El autismo, hoy en día definido como trastornos del espectro autista, fue descrito inicialmente en 1943. Se caracteriza por alteraciones en la comunicación, la interacción social y un espectro restringido de intereses del paciente. Generalmente se identifica en etapas tempranas del desarrollo a partir de los 18 meses de edad. Actualmente el autismo se considera un desorden neurológico con un espectro que abarca diferentes grados que se asocian con factores genéticos, no genéticos y del medio ambiente. Dentro de los factores genéticos se han referido diversos síndromes relacionados con este trastorno. Asimismo, el autismo se ha estudiado a nivel genético, neurofisiológico, neuroquímico y neuropatológico. Las técnicas de neuroimagen han mostrado múltiples anormalidades estructurales en estos pacientes. También se han observado alteraciones relacionadas en los sistemas serotoninérgico, GABAérgico, catecolaminérgico y colinérgico. En este trabajo se presenta una actualización de la información de los aspectos genéticos y neuroendocrinos del trastorno del espectro autista.
Autism spectrum disorder (ASD) was described in 1943 and is defined as a developmental disorder that affects social interaction and communication. It is usually identified in early stages of development from 18 months of age. Currently, autism is considered a neurological disorder with a spectrum covering cases of different degrees, which is associated with genetic, nongenetic and environmental factors. Among the genetic factors, various syndromes have been described that are associated with this disorder. Also, the neurobiology of autism has been studied at the genetic, neurophysiological, neurochemical and neuropathological levels. Neuroimaging techniques have shown multiple structural abnormalities in these patients. There have also been changes in the serotonergic, GABAergic, catecholaminergic and cholinergic systems related to this disorder. This paper presents an update of the information presented in the genetic and neuroendocrine aspects of autism spectrum disorder.
1. Introduction
Autism today, defined as autism spectrum disorders (ASD), was initially described by Dr. Leo Kanner in 1943. It is characterized by disorders in social interaction and communication along with a restricted spectrum of interest of the patients. Diagnosis is done in an infantile stage. Patients are described as distracted children who have an unusual manner of relating, with scant language or little communication, and attached to routines. Children present repetitive strange behaviors, unusual ways of playing and lack of emotional reciprocity towards people.1 At present, autism is considered to be a wide-spectrum neurological disorder that encompasses cases of different degrees associated with genetic and environmental factors whose manifestation is variable. It is generally identified in early stages, from 18 months of age. Moreover, it has been proposed that alterations in multiple genes in combination with the presence of non-genetic factors are the cause for the development of the phenotype corresponding to autism, which represents, in itself, a set of atypical genetic alterations that generate a same phenotype.2
2. Diagnosis
The diagnosis of autism, or ASD, has as a basis the clinical study. Until now a totally reliable biological marker has not been identified.3 However, and based on the different symptoms indicative of ASD, different specialists have suggested a comprehensive strategy, structured and systematic, both for diagnosis as well as for treatment, so as to identify the different abilities and specific limitations of each patient with ASD.4 Some of the symptoms present in autistic patients are also present in children with mental disability without autism. For example, patients with autism may demonstrate different degrees of cognitive deficit and, in turn, patients with intellectual disability may develop stereotypes and difficulties in communication characteristic of patients with ASD.
However, this poses a problem for the accurate diagnosis of autism.5 Different tests are required for the timely diagnosis of this set of pathologies. For this, medical specialists, as well as field researchers, have designed various questionnaires. In addition, there are the criteria established by the American Association of Psychiatry in the Diagnostic and Statistical Manual of Mental Disorders or DSM-V-TR, recently published, in which the diagnostic criteria have been improved by consensus and simplification. Also, integrated into this manual are the last findings derived from genetic and neuroimaging studies, specific for each of the ASD. Similarly, symptoms that present themselves and encompass various categories of the diagnosis are recognized and detailed, thus broadening the clinical perspective. Specific criteria have been consolidated and include autistic disorder, Asperger syndrome and generalized developmental disorders along the autistic spectrum. Finally, classification of other disorders has been optimized such as bipolarity and depression, all this in order to contribute consistently with the diagnosis in clinical practice.6
The specific criteria for the diagnosis of ASD are derived from three domains.
