Brolucizumab, a new generation anti-VEGF, has demonstrated efficacy and safety in AMD in the pivotal HAWK and HARRIER trials. Post-marketing, previously undetected adverse events related to intraocular inflammation have been reported. An independent post hoc review of the pivotal trials puts the rate of IOI at 4.6%. The aim of this paper is to propose a set of recommendations for implementing the management of brolucizumab in clinical practice.
MethodsThe recommendations made by the authors are based on their clinical experience, critical review of (i) the pivotal trials, the post-hoc analysis of the Safety Review Committee, (ii), and (iii) the published literature.
ResultsIn the pivotal trials, brolucizumab showed sustained functional gains, superior anatomical outcomes with potentially longer intervals between injections and a well-tolerated overall safety profile. Adverse events reported post-marketing include retinal vasculitis and retinal vascular occlusion. Based on the available information, experts recommend (i) ruling out non-recommended patient profiles (prior history of ORI), (ii) screening the patient prior to each injection to rule out active ORI, (iii) monitoring the patient for early warning signs, and (iv) treating immediately should any adverse events develop.
ConclusionsThe adverse events reported are rare, but may be associated with severe and irreversible loss of visual acuity. The recommendations made are intended to facilitate the management of brolucizumab in the routine practice of retinologists, to ensure patient safety and, should any adverse events occur, to minimise their impact on vision.
Brolucizumab, un anti-VEGF de nueva generación, ha demostrado su eficacia y seguridad en degeneración macular asociada a la edad neovascular exudativa (DMAEn) en los ensayos pivotales HAWK y HARRIER. Tras su comercialización, se han reportado eventos adversos relacionados con la inflamación intraocular no detectados previamente. Una revision post hoc independiente de los ensayos pivotales cifra la tasa de IIO en 4,6%. El objetivo de este trabajo es proponer una serie de recomendaciones para implementar el manejo de brolucizumab en la práctica clínica.
MétodoLas recomendaciones realizadas por los autores se han basado en su experiencia clínica y la revisión crítica de: 1) los ensayos pivotales; 2) el análisis post hoc del Comité de Revisión de Seguridad, y 3) la literatura publicada.
ResultadosEn los ensayos pivotales, brolucizumab mostró ganancias funcionales sostenidas, resultados anatómicos superiores con intervalos entre inyecciones potencialmente más prolongados y un perfil de seguridad global bien tolerado. Los eventos adversos reportados tras la comercialización incluyen vasculitis retiniana y la oclusión vascular retiniana. De acuerdo con la información disponible, los expertos recomiendan 1) descartar los perfiles de pacientes no recomendados (historial previo de IIO), 2) explorar al paciente antes de cada inyección para descartar la presencia de IIO activa, 3) monitorizar al paciente para detectar precozmente los signos de alerta, y 4) tratar de inmediato en el caso de que se desarrolle algún evento adverso.
ConclusionesLos eventos adversos reportados son poco frecuentes, pero pueden estar asociados con una pérdida severa e irreversible de agudeza visual. Las recomendaciones realizadas pretenden facilitar el manejo de brolucizumab en la práctica habitual de los retinólogos, garantizar la seguridad del paciente y, en caso de que se produzca alguno de los eventos adversos, minimizar su impacto sobre la visión.
