Con el avance en el tratamiento de los diversos tipos de neoplasias malignas pediátricas, la supervivencia de los niños que cursan con este padecimiento es cada vez mayor. Se estima que en nuestro país, en los próximos años, 1 de cada 1,000 habitantes menores de 25 años será un sobreviviente de cáncer. Debido a esto, ha surgido la necesidad de mejorar el cuidado de la salud de esta población. Algunos aspectos para considerar son la vigilancia y el tratamiento oportuno de las alteraciones endocrinológicas. Tanto la neoplasia en sí como la quimioterapia, la radioterapia y los procedimientos ablativos pueden dañar estructuras relacionadas con el sistema endocrino. En estos pacientes son frecuentes las alteraciones del crecimiento por diversos mecanismos, las alteraciones tiroideas primarias o secundarias, las alteraciones de la función sexual y reproductiva, el daño a la salud ósea y el aumento del riesgo cardiovascular. Los efectos secundarios endocrinológicos pueden presentarse a corto plazo o hasta 10 años o más después de concluido el tratamiento. En este trabajo se propone una guía para vigilancia y referencia oportuna al especialista.
Advances in treatment of childhood cancer have increased the rate of cure and survival in these patients. It is believed that in the near future 1/1,000 inhabitants <25 years of age will be a cancer survivor. Because of this, it is necessary to improve health care in this population, with endocrinologic disorders being one of the affected systems needing special attention. Both the neoplasm and its treatment including radiotherapy, chemotherapy and ablative procedures may injure endocrine system-related structures. Growth disorders are frequent in these patients so are primary or secondary thyroid disorders, sexual and reproductive dysfunction, bone health problems and increased cardiovascular risk. Endocrinologic side effects may present in the short term or >10 years after conclusion of treatment. In this paper we propose a guideline for surveillance and timely referral to a specialist for these patients.
1. Introduction
The annual incidence of cancer in children <14 years of age is between 70 and 160 cases per million.1 In 2008, there were 2039 new cases of cancer reported in this age group in Mexico, which corresponds to ∼64 cases per million, 65% of these being hematologic malignancies and 10% central nervous system neoplasms.2 It is highly likely that this figure has been underreported