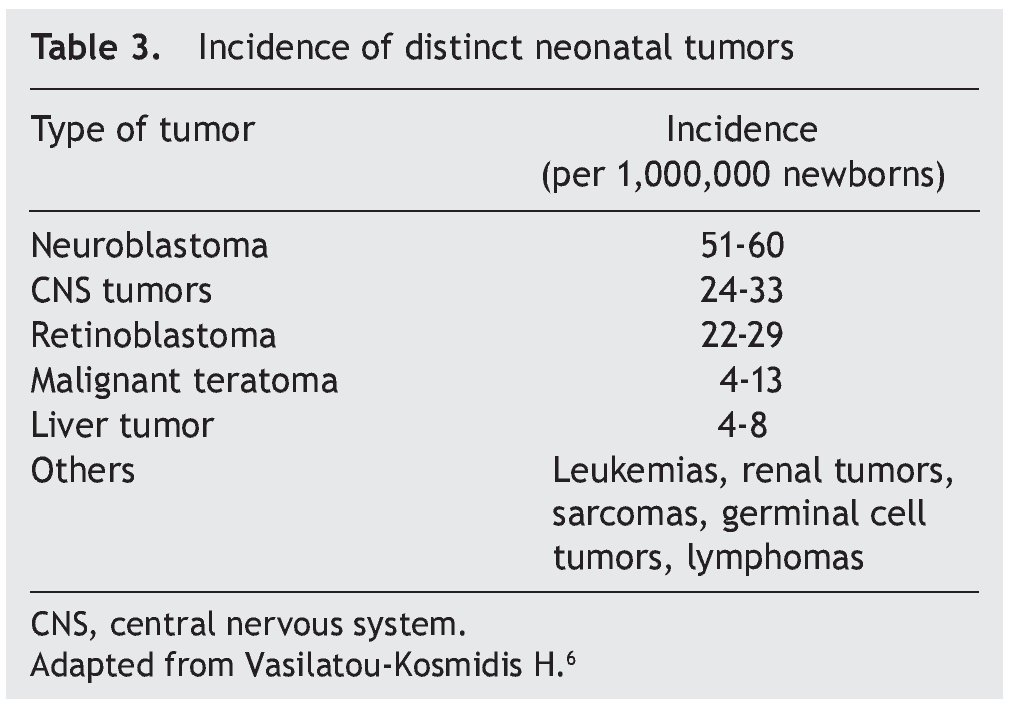
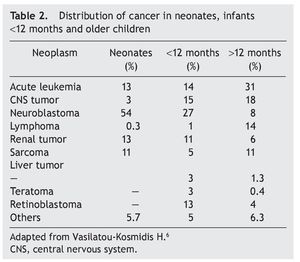
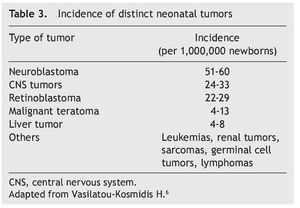
Neoplasms in the pediatric group constitute 2% of their total. Of these, 17% correspond to tumors diagnosed during the neonatal stage, with an incidence of 2.8-3.74 cases per 100,000 live newborns per year. Of the 7000 children diagnosed per year with cancer in the U.S., 10% are diagnosed during the first year of life, 2% during the first month and only 1% in the first day of extrauterine life. According to the report by Vasilatau-Kosmidis in 2003, congenital neoplasms correspond to 1/12,500 live newborns, similar to what has been published in other studies, with different distribution of the types of cancer in accordance with the age of the patients (Table 2) and incidence of different neonatal tumors. (Table 3).5,6
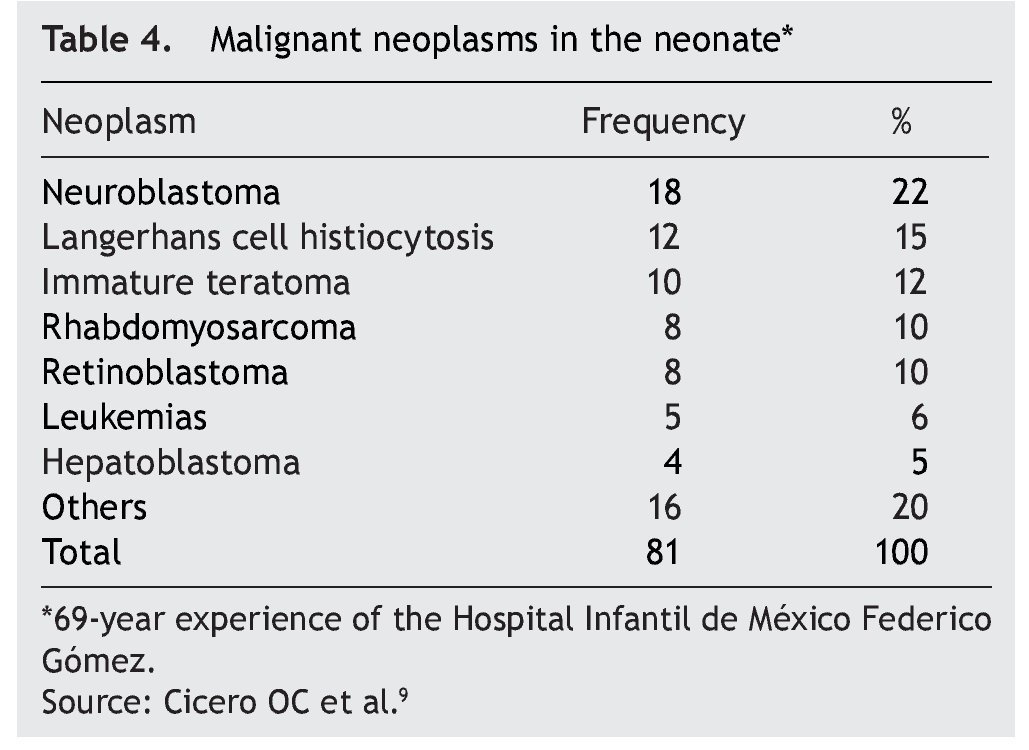
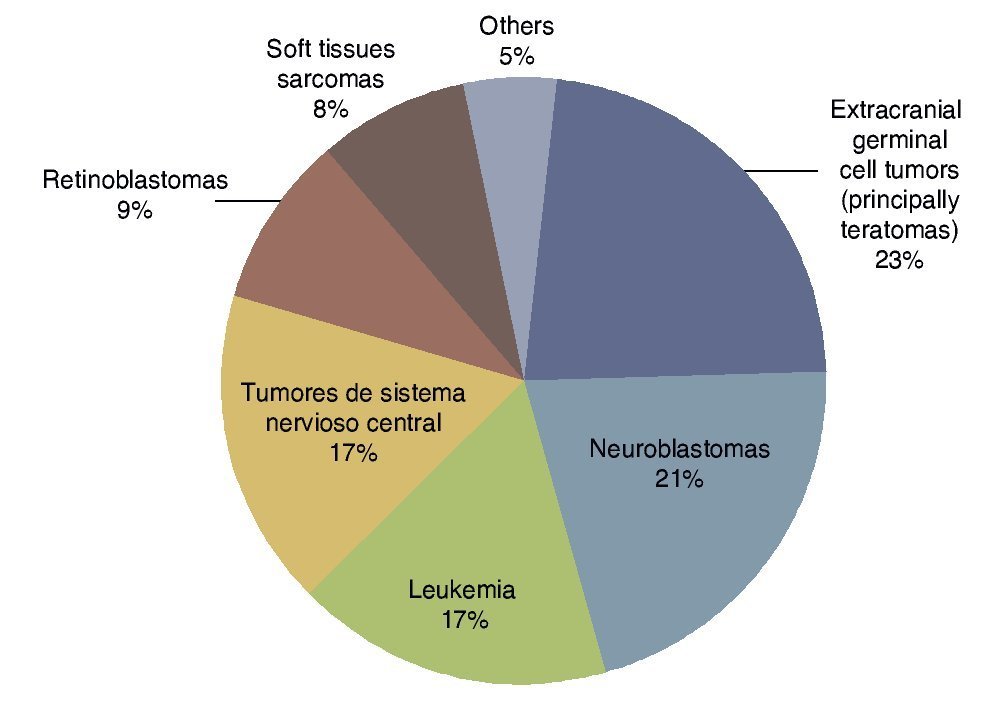
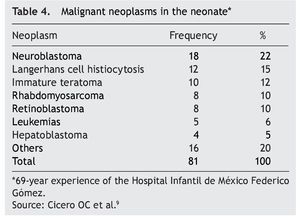
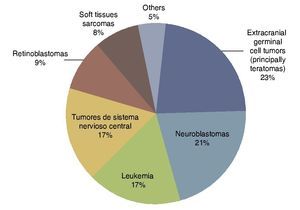
According to the data from the National Cancer Institute’s Surveillance Epidemiology and End Results Program (SEER), the incidence of cancer in children <12 months shows an increase year after year since 1970 up to 15%. There have been variations in the distribution by gender, as the patients of the male gender have had an increase of up to 30% of the central nervous system (CNS), retinoblastomas and neuroblastomas tumors, whereas in females there has been an increase of only 6%, based on liver tumors.7 In the UK, between 1993 and 2007 there were 303 newborns reported with cancer during the first month of life. This results in an incidence of 28 newborns with cancer per million live newborns (Fig. 1).8 Table 4 shows the 69-year experience in the Hospital Infantil de México Federico Gómez (HIMFG).90
Figura 1 Distribution of the types of neonatal cancer in the UK (1993–2007). Source: Vormoor J, Chintagumpala M8.