The development of anti-VEGF (vascular endothelial growth factor) therapy was a paradigm shift in the treatment of exudative neovascular age-related macular degeneration (ENAMD).1,2 Since its introduction in 2006, the incidence of blindness among AMD patients with ENAMD has been reduced by more than 50%.3–6 However, there are still significant unmet needs in the management of ENAMD. Currently, evidence from real clinical practice in Spain with ENAMD patients shows that almost 70% of the intervals between specialist visits and almost 50% of the intervals between injections are no longer than 8weeks after 2years of follow-up.7 This places a high burden of disease on clinicians, patients and caregivers,8 potentially impacting on the appropriate follow-up and treatment of patients, and leading many to a situation of undertreatment with suboptimal visual acuity gains.9 Furthermore, although the presence of retinal fluid is considered a criterion for retreatment by the main scientific societies.10–13 evidence from actual clinical practice shows that more than 50% of AMD patients' eyes have persistent fluid after 2years of follow-up14 and 35% still have persistent fluid after a decade of treatment.15
Brolucizumab is a single-chain antibody that was developed with the aim of exerting a more potent and longer-lasting effect on macular neovascularisation to reduce the periodicity of injections and thereby reduce the burden of disease.16 The efficacy of brolucizumab was demonstrated in its pivotal HAWK and HARRIER trials, the most recent and robust clinical evidence for the drug in AMD. Brolucizumab 6mg showed non-inferiority in visual acuity gain to aflibercept at week48 (primary endpoint), a gain that was maintained over time until week96. At week16, after the loading phase (same number of injections for both drugs), the brolucizumab arm showed a lower percentage of patients with presence of disease activity. Brolucizumab 6mg was superior to aflibercept in terms of retinal anatomical improvement at weeks16 and48, with results maintained until week96: reduction of central retinal thickness (CRT) and resolution of intraretinal fluid (IRF)/subretinal fluid (SRF) and below retinal pigment epithelium (sub-RPE). These results were obtained with more than 50% of patients treated with brolucizumab at an interval every 12weeks immediately after the loading phase until the first year. More than 75% of these patients were maintained at a 12weekly interval until the end of the study.17,18
Brolucizumab 6mg showed a higher rate of intraocular inflammation (IOI) than aflibercept 2mg (4.5% vs. 0.9%). More than 90% of IOI cases were mild or moderate and 85.3% resolved without sequelae. Retinal arterial occlusion (RAO) events had a rate of 0.9%. However, the proportion of patients who lost ≥15letters was similar with both drugs at week96. At the close of both studies, brolucizumab showed a well-tolerated overall safety profile.17,18 Since then, brolucizumab has been approved in more than 70 countries worldwide.
In February 2020, the American Society of Retinal Specialists (ASRS) presented a safety update detailing 14 cases of retinal vasculitis (RV) in patients treated with brolucizumab (following the marketing of approximately 46,000 injections of the drug), of which 11 were reported with retinal vascular occlusion (RVO).19 Since then, Novartis has received post-marketing reports of rare RVO and RVO-associated adverse events (RVO AEs) following brolucizumab administration.20–24 As of March 2021, the post-marketing incidence rate of RV and/or RVO was 15.6 per 10,000 injections,25 equivalent to 0.16% of treatments sold. In response to these reports, Novartis formed a nine-member Safety Review Committee (SRC), comprising global retina and uveitis specialists, ophthalmology and imaging experts from two external data monitoring committees, and an independent observer from the ASRS. The purpose of the SRC was to provide an independent, unblinded post hoc review of the IRI, endophthalmitis and RVO events detected in the HAWK and HARRIER studies. The SRC reviewed all patient images (60 eyes), determined whether the adverse events were drug-related or not and classified them as IOI, VR and/or OVR, regardless of the terminology of the version of the Medical Dictionary for Regulatory Activities [MedDRA]) used in the trials. The SRC concluded that any form of IOI was identified in 50 of the 1088 eyes treated with brolucizumab (4.6%, similar to that found in the HAWK and HARRIER studies). Of these 50 eyes with IOI, 36 eyes had concomitant RV (3.3%), of which 23 had concomitant RVO (2.1%). Despite the risk of vision loss associated with these events after brolucizumab injection, the overall incidence of moderate vision loss due to OVI remained low (0.7%) and the proportion of patients who lost≥ 15letters was similar between both treatment arms (brolucizumab, 7.4%; aflibercept, 7.7%).26 Due to these findings, the brolucizumab label was updated.27 In the European Union, this update includes the terms “retinal vasculitis” and/or “retinal vascular occlusion”, usually in the presence of intraocular inflammation, in sections 4.4 (special warnings and precautions for use) and 4.8 (undesirable effects).28 The label warns that patients who develop these events should discontinue treatment and AEOI should be treated immediately.28
To date, the first evidence-based studies on the efficacy and safety of brolucizumab in real clinical practice have been published. Two retrospective observational studies on the safety of brolucizumab over 6 months in mostly pre-treated patients have been made possible by the US IRIS and Komodo databases. The detected rates of AEOI in both studies were 2.4% for IOI (including VR) and/or OVR and 0.6% for VR and/or OVR. Both registries had similar population sizes (IRIS, 10,654; Komodo, 11,161) and results consistent with each other and lower than those of the pivotal studies.29 However, both studies have certain limitations: 1) there may be duplicate patients between studies; 2) there is no access to patient records; 3) some AEOI may not have been reported; 4) follow-up was only 6months; and 5) the causal relationship between brolucizumab administration and AEOI could not be determined.