because different epidemiological studies have reported a high incidence of leukemia in the Hispanic population. In Mexico City, an incidence of 57.6 cases per million has been reported for leukemia alone.3 Improvement in the treatment of cancer in the pediatric age group has increased long-term survival (20% in 1954 to 83% in 2006) at >70% in the case of acute lymphoblastic leukemia(ALL) and at >90% in Hodgkin's lymphoma (HL).4
According to this figure, in Mexico it is expected that each year >1500 children will recover and be cured from cancer. With these figures it is estimated that, in the following years, ∼1/1000 inhabitants <25 years of age would be a survivor of a malignant childhood neoplasm.
Children who are cancer survivors could again be healthy and experience normal growth and development. However, they can also have late effects from the cancer or from the treatment that allowed them to survive. For this reason, it has become necessary for primary care physicians to be trained to identify late effects of the neoplasm and of effects of chemotherapy, radiotherapy and surgical procedures that the patients were subjected to during their treatment.5
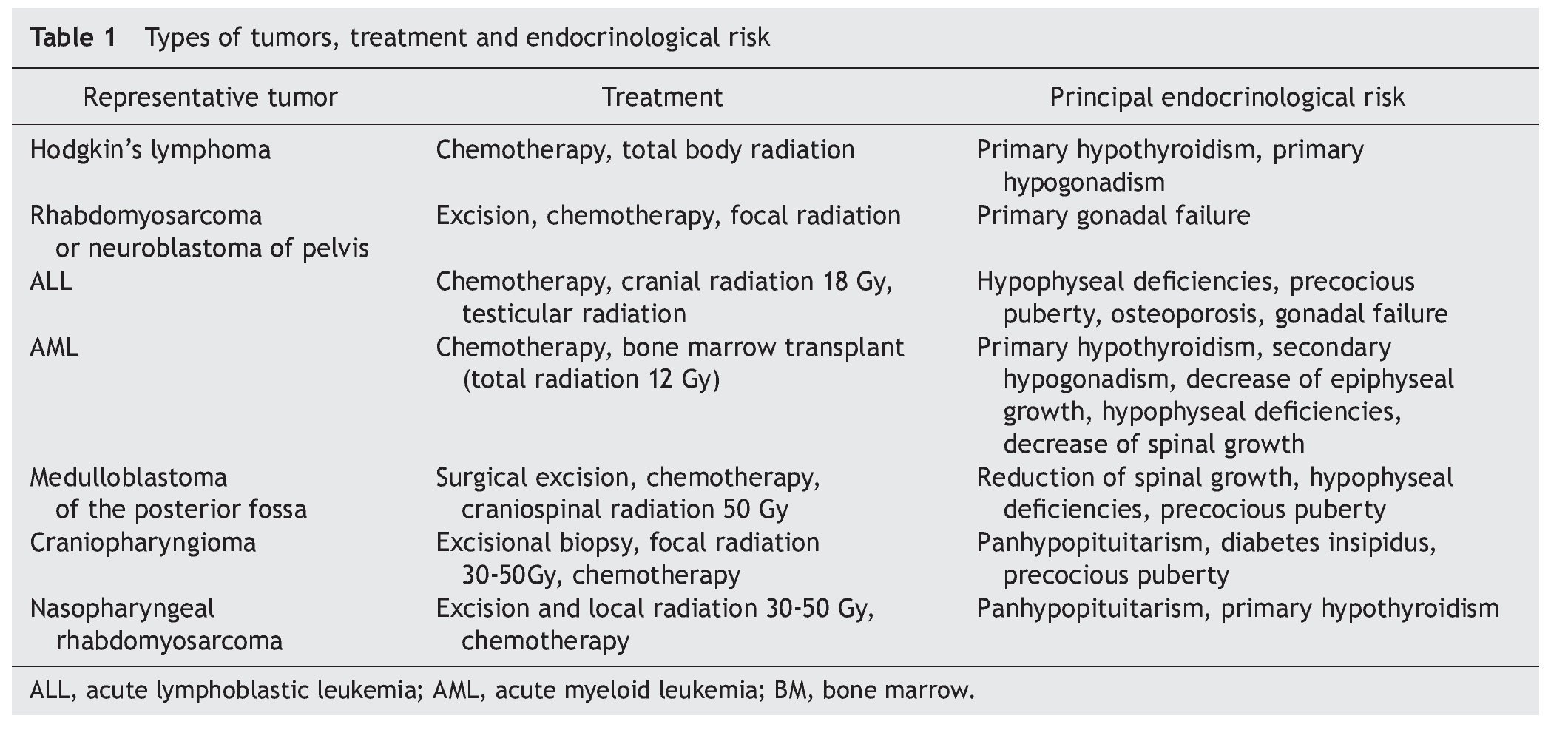
The endocrine system is particularly susceptible to experience long-term sequelae after resolution of the cancer; 57% of survivors have at least one endocrine disorder and 23% have more than one. Patients at higher risk are those who received hematopoietic stem cell transplantation because of the high doses of radiation and chemotherapy to which they are subjected.6 The most-well-described disorders are those of the hypothalamus-pituitary, thyroid and gonads. Diseases of these structures added to the multisystem damage caused by the cancer and its treatment predispose towards endocrine problems, among which is growth limitation, alterations in puberty, infertility, hypothyroidism and thyroid cancers, increase of cardiometabolic risk factors and decrease in bone mineral density (Table 1). The pediatrician should observe and monitor for the development of new expected and unexpected endocrine diseases for 10-15 years or even longer after termination of cancer therapy.7,8
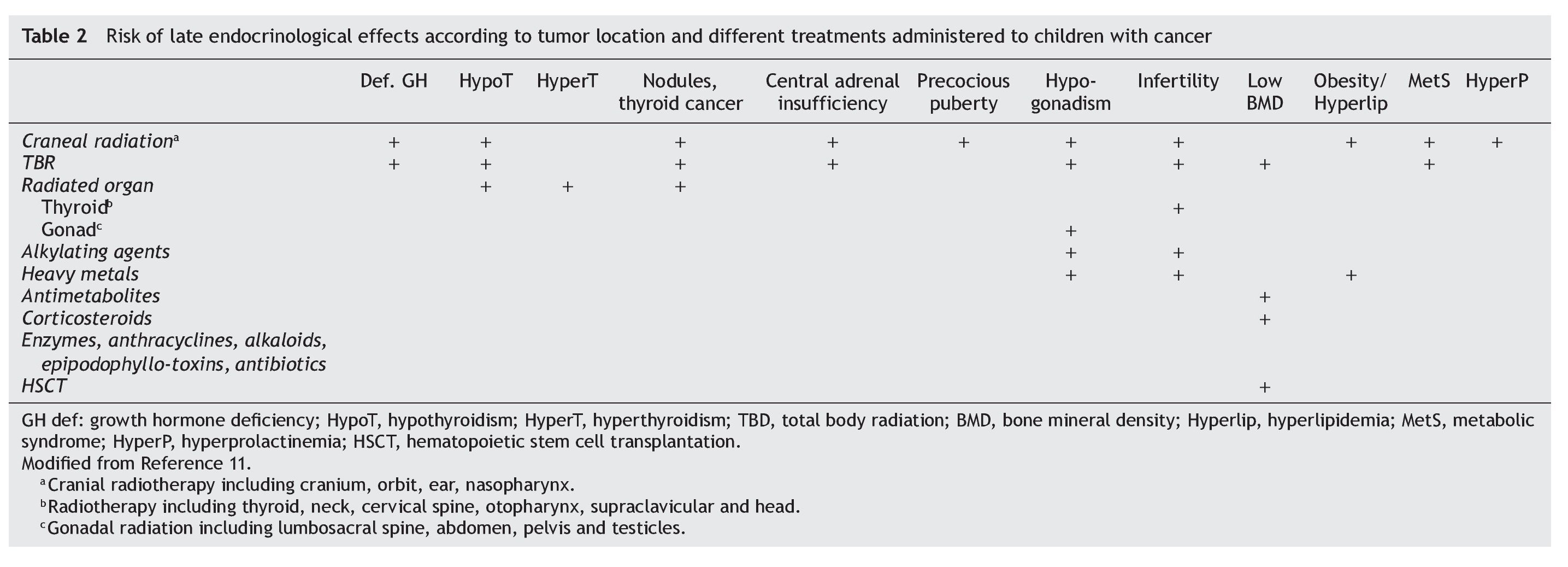
The risk of specific late endocrinological effects depends on the location of the tumor and therapeutic modalities used (Table 2). Both the tumor as well as the surgical procedures may require resection of the lesion or of fundamental structures for endocrine functioning.