Impairment of social interaction: a) impediment of the use of nonverbal communication such as eye contact, facial expression, and body posture; b) inability to develop relationships with age peers; c) inability to share or communicate affect and interests with other persons; d) limited interest or notion on reactions and emotions of others.
Qualitative alterations in communication skills: a) delay or lack of language acquisition; b) inability to initiate or maintain a conversation; c) language use in a stereotypic or repetitive manner or use of idiosyncratic language; d) nonexistent development of imitation or pretend games appropriate for age.
Presence of restrictive or repetitive patterns of behavior; a) exacerbated preoccupation by a restricted number of unusual interests; b) inflexible adherence to certain habits or routines; c) motor stereotypes; d) preoccupation or exaggerated attachment to parts of objects.
Diagnosis of autism is confirmed when the individual has a total of six or more behaviors from the three domains mentioned, including at least two from the first domain. Once the diagnosis is made, the confirmation and severity of the symptoms present is important. For this, a second questionnaire is generally applied, such as the CARS that is made up of 15 questions, each one with seven possible responses.5
Longitudinal studies in children at high risk of developing ASD by having an affected older sibling as well as the retrospective analysis of videos of children with 1 year duration of this disease confirm that in some of these patients the characteristic symptoms can be identified from 6 and up to 12 months of age,7 with the mentioned symptoms being more evident from 18 months of age.8 It is important to mention that even when the literature mentions as initial symptoms the diverse alterations in language and communication, the above-mentioned longitudinal studies have demonstrated that other not-so-characteristic symptoms can be identified before 6 months such as irritability, hypo- or mid hyperactivity, hypo- or hyperactivity, as well as deficit in developing coarse movements.9 Therefore, it is important that first-level care physicians are aware of the signs within the symptomatic spectrum that a child with ASD may have so as to be able to carry out, in the first instance, the pertinent screening tests followed by diagnostic confirmation as early as possible.