The increase in the incidence of neonatal tumors observed in this and other studies is based on the increase of solid tumors (teratomas and neuroblastomas). The number of CNS tumors as well as the cases of leukemia has remained constant.10-16 The increase in the diagnosis of these pathologies is related with the increase in use of routine imaging techniques during pregnancy such as fetal ultrasound, as well as the application of screening diagnostic methods.17-19 A clear example of this is seen in Japan and Canada where the use of catecholamines for the diagnosis of neuroblastoma has been standardized.20-23
3. Prenatal diagnosis
With the development of perinatology and maternal/fetal medicine, prenatal detection of many tumors is currently possible such as abdominal, mediastinal and tumors of the CNS.24 Ultrasonography is the first line of approach and can detect up to 70% of fetal anomalies.25 The next step in prenatal diagnosis is fetal magnetic resonance (MR). Fetal echocardiography with Doppler has also been useful for determining cardiac malformations and hemodynamic states secondary to tumor activity or by volume sequestration. It may play an important role in intracardiac tumors. With cordocentesis, fetal cord blood may be obtained for chromosomal analysis as well as for other measurements. Also, with chorionic villous biopsy a karyotype can be obtained, searching for chromosomal microarrays and specific mutations.
It is common in CNS tumors to find polyhydramnios secondary to hypothalamic dysfunction, which decreases swallowing of amniotic fluid. The mass in question is localized by ultrasound or indirect data are found such as hydrocephalus secondary to compression. In one of every five tumors there may be hemorrhage.
Tumors of the face and neck have a wide range of differential diagnosis. Included are the teratomas, lymphangiomas, congenital goiter, thyroid tumors, thymic tumors, neuroblastomas and hamartomas. The most common lesions to acquire a substantial size are the teratomas. Their detection and measurement are important because it allows for planning the resolution of the pregnancy in a third-level care hospital. Airway compromise places life at risk. The EXIT procedure has been described in which a cesarean delivery is carried out and access is gained to the airway while the fetus is under placental circulation. If performed adequately, the placental circulation allows up to 2 h to perform a laryngoscopy, bronchoscopy, tracheostomy and even resection of the tumor.26 Cardiac tumors may be found during routine examination and may also be accompanied by cardiomegaly, pericardial effusion, arrhythmias or heart failure.