2. Radiotherapy
The impact of radiotherapy depends on the field involved, total dose, administration scheme and age of the patient when administered. As a general rule, any structure within the radiated field could experience functional changes. For example, radiation for Hodgkin's lymphoma directly affects thyroid and gonadal function if applied to the neck or abdomen, respectively. Craniospinal radiation for medulloblastoma affects the function of the hypothalamus, thyroid and spine growth. In addition, there is a well-established relationship between the dose of cranial radiotherapy and the development of pituitary hormone deficiencies. There is also a well-established relationship between the cranial radiotherapy dose and development of deficiency of hormonal rhythms. The growth hormone (GH) axis is the most sensitive, being damaged with doses from 18 Gy, followed by the gonadotropic, thyroid and, finally, corticotropic axis.9
3. Chemotherapy
Alkylating agents and heavy metals cause female and male gonadal dysfunction related to the dose. Busulfan, procarbazine and mechlorethamine are particularly gonadotoxic. Cyclophosphamide is one of the most frequently used alkylating agents. Patients who received a dose >7.5 g/m2 had a greater risk of gonadal toxicity. As with radiation, germ cells are injured at doses lower than those of Leydig cells. Also, the combination of chemo-with radiotherapy increases the risk. Gonadal dysfunction in females due to the use of alkylating agents may result in delayed or detained puberty, premature menopause or infertility.
4. Growth in cancer survivors
It is expected that the growth rate decreases during the course of a moderate or severe active disease such as cancer. Ideally, after resolution of cancer, there should be a catch-up growth, which returns the patient to his/ her familial growth track in order to reach the genetic target height. There are multiple factors that limit this catch-up growth in a child who has had cancer; in first place are the nutritional deficits, the inflammatory state, the psychological stress caused by the cancer itself, and the concurrent diseases. These insults may be of such magnitude that damage in growth may not be recoverable. Additionally, there are the toxic effects of chemotherapy and radiotherapy on bone. Finally, there is the damage in the glands that secrete hormones directly involved in growth.