3. Prevalence
Epidemiological studies in the international literature have identified patients with ASD in the countries included, with similar degrees of prevalence.10 Autism disorder is estimated in 2/1000 individuals.4 Currently, there are ~1/175 children worldwide born with this disorder although the frequency varies in each country. In the U.S., based on data from the Community Report from the Autism and Developmental Disabilities Monitoring Network of the Centers for Disease Control and Prevention, it is indicated that one of 68 children is diagnosed with autism. Males have a five times greater risk of developing ASD than females. In a similar manner, data from this report indicate that the majority of the diagnoses are made after 4 years of life, and the prevalence is greater in Caucasian children than in blacks or Hispanics.11 For 2013, an estimate of the prevalence for autism in Mexico was 1/300 children. One can predict at least 115 million children with autism in Mexico, with a risk factor of 6200 new cases per year.12
4. Genetics of ASD
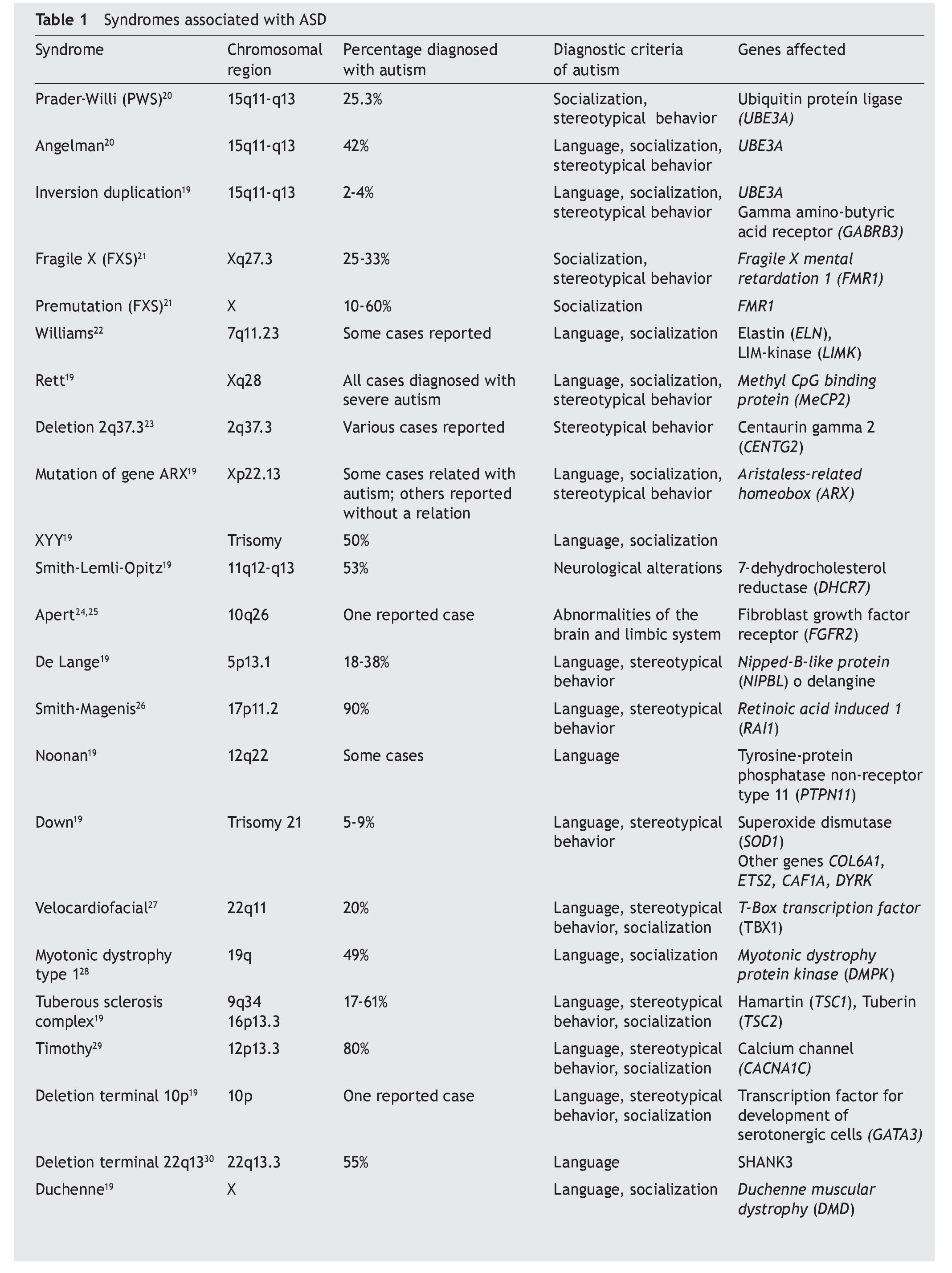
Autism takes into consideration genetic alterations that tend to be heterogeneous, among its principal causes. These alterations present themselves according to different levels of the organization of the genetic material. Genetic material during cell division is organized in superstructures called chromosomes, which demonstrate the so-called chromosomal alterations in which macro- or microdeletions, duplications, insertions and inversions of the genetic material may take place. It should be noted that even at the molecular level there may exist de novo point mutations in the DNA sequence that alter genes or promoters and affect genetic expression. Many of these are related with the development of the nervous system. The principal syndromes related with autism are mentioned below.