When solitary lesions are found, one must consider rhabdomyomas, teratomas or fibromas. It is expected that in a patient with multiple rhabdomyomas, these will evolve over time. However, strict surveillance should be carried out because of the risk of CNS tumors upon finding a probable tuberous sclerosis.27
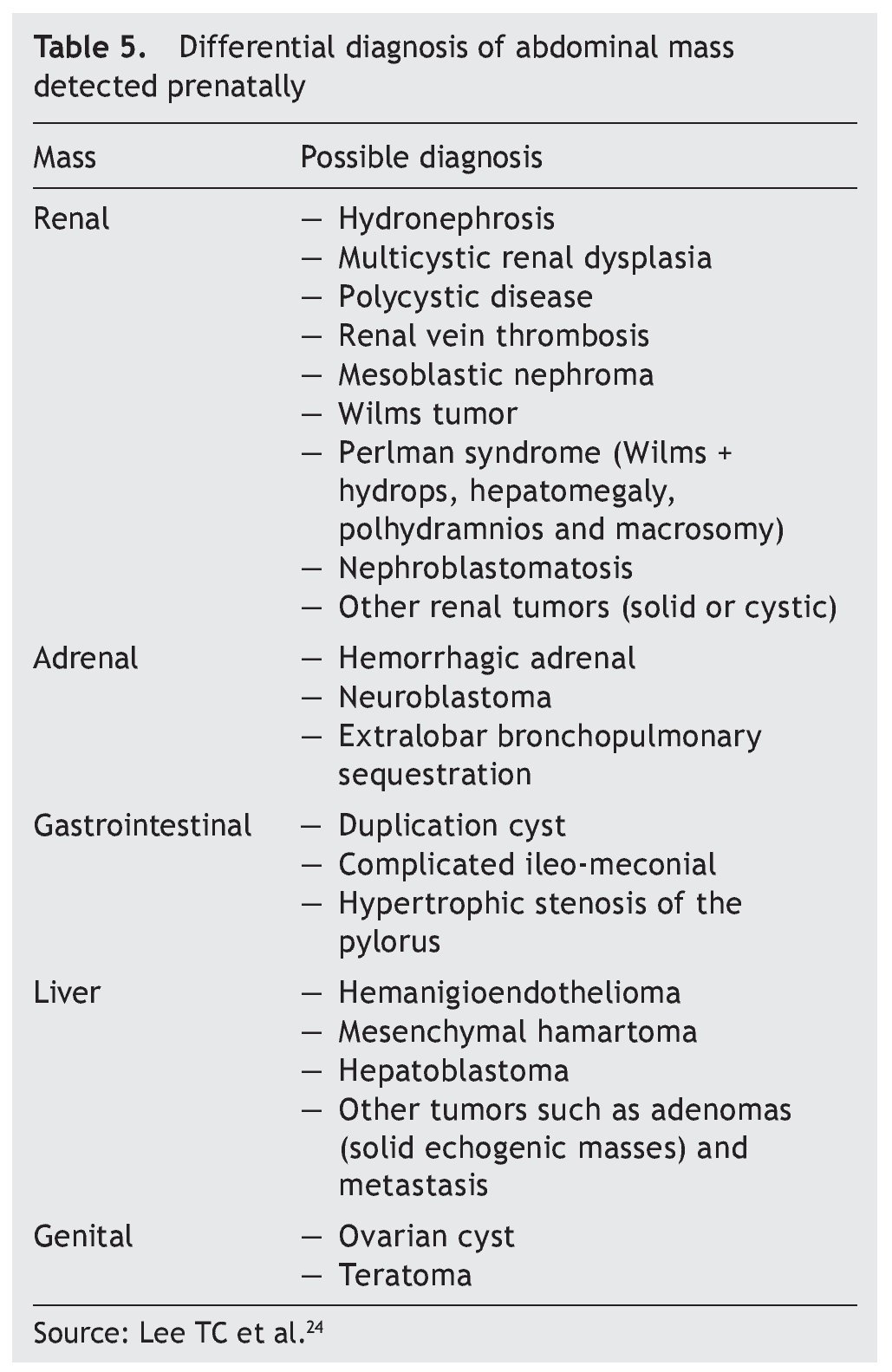
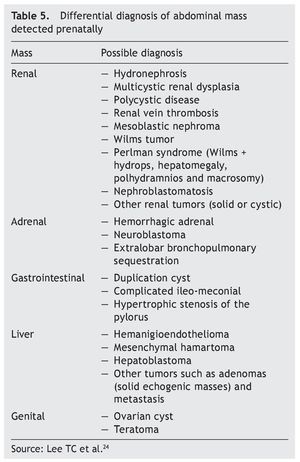
When mediastinal tumors are found, teratomas are considered but they also may be thymic cysts, intrathoracic goiter, neuroblastoma or intestinal duplications. In the case of pulmonary masses, one must determine the histology after birth to differentiate cystic adenomatous malformations, bronchopulmonary sequestration, bronchial atresia, congenital lobar emphysema or, most rarely, pleuropulmonary blastoma or tumor of the interstitial tissue. Table 5 shows the differential diagnosis of an abdominal mass detected prenatally.
In the case of leukemias there are ultrasound findings such as hepatosplenomegaly and fetal hydrops as well as polyhydramnios that help in the suspicion of antenatal leukemia, with the diagnosis being corroborated with fetoscopy and cordocentesis (sampling cord blood and carrying out Wright-Giemsa stain). Fetal anemia can be diagnosed and followed up with a Doppler of the mid-cerebral artery. There are reports of a fetus with Down’s syndrome with prenatal diagnosis of leukemia at 33 weeks gestation.
In cases of cancer of genetic origin such as retinoblastoma, there is a possibility today of carrying out the diagnosis of preimplantation, analyzing the embryos, and performing PCR to detect mutation of the RB1 gene. In this manner the embryos not affected are detected and are transferred to the uterus on day 5.28,29
For detection of neuroblastoma in countries such as Japan and Canada, it was attempted to quantify the number of patients with neonatal screening by measuring the metabolites of catecholamines in the urine.30 It was found that ∼50% of all neuroblastomas that would reach a size detectable by screening would have a spontaneous regression. It was also concluded that screening is not recommended because of the cost-benefit effect; however, in Japan it is still being discussed.
