The continuous subcutaneous insulin infusion (CSII) is an established form of insulin therapy for patients with type 1 diabetes mellitus. The vision, the development of an artificial pancreas (closed-loop) is not yet realized. One important precondition for this to achieve, the availability of continuous glucose monitoring (CGM), is given. The combination of CSII and CGM turns CSII into a new form of therapy, the sensor-augmented pump therapy (SaP). The superiority of SaP over classic CSII has been proven in randomised, controlled clinical trials. In this article, we review this new possibility of insulin therapy.
continuous glucose monitoring
continuous subcutaneous insulin infusión
low glucose suspend
multiple dose injection
randomised, controlled clinical trials
sensor-augmented pump therapy.
The development of an artificial pancreas was first proposed by A.H. Kadish1 more than four decades ago. This so-called closedloop system appeared and still appears to be the best possible solution for insulin treatment, especially for patients with type 1 diabetes. This kind of system was realised as early as the 1970s with the Biostator. This was a device as large as a table, to which the patient was connected. Blood sugar was measured in blood obtained from a vein and insulin was infused directly via a venous access, just like glucose in the case of falling blood sugar levels. At that time it was optimistically thought that the equipment only needed to be sufficiently reduced in size for patients to be offered a portable system suitable for everyday use. It was expected that the substantial obstacles, namely the fact that sensors for continuous glucose measuring were not yet available and the problem of the unfavourable risk-benefit ratio associated with infusion of insulin and glucose via a venous port, would soon be overcome.2
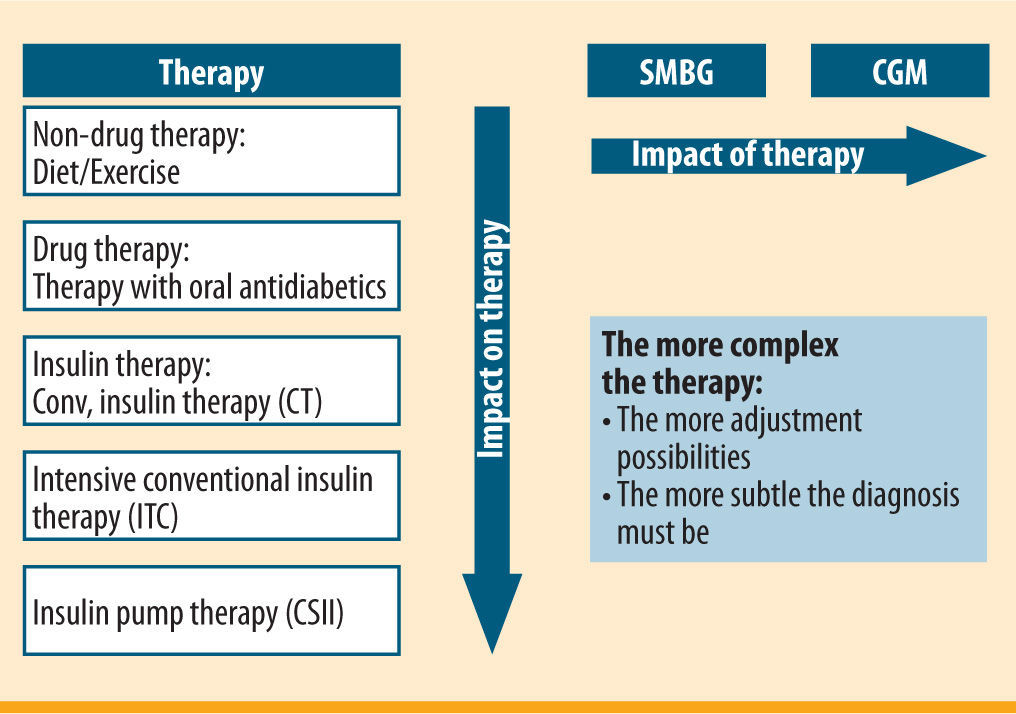
Pursuit of the goal of physiological insulin administration led to the development of small insulin pumps and hence to insulin pump therapy (CSII-continuous subcutaneous insulin infusion). After a hesitant start in the eighties, an established form of insulin therapy was developed.3 At present between 10% and 20% of patients with type 1 diabetes are treated with CSII in a number of western countries, such as Germany, Netherlands, France, Switzerland and Austria.3 In 2009 this rate was actually more than 35% in the USA.4,5 One of the essential preconditions for the widespread use of CSII was the wide availability of self-monitoring of blood glucose levels. This made it possible to adjust the insulin to the patient's current need and to control functioning of the infusion system (pump, reservoir and infusion set). However, this was an open system (open-loop), which means insulin delivery is not controlled by a glucose sensor and relevant algorithms. In a sense, it is therefore a compromise on the vision of an artificial pancreas.