4.1. Prader-Willi Syndrome (PWS)
PWS is the consequence of a deletion of chromosome 15, region q11-q13, whether due to mutation of the paternal gene or unilateral disomy of maternal origin, i.e., when the paternal allele is not expressed or there is an alteration in the methylation pattern. This syndrome presents itself in 1-4% of cases of autism, and its symptoms are hypotonia, intellectual disability, obesity, food cravings, obsessive-compulsive disorder, and low sociality. These are individuals who talk excessively and have high levels of oxytocin.13
4.2. Angelman Syndrome
This syndrome is seen in 2-4% of autistic persons, and the same region as in the PWS is found to be affected, although the alteration comes from the maternal side. There could be an inversion, duplication or mutation of gene UBE3A/E6AP. This gene codifies for ubiquitin E3 ligase that participates in the pathway of degradation of proteins in the neurons. Individuals who are carriers of this alteration present hyperactivity, flapping of hands, convulsions, intellectual disability, epilepsy, strabismus and very low language ability. They may also have cryptorchidism or microcephaly.14,15
4.3. Fragile X Syndrome
With an incidence of 4-8% in patients with a diagnosis of autism, this syndrome is characterized by intellectual disability, macroorchidism, perseverating and repetitive language, poor eye contact and characteristic facial dysmorphias. The alteration of the gene FMR1 (fragile X mental retardation1) located in chromosome X usually comes from a demethylated state from the maternal side, and the conditions are principally found in males. A series of repeated trinucleotides (CGG) (5-45 times) at the 5’ end of the mRNA are found in this gene, which regulates the gene translation. However, duplications of this region could increase to > 200 repetitions of trinucleotides, causing the syndrome. This increase of trinucleotides in mRNA of the gene prevents its translation and because the junctional RNA protein that codifies negatively regulates the gene messengers that modulate the synaptic plasticity, intellectual development is seriously affected.16
4.4. Timothy Syndrome
Spontaneous mutations of the CACNA1C gene have been detected, which interfere with functioning of the calcium channels. This gene is located on chromosome 12, region p13.3. In addition to the autism phenotype, lethal arrhythmias, congenital heart disease, immune deficits, hypoglycemia and cognitive deficits present themselves.17
4.5. Rett Syndrome
Rett syndrome principally affects females. In the case of male heterozygotes, it is lethal. In this syndrome the MeCP2 gene is found mutated in the long arm of chromosome X. This gene is related with neuronal development. This syndrome is characterized by a severe autism phenotype, psychomotor regression, stereotypic movements, ataxic walk and lack of social interaction. The MeCP2 protein is responsible for “silencing” the methylated chromatin in the cytokines of the CpG pairs.18
4.6. Other genetic alterations
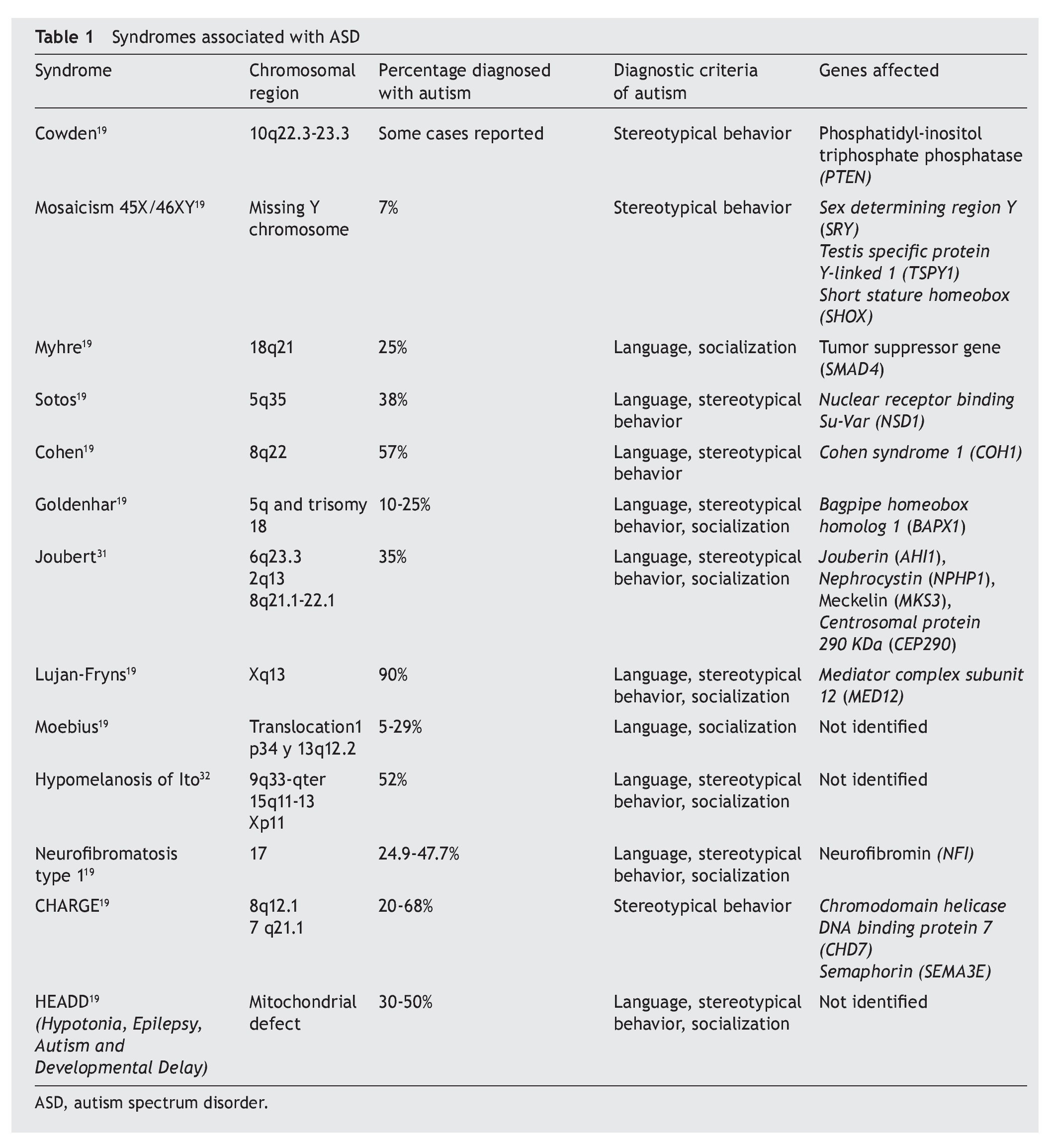