4. Diagnostic difficulties of neonatal tumors
Pathologists generally establish the diagnosis of a malignant disease based on histological criteria, which cannot necessarily be extrapolated to pediatric patients. This happens especially in tumors that present themselves during the first 28 days of life. Normally, the development of organs and tissue show an increase in mitotic activity with the appearance of immature structures that could be confused with malignant neoplasms. Also, during the neonatal period the histological characteristics of a tumor are not always indicative of its biological behavior or of its evolution. Particularly in this age group the location plays a predominant role on patient morbidity and mortality because there are tumors that even though they are not histologically malignant, they may cause death due to the site where they originate as well as the adjacent structures that are affected. It must be noted that there are a large number of diseases that can simulate the appearance of a tumor and which have the same benign characteristics in those patients. Examples of this are diseases with gastrointestinal involvement (intestinal atresia, anorectal malformation, meconial obstructions, cystic fibrosis, lymphangioma, etc.) in up to 15%; genitourinary (hydronephrosis, hydroureter, urachal cyst, urachal diverticulum, multicystic kidney, renal polycystic disease, renal vein thrombosis, hydrocolpos, ovarian cyst, ovarian torsion, etc.) in 55%; adrenals (5%) and hepatobiliary (5%), among others. In a similar manner, some malignant neoplasms can present a benign behavior such as the case of neuroblastoma, even when spontaneous involution is seen.31
5. Pathological etiology of neonatal tumors
The pathological etiology of neonatal tumors differs from what is reported in other stages of life.1,3 In the general population, neoplasms appear during the second half of life as longer periods of exposure and latency are required due to the ability of carcinogenic agents (physical, chemical or biological) to cause mutations in tumor suppressor genes as well as in protooncogenes.32-34 Unlike adults, during the pediatric age there is a greater susceptibility to these agents caused by a greater cell division with less time for DNA repair and greater clonal proliferation, less mutational repair activity, physiological immaturity in hormonal mechanisms, detoxification and immunosurveillance and a greater predisposition to carcinogenic agents in the induction of developmental anomalies.35-37
As mentioned previously, neonatal tumors are composed of embryonic tissue that remains unchanged through time and which makes one think that its genesis could be due to failures in the mechanism of maturation during the first stages of life. Among the principal mechanisms of oncogenesis described in neonatal tumors are those prior to conception where environmental factors (ionizing radiation, non-ionizing radiation, infectious agents, medications, drugs, tobacco, alcohol, diet and parental occupation) could act on germinal cells of the progenitors causing alterations.38 These agents affect the cells of the cells of the organism including germinal cells (eggs and spermatozoids), causing genetic changes before conception. This favors the appearance of neoplasms in their offspring in a variable time period, from the neonatal period until adulthood. In males, spermatogenesis begins during puberty and ends at an advanced age, with a long period of exposure to different carcinogenic agents. On the other hand, in females the ova are formed during gestation so the creation of germinal cells is halted at birth, which produces a short period in which carcinogenic agents may act. For this reason, when epidemiological studies attempt to relate the different environment exposures, and more specifically, those related with prenatal occupations with the pediatric tumors, it was with the fathers and not the mothers where positive results were obtained.39
A special type of preconception oncogenesis is transgenerational. This gives rise to hereditary or familial cancers where the mutation or mutations are inherited with a dominant or recessive character. This significantly increases the appearance of early neoplasms with a greater frequency of lesions in the same organ, bilateral involvement in pair organs and the simultaneous presence of multiple primary cancers.40
Depending on the time that the cells are affected, this can be primarily divided into intrauterine where the changes take place during the time in which gestation lasts and necessarily implies passage of mutagenic substances through the placental barrier towards the fetus, causing a subsequent neoplasm during a variable period of time (even up to adulthood).41,42 Some toxic, mutatogenic, and carcinogenic agents cause different effects according to gestational stage in which they act: causing abortions (in the first weeks), malformations (between the second and eighth weeks) or tumors (between 6 and 40 weeks).
The second stage corresponds to the maternal-fetal transmission of tumor cells. In this case there is no mother-child transmission of the mutations as such. For the most part, the placenta acts as a barrier that prevents the passage of metastatic tumor cells from the mother to the fetus and vice-versa. Metastasis of this type has been rarely described in melanomas, choriocarcinomas, lymphomas, bronchogenic carcinomas and breast epithelioma. Finally, there is the stage of postnatal presentation where the short period of exposure and latency makes one think that during this stage there is no oncogenesis.
Another very peculiar characteristic of neonatal tumors is the term used for the first time by Bolande in 1985, known as “grace period of neonatal oncology”.35
It refers to the fact that the majority of the histological types of neonatal tumors have a less aggressive biological behavior than in later stages of life, so that age per se is an important and independent prognostic factor for tumor type, histological pattern and extension. This is due to two different phenomena that cause partial or complete disappearance of the tumor: spontaneous regression (total disappearance of tumor cells) and cytodifferentiation (malignant or benign cell maturation).