The most important advantage of CSII is that, using solely short-acting insulin, it adjusts the basal insulin dosage to the diabetic's individual physiological insulin requirement, which can only be depicted with variable basal rate programming in patients with type 1 diabetes.6 This results in a number of advantages over multiple dose injection therapy (MDI):
- •
In most pump patients, insulin delivery based on demand leads to close-to-normal glycaemia with HbA1c around 7%, without increasing the risk of hypoglycaemia.7-9 The lower HbA1c compared with MDI lessens the progression of diabetic complications and may even cause their regression to some extent.10,11 Markedly smaller blood sugar fluctuations can also be seen, which in turn is likely to be associated with a reduction of vascular risk.12-14
- •
Even diabetic patients who achieve their target blood sugar levels with difficulty or not at all on MDI usually achieve comparably better control with the aid of an insulin pump.
- •
Various options for bolus delivery allow optimal insulin adjustment to meals with a variable glycaemic index.15-17 High postprandial blood sugar peaks, which are a risk factor for the development of macrovascular diseases (proven at least for type 2 diabetes),18,19 are more likely to be avoided.
- •
The pump allows patients to achieve a more flexible daily profile that is influenced far less by the insulin therapy. This leads to an increase in the diabetic's exercise tolerance and functional capacity. Consequently everyday work activities (e.g. business travellers, doctors, etc.) can be managed better.
- •
An increased insulin requirement (e.g. during infection) or reduced requirement (e.g. during sport) can be simply responded to by temporary adjustment of the basal insulin dose.
The conceptual and clinical advantages as an improvement in HbA1c and/or a decrease in hypoglycaemic events are all the more impressive the worse the initial situation was on the previous MDI.20 The advantages of CSII have been demonstrated by a large number of experimental and clinical trials but particularly by meta-analyses of randomised, controlled clinical trials (RCTs).21,22 In the meta-analysis by Pickup et al. 21 on 22 studies with a high evidence level, it was shown that in patients with frequent hypoglycaemic episodes their rate decreased by a factor of 4.19, hence to 23.9%. At the same time HbA1c improved by an average of 0.61%. This improvement was also confirmed by the meta-analysis of Jeitler et al. 22 (mean HbA1c improvement of 0.55%). Hence CSII versus MDI is not only demonstrably more physiological but also more successful, even if not every patient can realise these advantages.
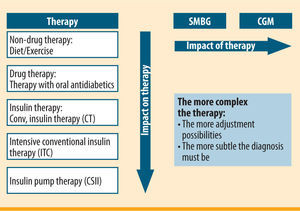
Use of continuous glucose monitoring (CGM) in CSII for sensor-augmented pump therapy (SaP)Patients usually measure their blood sugar after getting up/before breakfast, again before meals and finally before going to bed. They will take additional measurements sporadically, if their blood sugar is not within the desired range or they feel they are having or about to have a hypoglycaemic episode. A total of 5-6 blood sugar measurements a day form the diagnostic basis for CSII. However, CSII has various possible ways of controlling insulin delivery by means of different bolus options or temporary change to the basal rate. If these are utilised, a more extensive database of glucose levels than that provided by self-measurement of glucose at specific time points is valuable. The use of a glucose sensor with continuous, automatic glucose measurement at intervals of a few minutes (CGM) has been demonstrated to be very advantageous for this purpose, more than with all other treatment options (figure 1). The use of a sensor means not only that the open-loop is gaining the essential component for further development into a closed-loop system, but it also turns CSII into a new form of therapy, namely sensor-augmented pump (SaP) therapy.