In general, autism is associated with various syndromes in which there are changes in behavior, language development or socialization, for which the genetic diagnosis serves to detect if the individual suffers from any of these syndromes (Table 1).19-32 In addition to the symptomatology of the syndrome itself, a percent of cases are diagnosed with autism if the diagnostic criteria prevail.33
Many of these syndromes also have components such as mental delay, epilepsy and cardiac alterations, among others. In this regard, autism also has a high percentage of mental retardation (75%) and an epilepsy component to a lesser percentage (42%). The majority of the mutations related with autism correspond to genes that participate in neuronal development and synaptogenesis.
As for the association of autism with epilepsy, mutations in genes involved in the excitatory system (glutamate) and neuronal inhibitor (GABA) have been identified. The gene for the glutamate receptor type 6 (mGluR6) is found to be in linkage disequilibrium in some individuals with autism, i.e., they do not segregate independently and have low recombination because the two loci involved tend to be on the same chromosome. On the other hand, decrease of enzymes of the GABAergic system and availability of GABA in autism has been detected. Also, alterations in the 15q11-13 region include genes of the GABAA receptors. The family of neuroligines (NLGN1, NLGN2, NLGN3, NLGN4X and NLGN5, genes distributed in chromosomes 3, 7, X and Y) also play a relevant role in synaptogenesis and disequilibrium between neuronal inhibition and excitation. Studies of association of polymorphisms in NLGN3 and NGLN4 with autism have not found a clear relationship. However, isoforms of these genes have been found related with autism.34 Genes described in some syndromes are also factors that trigger epilepsy in patients with autism such as CDK5, FMR1, ARX (aristaless-related homeobox, implicated in brain development, proliferation of neuroblasts and migration of GABAergic interneurons), and the MeCP2 gene which, in turn, regulates DLX5. Also, other genes with mutations that encode for subunits of the voltage dependent SCN1A (alpha 1) and SCN2A (alpha 2) neuronal sodium channel trigger convulsive crises in autism.35
Tuberous sclerosis complex is produced by mutations in TSC1 and TSC2 genes. In it, symptoms such as epilepsy, autism and neurocognitive disorders present themselves. TSC1 and TSC2 proteins modulate cell growth mediated by signaling pathway of mTOR, which also modulates synaptogenesis.36
Language development is one of the critical components of autism. Various genes related with language are found to be altered. The alterations described have been localized in the loci AUTS and include genes implicated in brain development. The locus AUTS3, localized in the region 13q13.2-14.1, contains genes for neuronal migration and development (NBEA, MAB21L1, DCAMKL1 and SMAD9). The locus AUTS1B (7q31) has at least two genes that are associated with autism. Among the candidate genes that affect the development of the central nervous system, WNT2 (7q31-33) is studied and are expressed in thalamus and FOXP2, which regulate genes for language and speech development. In this same locus are the MET genes, whose alternate promoter reduces in half its expression in autism, which affects maturation and growth of the neocortex (“new cortex” or the “most recent cortex”).