5.1. Risk factors
Neoplasms originate due to the variable combination of two types of determinants: genetic and environmental. Environmental factors are responsible for 98% of all cancers. Carcinogenic agents are classified into chemical, physical and biological. For their effect, long periods of latency are required (from a few years up to decades) for neoplasms to develop. At least two factors influence in this variability: first, the greater or lesser genetic susceptibility for developing cancer and, second, that a minimum of five to six mutations of protooncogenes and tumor suppressor genes are needed for the malignant transformation of a cell. Most pediatric cancers are developed after brief periods of time. In up to 40% they present during the first 4 years of life. The majority of authors associate genetic risk factors with 4-10% of infantile tumors. Environmental factors are responsible for the remaining cases, which explains the short period of latency for its action during pregnancy and prior to pregnancy on morphological and functionally im mature cell tissues.43,44
5.2. Genetic factors associated with pediatric cancer
Genetic syndromes are the cause of 1-2% of all cancers, with a greater frequency in the pediatric age (4-15%). Strictly speaking, cancers developed in patients who are carriers of specific mutations of the germinal cells are called hereditary or genetic and are therefore present in all the remaining somatic cells. These pathologies can be divided, according to their manner of inheritance, as recessives (ataxia telangiectasia, Fanconi’s anemia, xeroderma pigmentosum, Bloom sydrome), dominant (Li-Fraumeni syndrome, familial retinoblastoma, neurofibromatosis, familial Wilms tumor, familial neuroblastoma, multiple endocrine neoplastic syndrome, tuberous sclerosis, von Hippel-Lindau syndrome) or nonhereditary chromosomal (Down syndrome and sexual chromosomal aberrations).1
5.3. Environmental factors associated with pediatric cancer
Various factors have been associated with cancer in pediatric age although study results are controversial. Among the most studied factors are ionizing radiation,1,45-4 non-ionizing radiation,49-53 infections,54 medications, diet,55,56 smoking,57 alcoholism,58,59 work-related parental exposure (especially paternal exposure to pesticides, solvents, paints and work-related with motor vehicles have all been associated with a greater number of CNS tumors).60,61
6. Treatment of neonatal tumors
Physiology of the neonate requires that the necessary multidisciplinary treatment in neoplastic diseases be modified substantially in order to avoid toxicity and sequelae.62,63 Surgical treatment is most often used, which requires preoperative preparation and stabilization as well as advanced postoperative care.31 It is frequently followed by chemotherapy, which requires modification of the dose and route of administration of the different chemotherapeutic agents. Absorption, biotransformation and excretion of these drugs differ from that of other ages. As an example, decrease of gastric pH prolongs gastric emptying and the constant changes on mesenteric and peripheral vascular resistances make blood flow variable, modifying absorption and distribution of the drugs, whether administered orally or intravenously. The liver and renal immaturity alter the rate of elimination of the different chemotherapy agents (methotrexate, cisplatinum and cyclophosphamide and vincristine and actinomycin D, respectively). Plasma proteins, especially albumin, which normally transport and modulate the toxicity of the chemotherapeutic agents (methotrexate, 6-mercaptopurine, prednisone), tend to be saturated by the greater affinity and excess of bilirubin and free fatty acids, altering the distribution and increasing toxicity.64 The relative increase of the extracellular volume and the fat excesses in the newborn produce an increase in the bioavailability of many chemotherapeutic agents. To maintain the effectiveness and minimize toxicity, some authors and specialized medical centers use half of the dose normally recommended in pediatrics and calculate according to weight (in kg) instead of its classic calculation by body surface. Radiotherapy is normally omitted because it damages healthy tissues, causing serious short-term problems as well as deformities and greater oncogenic power.36
7. Types of neonatal cancer65
7.1. Fetal and newborn germ cell tumors
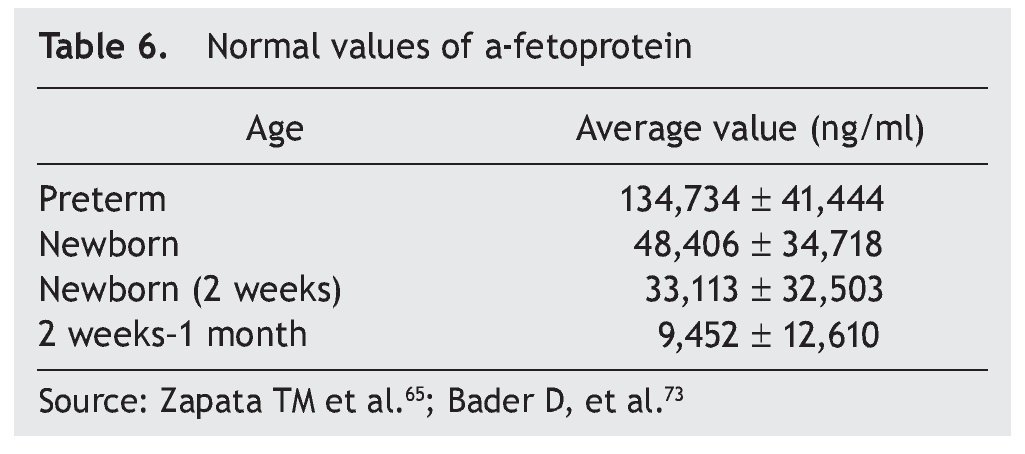
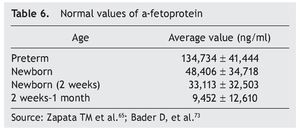