4.1. Decrease in growth due to the direct effect on bones
Radiation to the epiphyseal plates limits bone growth. Radiation to the neural axis causes decrease in vertebral growth and, therefore, a disproportionally short superior segment, whereas patients subjected to total body radiation such as bone marrow transplantation recipients can suffer generalized damage to the growth centers.
Regarding chemotherapy, it has been noted that methotrexate at high doses injures the growth plate, causing a decrease in the longitudinal growth of the long bones.10 Glucocorticoids, in turn, reduce the production of insulin growth factor type 1 (IGF-1) and induce resistance to this hormone in the growth plate. Also, C type natriuretic peptide decreases. This results in the reduction in chondrocyte proliferation and hypertrophy, matrix synthesis, and as a result a decreased linear growth. These phenomena directly affect the bone, independently of the inhibition of the growth hormone (GH) and gonadotropin secretion by these drugs.11
4.2. Growth decrease due to direct effects on endocrine glands
GH deficiency is the most common endocrine problem occurring after cranial radiation. It is recommended that growth of patients who received this treatment modality be monitored every 6 months11 and, in the case that low height or height velocity <10th percentile for age and gender is detected, specific tests be carried out to identify GH deficiency as well as to exclude other hormonal and systemic causes of decreased growth. Whenever GH or any other pituitary hormone deficiency is detected, it is necessary to assess the status of the rest of the hypothalamic-pituitary system. Hypothyroidism (primary, secondary or tertiary) is as frequent or more than GH deficiency and it alone may cause a growth limitation.
With regard to gonadal functioning, the insufficient production of sex hormones (androgens and estrogens) causes, in addition to delay or lack of development of sexual characteristics, lack of pubertal growth spurt, which results in a height deficit, more notable after the second decade of life. Precocious puberty can also occur as a result of a neoplasm or intracranial intervention, causing early closure of the epiphyseal plates and a final height lower than expected according to the genetic potential.
4.2.1. Thyroid in cancer survivors
Neck radiation with >10 Gy can cause hypothyroidism or, very infrequently, hyperthyroidism. A dose of >25 Gy predisposes to the development of thyroid nodules. The risk of cancer increases progressively up to the dose of 30 Gy, and then the risk decreases with higher doses.12
4.2.1.1. Hypothyroidism
It is estimated that 24% of cancer survivors have thyroid changes mainly associated with radiation to the neck or skull. Patients subjected to total body radiation have a prevalence of any type of hypothyroidism of up to 34%.13-16
Primary hypothyroidism, due to direct damage to the thyroid, is common after having received radiation for Hodgkin's lymphoma or for nasopharyngeal cancer. The thyroid tends to be small and increased in consistency, with elevated TSH levels and low free thyroxin (FT4).17
A history of radiation to the neck increases the risk of primary hypothyroidism 17 times with respect to the general population.18 The prevalence increases with time, for which it is necessary to periodically review thyroid function tests of survivors who received neck radiation even though many years may have passed since that time.19
Subclinical primary hypothyroidism is defined as normal FT4 levels and mildly elevated thyroid-stimulating hormone (TSH). Although management of this condition is controversial, it has been observed that patients with this profile who received radiation to the neck and have growth limitations improve their height velocity, lipid profile and vigor if receive replacement therapy with thyroid hormone to reach TSH concentrations of 1-2 mU/l.20,21
Secondary hypothyroidism, damage to the pituitary thyrotrophs, may occur after cranial radiation. In this form of hypothyroidism, there is no increase in TSH levels, which is a very sensitive marker of subtle thyroid hormone insufficiency. Biochemical diagnosis is made with certainty when FT4 is below the reference values without a concomitant increase in TSH. However, patients with partial TSH deficiency may have FT4 levels in the lower third of the reference values, which may delay diagnosis of secondary hypothyroidism. Diagnostic delay may be trivial for an adult, but in a child it may mean losing the opportunity for growth and potentially have functional implications. Mild TSH deficiency could precede the appearance of other hypothalamus-pituitary deficiencies and could be detected with a thyrotropin-releasing hormone (TRH) stimulation test prior to the manifestation of other clinical or biochemical evidences.