In 7q35, another gene crucial for language development is found, the CNTNAP2 of the protein associated with contactin of the family of neuroxins. Manifestations of symptoms of autism were observed in mice that do not express this gene. In a similar manner, decreased expression of this gene due to variations in its promoter region or loss of methylation sites presents in some autistic individuals. However, alterations in this gene are related with a large number of neuronal developmental disorders.37
The locus AUTS1A (7q36) contains the gene EN2 whose mutation implies the reduction of Purkinje cells and cerebellar hypoplasia. The long arm of chromosome 2 contains the locus AUTS5, which is linked to delay for construction of phrases and from which the responsible gene has not yet been identified, but it has been associated with polymorphisms of the gene RAPGEF4 in 2q31-32. The region 15q11-13 with the locus AUTS4 contains the genes UBE3A, ATP10A, GABRB3, GABRA5, GABRG3. It is also subject to disorders in its pattern of methylation (genomic imprint) that implies a language disorder.38
The genetics of autism reveal the participation of genes implicated in the development of the central nervous system and the implications for language development, socialization, behavior and even neuronal disorders. The new molecular tools such as the identification in the variation of the number of copies, the de novo mutations, microarrays of genetic expression, genome sequentiation and massive sequentiation will allow for the examination of the majority of cases of idiopathic autism.39 Identification of genes related with the development of language, socialization and behavior will allow for establishing the interactions between them and to establish the molecular mechanisms that participate in autism.40,41
5. Neuroendocrine aspects of ASD
ASD are disorders in neurodevelopment characterized by alterations in social interaction, communication and repetitive behavior. These disorders affect 1% of the population and its prevalence is greater in males. Because of this, the majority of the studies have been carried out in males. The neurobiology of autism has been studied at the genetic, neurophysiological, neurochemical and neuropathological levels. Neuroimaging techniques have demonstrated multiple structural abnormalities, but these are not very consistent. Alterations have been found in the serotoninergic, GABAergic, catecholaminergic, and cholinergic systems, among others, although without specificity or diagnostic value.42 Bauman and Kemper, in 1985, showed the neuropathological findings of a 29-year-old male.43 In 1998 they completed a series of nine cases, of which four suffered from epilepsy and five from intellectual disability, without obvious malformations and normal myelinization. Compared with non-autistic subjects they demonstrated a reduction in neuronal size and increase in cellular density in the limbic and cerebellar system. Also, they observed a decrease in the extension of the dendritic branches in pyramidal neurons CA1 and CA4 of the hippocampus.44
Recent studies have established that the head circumference in autistic neonates is normal at birth, but at 2 years of age they show elongation of the head, and at 3 to 4 years it increases ~5-10%.45,46 This increase in head circumference has been associated with a decrease in cortical layers and maturation of the cortex. Another theory proposes that there is a secondary response to events of neuronal remodelation that induces overgrowth.46 There also exists a dysfunction in cortical areas, including the frontal lobe, temporal lobe and cingulate cortex, affecting and promoting problems of attention and of executive function responsible for planning and organization, resulting in the lack of autonomy and decision making as well as dependence of autistic persons.47 Others authors relate the dysfunction of the hippocampus and amygdala (the medial temporal lobe structures), which affects memory or recognition and verbal encoding, depending on the severity of the autism.48