A glucose sensor used for CGM is minimally invasive, which means a small needle electrode is pushed under the skin and measures glucose in the interstitial space with the aid of chemical principles similar to self-monitoring of blood glucose, i.e. conversion of glucose into gluconic acid and hydrogen peroxide with the aid of the enzyme glucose oxidase, dissociation of the hydroxide peroxide at an electrode and measurement of the resulting flow of current dependent on the glucose concentration.23 A measurement is taken every 10 seconds. These individual measurements are combined to form a mean measurement over 5min and indicated on a display. A sensor is used for 6 days.
This gives the patient an opportunity to influence his glucose profile directly, which mainly includes active avoidance of hypoglycaemic events. Adjustable alert thresholds for hypoglycaemic and hyperglycaemic levels help patients to do this. The advantages of CGM with current glucose levels have been demonstrated in several RCTs. Among these, the JDRF (Juvenile Diabetes Research Foundation) studies are hitherto the largest and methodologically best RCTs involving CGM.24,25 As a result of the application and dependent on the level of use of CGM, the HbA1c levels in JDRF I study (baseline HbA1c >7-10%, 322 patients with type 1 diabetes) improved over 6 months as a result of adequate use of CGM in adults aged over 25 years, being the effect only significant in this age group with a 0.5% HbA1c improvement.24 In well-controlled patients with type 1 diabetes (JDRF <7 study, 129 patients with type 1 diabetes), this value did remain constant at 6.4% although the time spent in the range of hypoglycaemic glucose levels (≤ 70mg/dl) was reduced by 41% from a daily average of 91min to 54min. In this trial, all three age groups showed good compliance in terms of adequate use of the CGM system, which is why the positive effect was relevant irrespective of the age group.25
These improvements become visible particularly on CSII because of the potential fine control of therapy. The Paradigm® VEO system comprises the insulin pump, with the option of transferring the current glucose levels recorded by a glucose sensor via a wireless interface to the system display (figure 2). These data can also be incorporated into the bolus delivery calculation (Bolus Wizard) and, after suitable confirmation by a conventional blood sugar measurement, entered into the insulin bolus calculation. As with the previous model (Paradigm® REAL-Time), under normal conditions the glucose sensor does not yet intervene automatically in glycaemic regulation but offers the patient a complete overview of his glucose profile. The system hence works as an “open system”, either in CSII mode (i.e. without the CGM component) or in SaP mode (if a glucose sensor is being used).
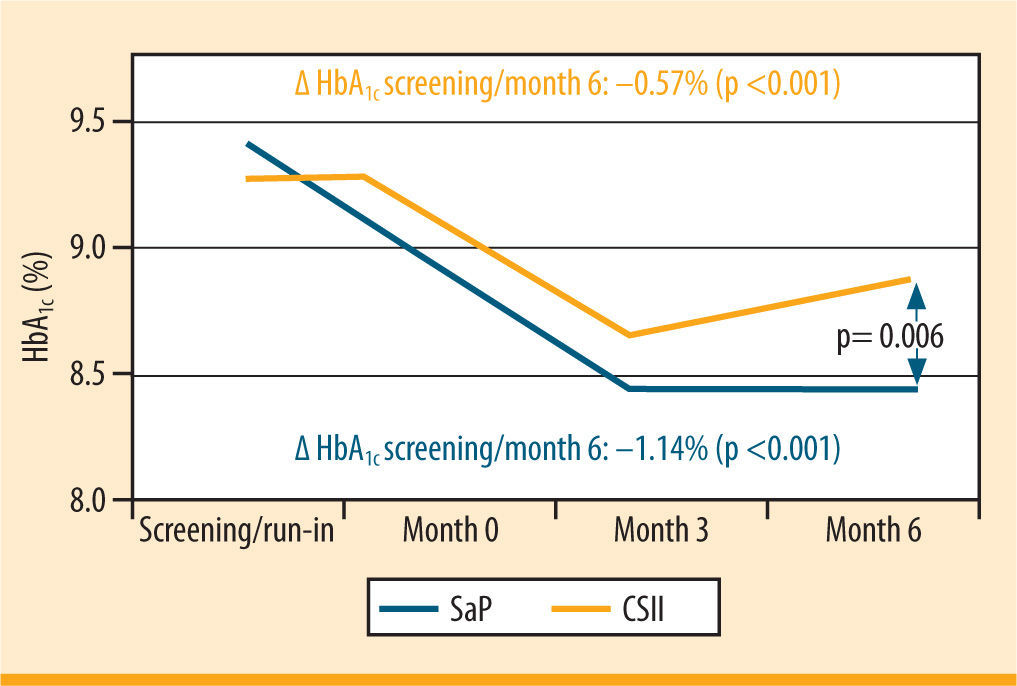