Tertiary hypothyroidism occurs due to hypothalamic lesions. It is difficult to differentiate it from secondary hypothyroidism and they frequently coexist. It may present biochemically with the same thyroid profile as secondary hypothyroidism or there may be a slightly elevated TSH concentration that has decreased bioactivity due to altered patterns of glycosylation. It is possible to diagnose tertiary hypothyroidism using a thyroid-releasing hormone (TRH) stimulation test in which a favorable TSH response is observed, although generally it is not necessary or practical to make the differential diagnosis. Finally, it is possible that a patient who has received head and neck radiation may present a mixed, primary and central hypothyroidism.
In order to timely diagnose hypothyroidism, it is recommended that growth, TSH and FT4 concentrations be monitored annually. Diagnosis is based on a persistently low FT4 concentration or in the lower third of the reference values as well as compatible signs and symptoms. It is important to rule out adrenal insufficiency before starting thyroid replacement therapy; otherwise, administration of thyroid hormones may precipitate an adrenal crisis in a patient who is deficient in adrenocorticotropin hormone (ACTH).
Nodule and thyroid cancer. Patients who received neck radiation have a greater risk of developing nodules and thyroid cancer. Up to 27% of the patients who received neck radiation developed thyroid nodules during a 20-year follow-up. Of these, up to 38% may be malignant.22
5. Puberty and reproductive function in cancer survivors
Changes in the normal process of puberty in cancer survivors can be of three types: 1) precocious or rapidly progressive puberty, 2) delayed, detained or absent puberty, and 3) infertility.
5.1. Precocious puberty
Precocious puberty occurs in patients who have lost the hypothalamic gonadotropin-releasing hormone (GnRH) secretion inhibition as a result of the presence of the tumor (for example, a hypothalamic glioma or of the optic pathway), elevated intracranial pressure, surgery or cranial radiation.11,23,24 Precocious puberty is associated with brain radiation doses of 18-30 Gy, whereas doses >30-40 Gy generally cause GnRH deficiency and, thereby, delayed puberty. Breast development in girls <8 years or testicular growth in males <9 years must be approached as precocious puberty. Some children may develop transitory growth of the mammary bud due to nutritional recovery. These children should be examined each 3 to 6 months. If mammary bud growth persists for >12 months, it may signal the beginning of puberty.11 Treatment of precocious puberty with GnRH analogues should be indicated and supervised by a pediatric endocrinologist.
5.2. Delay in pubertal development
Delay in pubertal development, i.e., lack of breast develop ment in girls >13 years or testicular growth in males >14 years of age, can be functional or secondary to a hypothalamus-pituitary or gonadal lesion. The functional delayed puberty may be a variant of the normal (nonpathological) or may be due to malnutrition, chronic disease, depression, anxiety, or hypothyroidism and cannot be biochemically differentiated from hypogonadism of hypothalamus-pituitary origin (hypogonadotropic). Accurate diagnosis is determined by the evolution and progression of puberty when the functional problem that gave rise to it improves.
Brain radiation frequently causes hypogonadotropic hypogonadism due to a lesion of the hypothalamus with a decrease in GnRH secretion or due to lesion of the pituitary gonadotrophs with decrease in secretion of luteinizing hormone (LH) or follicle-stimulating hormone (FSH). Hypergonadotropic hypogonadism secondary to a gonadal, testicular or ovarian lesion generally is caused by highly cytotoxic chemotherapy or gonadal radiation. The most gonadotoxic chemotherapy agents are alkylating agents such as cyclophosphamide, mechlorethamine, and procarbazine. The manner of presentation of gonadal damage in males is frequently delayed puberty, whereas in females premature ovarian failure or early menopause occurs.
Total body, abdominal, pelvic and lumbosacral radiation, particularly in postpuberal females, may compromise ovarian function, especially if the patient received a prepubertal dose >10 Gy or a postpubertal dose of 5 Gy or high doses of alkylating agents. The testicles are particularly sensitive to radiation, with reversible azoospermia observed with doses from 1-3 Gy, permanent azoospermia from 6 Gy and Leydig cell damage with doses >20 Gy.25
Treatment of boys and girls with hypogonadism includes testosterone and estrogen replacement therapy, respectively, by a pediatric endocrinologist, with care to not obscure the prognosis for height due to an accelerated fusion of the growth plates by sex hormones.