Germ cell tumors are a challenge because their intrinsic pluripotentiality results in a broad histological spectrum and biological behavior. They currently represent (in competition with neuroblastoma) the most common type of neonatal tumor. Due to its size, location and complexity, optimal management requires a multidisciplinary team of obstetricians, neonatologists, surgeons, radiologists and oncologists.66 Approximately 95% of germ cell tumors are composed of mature or immature teratomas, curable only with surgery. Alpha-fetoprotein is a reliable marker for the detection of disease recurrence for initiating adequate management (Table 6). The need for negative surgical margins is controversial because often they can be difficult to achieve and recurrences are infrequent (even in stage 2). If chemotherapy is necessary, a scheme based on carboplatin should be given, adjusted and minimized to avoid toxicity. In the rare instance of a newborn with metastatic disease, the behavior to follow is neoadjuvant chemotherapy after diagnostic biopsy.
7.2. Neuroblastoma
The second, and in some series, most common cancer in the newborn period is neurobastoma.67 In general it has a good prognosis, especially in those cases without amplification of the MYC oncogene. Cases in which there is amplification (regardless of stage) they should be treated as high-risk patients with an intensive regimen of chemotherapy. Clinically and radiologically the presentation is similar to other ages, and symptoms related with the production of catecholamines and radiological presence of calcifications is observed. Timely diagnosis and urgent treatment are fundamental in cases that present with spinal compression from spinal mass or coagulopathy due to hepatic infiltration in metastatic disease. Metastatic disease may have spontaneous regression in this age group, so it is often managed with observation. Surgical approach should be conservative and carried out when there are well-established risk factors. With regard to Pepper syndrome (localized primary tumor with metastasis limited to the liver, skin and bone marrow), there is a high mortality during this stage of life.
7.3. CNS tumors
Prenatal diagnosis of these tumors has currently increased. Neonatal symptoms differ from other stages of life. Due to open skull sutures, it is noted that the most common sign or symptom is increase in head diameter without secondary intracranial hypertension. Hydrocephaly should be considered as an alarm sign. Focal neurological signs are rare in newborns. Teratomas are the most common histological type followed by astrocytomas, medulloblastomas, choroid plexus tumors and craniopharyngiomas. Tumors of the choroid plexus (generally papillomas) have a better prognosis and in some cases aggressive surgery is curative. The remaining histological types have a poor prognosis although some strains may respond to aggressive surgery and chemotherapy. Participation of a multidisciplinary team is necessary because neuroendocrine complications are common.68,69
7.4. Newborn leukemia
Newborn (or congenital) leukemia is that which is diagnosed in the first 30 days after birth.70 Its incidence is reported to be 1-5 per million live newborns (<1% of the total leukemias). The experience of various countries over 20-to 50-year periods comprises only a few dozen cases. The international literature reports the incidence of myeloid leukemias from 56% to 64% and lymphoid from 21% to 38%; 50% of myeloid leukemias are monoblastic (M5). Among the clinical findings for diagnosis are hepatosplenomegaly (80%), hyperleukocytosis (85%), cutaneous leukemia (60%). All patients should have a differential diagnosis done for infections by the TORCH complex, group incompatibility, and other cancers such as neuroblastoma and histiocytosis of Langerhans cells, etc. The international literature has described a survival rate of <10% for lymphoids and 25% for myeloids.
7.5. Transitory myeloproliferative syndrome of the newborn associated with Down’s syndrome
This condition represents an uncontrolled proliferation of myeloblasts that occasionally may be difficult to differentiate from newborn or congenital leukemia, although this syndrome tends to resolve spontaneously within the first 3 months of life. It is caused by mutations in the coding gene of the GATA 1 protein when it produces a truncated protein as it alters the regulatory function of megakaryopoiesis.71 The majority of newborns do not require treatment.