The superiority of SaP over classic CSII has been demonstrated in RCTs, for example in the multicentre REAL Trend study (figure 3)26 In this study, the patients had previously been treated with ICT, on which they displayed inadequate glycaemic control. On SAP, their HbA1c improved by 1.23% over the course of the 6 months of the study for the group of 91 patients who wore the sensor ≥70% of the time or by 1.14% for all patients (n= 115). By comparison, the percentage improvement on CSII was only 0.55%.
HbA1c trend in patients, wearing the sensor ≥70% of the time (Real-Trend study: randomization of patients with MDI in CSII or SaP)26
The Paradigm® VEO system is the first to offer the possibility of treatment control by means of a glucose sensor. The system initially sounds an alert if there is a risk of or an actual hypoglycaemic event depending on the threshold setting. If the patient does not respond to these alerts because he is deeply asleep, for example, the insulin pump automatically suspends insulin delivery for 120min, a function known as LGS low glucose suspend. After this period of time, it automatically switches back on if the patient has not already done so manually. By this mechanism, hypoglycaemia can largely be avoided.27 Hyperglycaemia or even diabetic ketoacidosis are unlikely to arise because of the relatively short suspension of 120min maximum. It is therefore the first time that a glucose sensor intervenes directly in therapy, making it a major advance towards an automated insulin pump system and hence towards an artificial pancreas.
There is currently intensive research work on further steps towards a closed-loop system. In the creation of an “artificial pancreas”, the key challenge is to develop algorithms for controlled insulin delivery based on measured glucose levels, while subcutaneous measurement and subcutaneous insulin delivery are favoured in view of the cost-benefit-risk relationship and the available hardware. The measurement of glucose in the interstitial fluid and the resulting physiological time lag to the blood glucose concentration as well as the non-physiological infusion of insulin into subcutaneous tissue pose a special challenge to this algorithm.
It should further be noted that insulin must be delivered in such a way that the glucose concentration can be predicted as lying in the normoglycaemic range. Previous studies show that normoglycaemic glucose regulation is achieved solely in the basal phase without food intake and without physical activity but unphysiologically high glucose levels occur after meals. The problem is essentially due to subcutaneous exogenous insulin, which unlike endocrine insulin is peripherally active first and only then hepatically active so that gluconeogenesis is not immediately halted. Thus, its pharmacodynamics is independent of the glucose stimulus. For this purpose, the short-acting insulin analogues currently available on the market are still too slow. In any event, however, the activities and the number of studies and publications on this subject have markedly increased.