5.3. Infertility
Infertility may be secondary to hypogonadism of central origin (hypogonotropic) or gonadal (hypergonadotropic) or due to lesion of the germinal epithelium, which is responsible for maintaining germ cells (sperm and oocytes). This lesion may be caused by radiation or chemotherapy. Chemotherapy agents associated with infertility include mechlorethamine, chlorambucil, melphalan, busulfan, cyclophosphamide, ifosfamide, procarbazine, nitrosoureas and platinum. Radiation dose >250 cGy to the testicles or >1000 cGy to the ovaries may cause infertility. Spermatozoids are more sensitive to damage by chemo- and radiation therapy than oocytes and Leydig cells, which explains why males experience infertility more frequently than females and experience infertility with a higher frequency than hypogonadism. Increases in FSH and inhibin B levels are markers of damage to the germinal epithelium in males. It is important to sensitively inform the adolescent about the reproductive prognosis and available assisted reproductive options and to offer spermatobioscopy to patients who are interested.
6. Adrenocorticotrophic function in cancer survivors
The pituitary corticotrophs are less sensitive to radiation therapy compared with the somatotrophs, gonadotrophs and thyrotrophs. However, ACTH deficiency has more serious consequences if not detected in time. It is rare if cranial radiation >24 Gy has not been received but should be suspected whenever there has been an intracranial tumor, regardless of whether radiation was received or if there is documentation of other pituitary hormone deficiency. Monitoring of this hormone deficiency should continue for 10-15 years after resolution of the neoplasm because damage to the corticotrophs manifests itself gradually over several years. Monitoring is done annually with review of symptoms (failure to thrive, anorexia, dehydration, hypoglycemia, lethargy, hypotension) and with cortisol determination at 8 a.m., with evaluation by a pediatric endocrinologist if <10-18 mg/dl.26 The most severe deficiency is manifested by hypoglycemia and adrenal crisis precipitated by a concomitant disease. There may be mild or moderate hyponatremia; severe hyponatremia and hyperkalemia do not present themselves as the mineralocorticoid function is preserved. It is important to evaluate the corticotropin axis before thyroid hormone replacement so as to avoid an adrenal crisis.
7. Cardiometabolic risk in cancer survivors
As compared to the general population, survivors of childhood cancer have a high risk of developing cardiovascular disease, at least up to 45 years after the original diagnosis of neoplasm. Vascular damage induced by chemo- and radiation therapy is, in part, responsible for early cardiovascular risk, although endocrine and metabolic abnormalities may be responsible for a large proportion of this risk as survival is increased. A higher prevalence of obesity in cancer survivors with respect to the general population has been described as well as lower levels of cholesterol, HDL, and higher levels of glucose, mainly associated with a history of cranial radiation and GH deficiency. It is also related with behavioral factors such as physical inactivity that is more common in cancer survivors, stressing the need to promote a healthy lifestyle in these patients.
Cranial radiation produces weight management problems often exacerbated by concurrent GH deficiency and hypothyroidism. The risk is higher in females and patients who received a radiation dose >18 Gy. It is recommended that the levels of glucose, insulin and fasting lipid profile be measured every 2 years in patients with overweight or obesity and every 5 years in patients with normal weight. Heavy metals such as carboplatin and cisplatin can cause dyslipidemia. For patients with a family history of dyslipidemia, overweight or obesity or GH deficiency the risk increases.27
Radiation induces anatomic pathological changes in the coronary arteries, similar to those observed in spontaneous atherosclerosis. This phenomenon is possibly due to the endothelial injury secondary to the generation of reactive species of oxygen by the ionizing radiation and the inflammatory response. Symptomatic coronary artery disease has been found in 10% of patients 9 years after mediastinal radiation. A risk of premature atherosclerosis and cerebral vascular events 10 times greater has been described in patients who are survivors of leukemia and intracranial tumors and received head and neck RT compared to the general population, particularly with doses >20 Gy.28
8. The bone in cancer survivors
A risk of fractures during the 5 years following the diagnosis of leukemia is twice as high as that of the general population29 and a 24% prevalence of low bone mineral density in patients in the third and fourth decade of life who suffered from ALL in the pediatric age group compared with 15% risk in the general population has been estimated.30
The etiology of bone mass deficit in patients who experienced childhood cancer is multifactorial and includes both direct and indirect effects of cancer and its treatment, resulting in bone loss, decrease in bone growth and decreased mineral accretion. Malignant infiltration, glucocorticoids, and methotrexate can directly interfere with bone metabolism. Suboptimal nutrition, lack of exposure to the sun and decreased physical activity are factors that also limit bone mineralization. On the other hand, the prevalent endocrine pathologies in cancer survivors such as deficiencies of GH, sexual hormones and vitamin D contribute to this deficit. The direct effects of radiation on bone mineral density (BMD) are not precisely known but are believed to be harmful due to damage to the bone matrix.