Studies conducted by Lai and colleagues at the Center for Autism Research at Cambridge University have suggested that autism affects different parts of the brain in women and in men. Using magnetic resonance imaging, they found that the anatomy of the brain of a person with autism differs depending on the gender.49 This could implicate physiological mechanisms that lead to a sexual dimorphism such as prenatal sex hormones and genetic mechanisms linked to gender. Because the frequency of autism in females is less than in males, this difference is an important example of the diversity within the “spectrum.”50
The importance of some neurotransmitters has been demonstrated, such as serotonin, in behavior disorders. In hyperkinetic children who have low plasma levels of serotonin, it has been proven that their clinical improvement depends on increase of serotonin. In the same manner, Schain and Freedman51 linked a high concentration of serotonin (26%) in autistic subjects studied. These levels of serotonin decreased when tryptophan content was restricted (aminoacid precursor) in the diet. Based on this, a common phenomenon obser ved in subjects with ASD is hyperserotoninemia.52 However, treatment with selective serotonin reuptake inhibitors (SSRIS) such as fluoxetine, paroxetine, fluvoxamine, and venlafaxine showed positive effects in the stereotype of repetitive behaviors, lack of social skills and in problems of communication.53
On the other hand, the dopamine system has been related with functions of analysis, planning and execution and also with motor activities, social and perception behaviors. Barthelemy et al. analyzed the urine levels of catecholamines in autistic subjects and found low levels of dopamine and high levels of noradrenaline, which induce passive behaviors as seen in autistic subjects.54 In the brain, children with autism showed weak connections in the areas that release dopamine in response to rewards compared with children without autism. In the left lobe of the brain, the autistic children showed weak connections in the nucleus accumbens and ventral tegmental area. On the right side there was a weak connection with the amygdala, which processes emotional signals.
In individuals with ASD with treatment of dopamine antagonists such as haloperidol and risperidone, which are antipsychotic medications, there has been an improvement observed in behavior of irritability and hyperactivity.55
On the other hand, in postmortem tissues of autistic subjects, a decrease of GABAergic connections in Purkinje cells was found in the cerebellum.43 Recent studies showed a decrease in (GABAA and GABAB) receptors and proteins in cerebellum and cortical areas, suggesting a deregulation of the GABA inhibitory system in autism, which affects the regulation of circuits and of behavior.56,57
6. Errors in metabolism in ASD
The principal metabolic alterations of an autistic phenotype are phenylketonuria, alterations in the urea cycle, alterations in purine metabolism and deficiencies of the enzyme succinate semialdehyde dehydrogenase. Some authors have found hyperuricemia in patients with intellectual disability and with personality disorders as well as other biochemical defects that could be caused, not only by the insufficient ingestion of its precursors, but also by a defective absorption such as can be seen in celiac disease, which is characterized by intolerance to fats and gluten. Intolerance to gluten causes damage in the intestinal epithelium resulting in bulky stools because of the fats and other substances that are not absorbed (steatorrhea) at the time in which a growth disorder is seen in autistics who suffer celiac disease. When these children were subjected to a gluten-free diet, the symptomatology of the autistic disease decreased. The presence of two diseases in the same patient does not necessarily mean that one is the consequence of the other, but that they may have the same genetic basis and, for that reason, they occur at the same time.