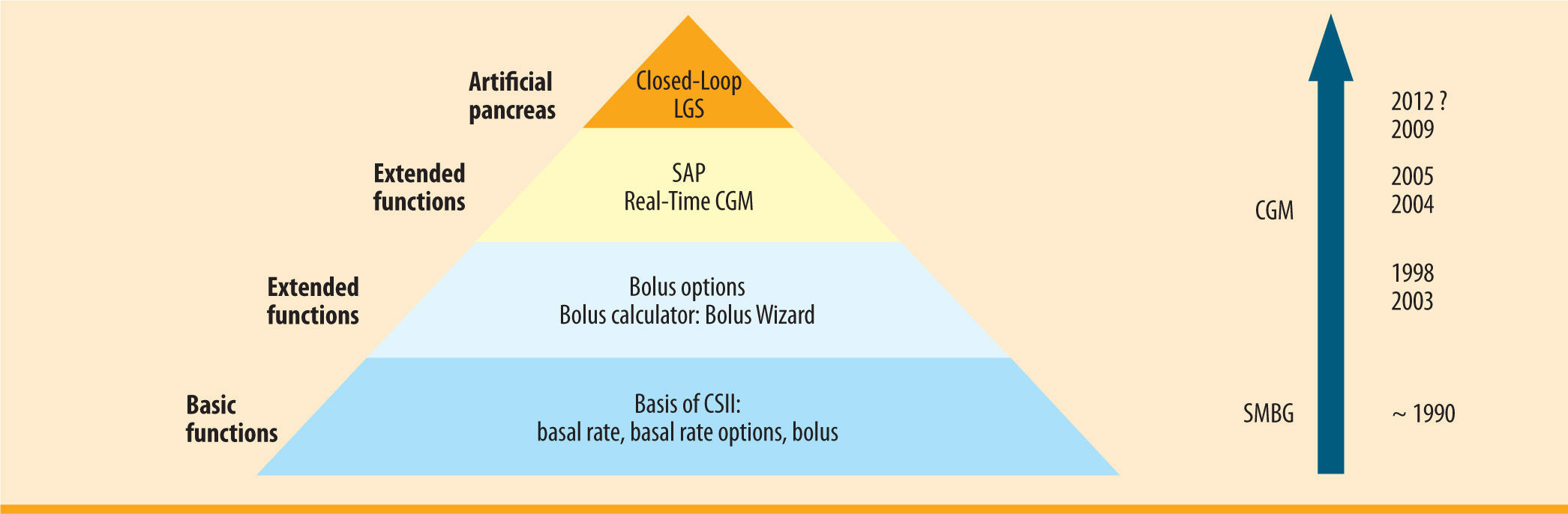
SummarySensor-augmented pump therapy is a major advance towards a closed-loop system. In the process, the glucose sensor, which is an essential component of the artificial pancreas, is being introduced directly into insulin pump therapy (figure 4). Assuming continuous use of the CGM component, the positive effects of CSII are markedly enhanced. Automated intervention when hypoglycaemia is ignored or not noticed means that the lowest level of a closed-loop has already been realised in a marketable product (LGS low glucose suspend). After decades of waiting, the diabetology vision of an artificial pancreas now seems within reach. ¿
Declaration of potential conflicts of interestDr. Martin Schonauer is a diabetologist, with no commercial interests in respect of the technology and therapy presented. Dr. Andreas Thomas is Scientific Manager of Medtronic, Diabetes Division, Germany.
- •
Sensor-augmented pump therapy, currently the most physiological form of insulin treatment, results from combination of continuous glucose monitoring with CSII.
- •
The latest type of insulin pump can interrupt automatically the insulin delivery for two hours in situations of ignored or unnoticed hypoglycaemia.
- •
For patients with hypoglycaemia unawareness, the low glucose suspend function is an additional safety feature of insulin pumps, which may help them to achieve close-to-normal glycemic controls.