Glucocorticoids at pharmacological doses affect the bone due to several mechanisms including decreased osteoblastic activity, increased bone resorption, interference with the GH/IGF, reduced muscle strength, and calcium balance disorder at the intestinal and renal level. In particular, patients with cumulative doses >9000 mg/m2 of prednisone have an increased risk of developing low BMD without recovery.
Methotrexate has cytotoxic effect on osteoblasts, which results in diminished bone volume and less new bone formation. Cumulative doses >40,000 mg/m2 are associated with an increased risk of osteopenia and failure to recover a normal bone mass after treatment.
Estrogens play a crucial role in achieving and maintaining peak bone mass because they prevent resorption and stimulate bone growth, both in males and females. Androgens have an important role in the bone apposition of the periosteum, which adds strength to the bone. Alkylating agents carry a dose-dependent risk of gonadal failure that indirectly impacts on the BMD because they produce deficiency of sex hormones. The risk of primary or secondary hypogonadism is even greater if it is combined with radiation to the gonads or to the hypothalamus-pituitary.
It is unknown to what extent the loss or lack of gain in bone during the period of the disease and its treatment is recoverable if hormonal deficiencies are corrected and nutrition and physical activity are optimized.31
In regard to the prevention and treatment of bone mass deficiency in children, it is recommended to increase exercise with resistance (with weight or force) as tolerated, ensuring an intake of 1500 mg per day of elemental calcium and 400 to 1,000 IU of vitamin D as well as to discourage smoking and consumption of alcohol and caffeine. It is also indispensable to substitute sex hormones in hypogonadic patients and with GH to deficient patients.
Patients with BMD of >2.5 SD below the mean could benefit from more specific treatments. Calcitonin and biphosphates are reserved for patients with recurrent fractures and as part of clinical trials. Studies of biphosphates in children are limited and have only been tested in patients with osteogenesis imperfecta. It is important to not underestimate the potential risks of medications at early ages. A baseline evaluation of BMD at 18 years of age is recommended.11,31
9. Hyperprolactinemia in cancer survivors
The dose of RT >40 Gy, mesencephalon surgery or a tumor in the hypothalamic area may predispose to the development of hyperprolactinemia, which interferes with the pulsatile secretion of gonadotropins. In women, hyperprolactinemia manifests with galactorrhea and menstrual irregularities and in males with galactorrhea and decreased libido. It is recommended that prolactin levels be determined only if there are compatible signs or symptoms observed.
10. Endocrinological surveillance in cancer survivors
Cancer survivors require continuous monitoring:
• Biannual:
— Precise measurement of weight, height, body segments and arm span
— Bone age if the patient is growing very rapidly or very slowly
— IGF-1 and IGF-BP3 if growth is too slow
• Annual:
— Evaluation of Tanner stage and interpretation of the stage and tempo of puberty
— LH, FSH, sex steroids, inhibin B (males) and anti-Müllerian hormone (females) if there is delayed or interrupted puberty
— FT4 and TSH
— Palpation and thyroid USG if there was radiation to the neck
— Nutritional counseling to prevent obesity and metabolic disorders and ensure adequate intake of calcium and vitamin D (annual or more frequent monitoring if there are alterations)
• Bone mineral density at age 18, 5 and 10 years after completion of chemotherapy, and more frequently with an abnormal BMD
Conflict of interest
The author declares no conflict of interest of any nature.
Received 14 January 2014;
accepted 14 February 2014