Finally, neurotoxins that cross the placental barrier are dangerous. Roberts et al. reported that if the mother is exposed to pesticides during pregnancy, such as in agricultural areas, there is a six-time increased risk that the fetus will develop autism.58
7. Treatment
7.1. Drug treatment
So far, there is no specific or curative treatment for autism. Existing treatments can be divided into pharmacological and psychological. All drug treatments are symptomatic. Many medications have been used in the management of this condition,59-61 but none is accepted unanimously or that is useful for all patients. Haloperidol could be useful for decreasing impulsivity and aggressiveness59,61 as well as the stereotypes and emotional lability, but it is important to be alert to its possible early and late collateral effects (dyskinesias, excessive sedation, etc.). It is advised that it be used intermittently or for short periods. Other reports have shown similar effectiveness for risperidone although with less secondary effects, a reason for which it is the drug most used at present.62
There are reports that indicate a high activity of endogenous opioids in the CNS of autistic subjects,63,64 and this has prompted the use of the opiate antagonist naltrexone.65,66 However, results have been poor and currently this is seldom used. Similarly, it is claimed that there are alterations in serotonin metabolism with significant elevation of serotonin concentration.67 This has given rise to the use of SSRIS such as fluvoxamine and sertraline68,69 with good results in decreasing repetitive thoughts and ritualistic behavior as well as a decrease in aggressive behavior and improvement in the use of language and social behavior. However, it should be mentioned that the beneficial effect may only be transient.
There is no medication that acts on the basic manifestations of autism. Sometimes some of the associated problems should be treated. Epilepsy is treated following the principles of epilepsy without any particular aspect. As most of the crises are focal complex, carbamazepine is one of the drugs indicated. When there is activity disorder with attention deficit, ritalin could be used (three daily doses of 0.4-1 mg/kg). For anxiety, buspirone (5 mg, three times a day) could be used. For aggression, naltrexone has been used (0.5 mg/kg/day). In recent years, studies with risperidone have been performed, an atypical antipsychotic that blocks the postsynaptic serotonin receptors. This could be more easily displaced by endogenous dopamine, which decreases the risk of the collateral neurological effects. The dosage used is 0.01-0.03 mg/kg weight in two daily doses during 8-week periods. Its favorable effect on auto- and heteroaggressivity, stereotypes, abnormal movements, inattention and hyperactivity is quite noticeable. The collateral effects are moderate somnolence and malaise, especially at treatment initiation. One problem that sometimes causes suspension of the medication is increased appetite and marked weight gain. In girls there may be amenorrhea, another indication for stopping the medication. When used in dosages > 3.5 mg/day and during prolonged periods, there may be dyskinesias and tremors.70
7.2. Psychological treatment
Psychological treatment plays a central role in the treatment of autism. The most accepted management currently is the initiation of an intensive and multimodal type of treatment as soon as possible: speech therapy, socialization programs, multiple sensory stimulation (auditive, visual and somatosensory), recreational therapy, etc. Unfortunately, there has been much quackery in this area, guised in pseudoscientific bases, which only bring confusion and false hopes in patients’ relatives (dolphin therapy, equine therapy, use of other pets, aromatherapy, music therapy, among others).59-61,71 Some treatments used with autistic subjects are educational and behavioral programs, which are centered on developing social abilities, speech, language, personal care and occupational skills. Mental health professions provide advice, training, and treatment based on the needs of each child because no generalizations can be made as each case has its own characteristics and particular needs. Specific treatment will be determined by the physician based on the following criteria:
• Age of the child, general state of health, and medical history
• Degree of the disorder
• Child’s symptoms
• Tolerance to determined medications or therapies
• Expectation for the progression of the disorder
• Opinion or preference of the parents
It is important to keep in mind that treatment of this disorder will be focused on the particular symptoms, with the purpose of improving the aspects that are deficient in the children. However, this does not mean that the treatment will eliminate the disorder or change the child’s behavior.71
Conflict of interest
The authors declare no conflicts of interest.
Received for publication: 8-27-14;
Accepted for publication: 1-15-15
http://dx.doi.org/10.1016/j.bmhimx.2015.01.010
Correspondence:
Dr. Christian Guerra-Araiza
E-mail: christianguerra2001@gmail.com