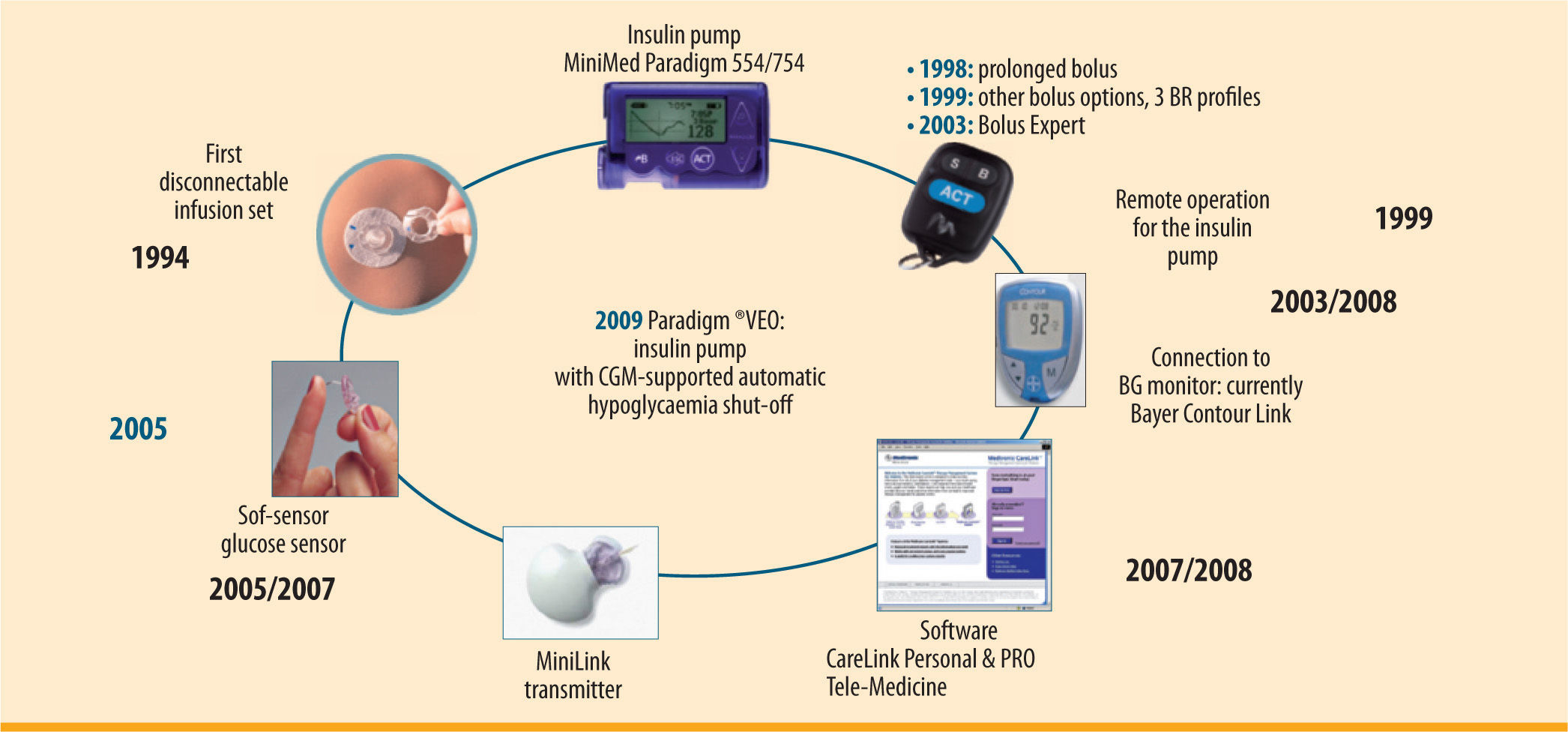
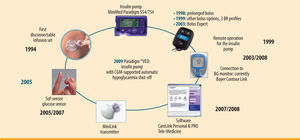 SaP) therapy resulting from connection to the glucose sensor' title='Innovations and components of the Paradigm® VEO insulin pump system with the option of sensor-augmented pump (
SaP) therapy resulting from connection to the glucose sensor' title='Innovations and components of the Paradigm® VEO insulin pump system with the option of sensor-augmented pump (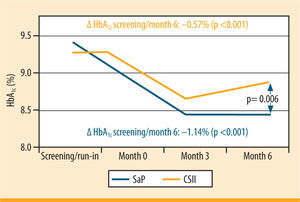 MDI in
MDI in 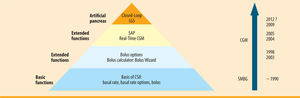 CSII from its initial basic functions via extended functions and the use of
CSII from its initial basic functions via extended functions and the use of 