7.6. Renal tumors
The most common tumor type is congenital mesoblastic nephroma (60%), which may be confused with Wilms tumor. Mesoblastic nephroma is not encapsulated and infiltrates normal renal parenchyma. It should be surgically resected as it is thought that it can progress to Wilms tumor if not completely removed. In fact, there have been reported cases of metastasis of this tumor. In second place is Wilms tumor (20%) followed by rhabdoid tumor (11%) and clear cell sarcomas. These tumors have a good prognosis when managed by expert oncologists, oncological surgeons and radiotherapists. Therefore, when such a tumor is suspected the patient should immediately be referred to the appropriate specialist.14,72
7.7. Liver tumors
The most common are the hemangioendotheliomas. These tumors are often accompanied by similar skin lesions, which typically regress after 1 year. In turn, this association is related with hypothyroidism as a result of the tri-iodothyronine deiodinase in these tumors. Asymptomatic lesions are managed conservatively. In lesions with hemodynamic involvement, treatment with propranolol, corticosteroids, and vincristine is provided. Hepatoblastoma represents only 1% of pediatric neoplasms.12,13 Of this percentage, ∼10% occur in the newborn period and are more common in premature infants with low birth weight. Treatment includes surgical resection with or without prior chemotherapy and consolidation with chemotherapy. α-Fetoprotein is a marker for this neoplasm (Table 6).73
7.8. Retinoblastoma
Retinoblastoma is the most common intraocular malignant tumor. Its actual incidence in the newborn is unknown. It may be sporadic or hereditary. Unilateral cases may be sporadic or hereditary, whereas bilateral cases are always hereditary; 90% of the cases are sporadic. The most common symptoms are leucocoria followed by strabismus, orbital inflammation, hyphema, fixed pupil and heterochromia of the iris. It is important to mention that, during the neonatal period, strabismus and inflammation of the orbit could appear as “normal” alterations, for which eye fundus examination with dilatation of the pupil is mandatory at birth. Diagnostic biopsy is strictly prohibited due to the risk of spreading the tumor. Computed tomography should be avoided due to the risk of a second neoplasm. Treatment includes many modalities such as systemic chemotherapy, laser, cryotherapy and brachytherapy. Enucleation is offered when there are few possibilities of preserving the eye.74
7.9. Soft tissue sarcomas
Vascular tumors are the most common and are generally benign although life-threatening complications may occur. Benign tumors can have a clinical and radiological appearance similar to malignant tumors, making the differential diagnosis difficult. Infantile fibrosarcoma is typical of the newborn stage and is considered to be a tumor of intermediate prognosis. Frequently, neoadjuvant chemotherapy free of alkylating agents and anthracyclines are offered to reduce the tumor for its subsequent surgical resection. Rhabdomyosarcoma is a severe form of cancer that requires multidisciplinary management. The prognosis in the newborn stage is poor.75
8. Prognosis
Diagnosis of a tumor in the neonatal period is not synonymous with death because apart from the cases that respond adequately to surgery or chemotherapy, there are tumors that present spontaneous regression or maturation (cytodifferentiation). Survival rate has improved over time. Patients with neuroblastoma have the highest survival rates recorded; however, death occurs in almost all patients with diagnosis of cerebral tumors and leukemias.5
Neoplasm in the newborn stage represents a group apart from that presented in the pediatric and adult age due to its peculiarities in incidence, degree of histological differentiation, therapeutic response and prognosis. For diagnosis, experience of the pediatric medical oncologists as well as the entire diagnostic team is necessary (inclu ding other specialties) and, of course, the pathology service with extensive knowledge of neonatal tumors and histology. Diagnosis represents a challenge due to the complexity of the anatomy and physiology of the newborn. From a pathological biological point of view, tumors of the newborn represent important models for the study of human oncogenesis and offer important information with respect to the pathophysiology of cancer in regard to genetics and patterns of inheritance.
Although oncogenesis is not completely elucidated, there are important associations with variable degrees of correlation. There are also risk factors that must concur to create the diagnostic possibility of these pathologies, forging medical awareness of the existence of malignant tumors in the neonatal stage. When an early diagnosis is achieved, there are effective treatment options to improve patient survival. Because these are rare diseases, the cumulative experience of many tertiary care centers is required so as to create an optimal level of management in these patients.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 23 May 2013;
accepted 30 May 2014
* Corresponding author.
E-mail: magazapata@yahoo.com (M. Zapata-Tarrés).