Other more recent observational studies, albeit with smaller population sizes, have concluded more mixed results: from reporting no AEOI30–32 to reporting mild inflammatory events with spontaneous resolution33,34 or inflammatory events of greater severity, but with varying rates.35–42 All this evidence in clinical practice reported so far also reflects that brolucizumab treatment results in anatomical improvement in all types of patients (naïve and pre-treated), favouring improvement of visual acuity in naïve patients and its stabilisation, or even its increase,34,41,42 in pre-treated patients with longer intervals.33,36–42
Although ocular inflammatory events have been widely reported in anti-VEGF treatment for ONAMD,43–55 evidence of non-infectious cases of RV or RVO is limited.56–59 Novartis therefore established an international think tank to understand the underlying causes of AEOI, the characteristics of patients at high risk for EAOI, and how to prevent, mitigate and treat EAOI. Although rare, these events can be associated with moderate to severe vision loss.26 This paper proposes a series of recommendations based on published evidence and the authors' experience to implement the management of brolucizumab in the routine clinical practice of retinologists, to ensure patient safety and, in the event that EAOIs occur, to minimise their impact on vision.
Patient profiling and risk-benefit stratification of brolucizumabInformation on the at-risk patient profile is still limited; however, the first evidence on the aetiology of brolucizumab-related AEOI has been reported. Its late onset (on average 166days since the start of treatment or 20days since the last brolucizumab injection) suggested an immune-mediated cause.25 Some of the independently published cases have suggested that inflammation after brolucizumab injection could be caused by local antibodies that may lead to the formation of immune complexes that, through a mechanism of delayed hypersensitivity, could cause vasculitis. In this regard, a recent study (BASICHR0049) has shown that blood samples from some patients with RV and/or RVO show: 1) increased levels of neutralising anti-drug antibodies; 2) lymphocytesT that are activated after exposure to brolucizumab, and 3) increased platelet aggregation in the presence of brolucizumab and VEGF-A concentrations above physiological levels.28 The authors of the paper suggest that these immune complexes could be formed by anti-drug antibodies, VEGF-A and brolucizumab.60 Other causes proposed by other authors prior to the publication of the BASIC study results are previous anti-VEGF treatment, previous IOI events, presence of certain human leukocyte antigens and comorbidities.20–23,29
So far, the HAWK and HARRIER studies are the largest and strongest clinical evidence of the efficacy and safety of brolucizumab. Of 1088 naïve patients treated with brolucizumab, 50 patients experienced treatment-related inflammatory adverse events.26 More than 90% of the EAOIs were reported as mild or moderate by the investigators and 85.3% resolved without sequelae.25 The mean age of patients presenting with this type of EAOI was 74years and the majority of patients were female (74%).25,28 A recent real-world clinical practice study based on patients with IOL reported to the ASRS revealed that in most cases the symptoms resolved and the mild loss of visual acuity was eventually recovered.61 Based on these data and in agreement with health authorities, brolucizumab has a favourable benefit-risk profile. However, special attention is recommended for the following patient profiles (Fig. 1):
- a)
Patient profiles by risk factor: intraocular inflammation. 1) Patients with active IIO prior to brolucizumab injection.28 A thorough examination of the patient prior to each injection is mandatory to detect signs of inflammation. If active OII is present, brolucizumab use is contraindicated.28,43,44 Inflammation should be treated according to standard medical practice.62,63 2) Patients with a history of prior IIO and/or RVO events from prior anti-VEGF treatments or other causes.28 Recent real-world clinical practice evidence studies revealed that patients with IIO and/or RVO events during the 12months prior to the first brolucizumab injection were more likely to have similar events in the 6months following the injection than patients with no history of such events.29 3) Although evidence is limited, patients with a history of autoimmune disease64 or with systemic inflammatory changes may be potentially excludable because they may have a greater tendency to vascular inflammation. A published case series showed that 20 % of patients with a history of inflammation had underlying autoimmune diseases such as multiple sclerosis, Raynaud's phenomenon, hypothyroidism, Graves' disease and psoriasis.22 In another series, 58 % of cases had arthritis, multiple sclerosis and hypothyroidism, all of which were autoimmune in nature.23 In addition, two case reports of brolucizumab-related vasculitis showed a history of arthritis and hypothyroidism.20,21
- b)
Patient profiling by benefit-risk criteria. The clinical benefits and risks of brolucizumab apply to each treated eye and each injection. 1) Patients with good preservation of visual acuity, absence of fluid and presence of macular atrophy. 2) Monocular patients. In the HAWK and HARRIER studies, the overall rate of any form of IOI associated with loss of≥ 15letters at the last visit was 0.7%.26 Although rare, these events may be associated with moderate to severe vision loss.20–24 3) Patients with bilateral involvement. When treating patients bilaterally, it is advisable not to inject brolucizumab into both eyes at the same time until the underlying causes of AEOI are better understood.22,64
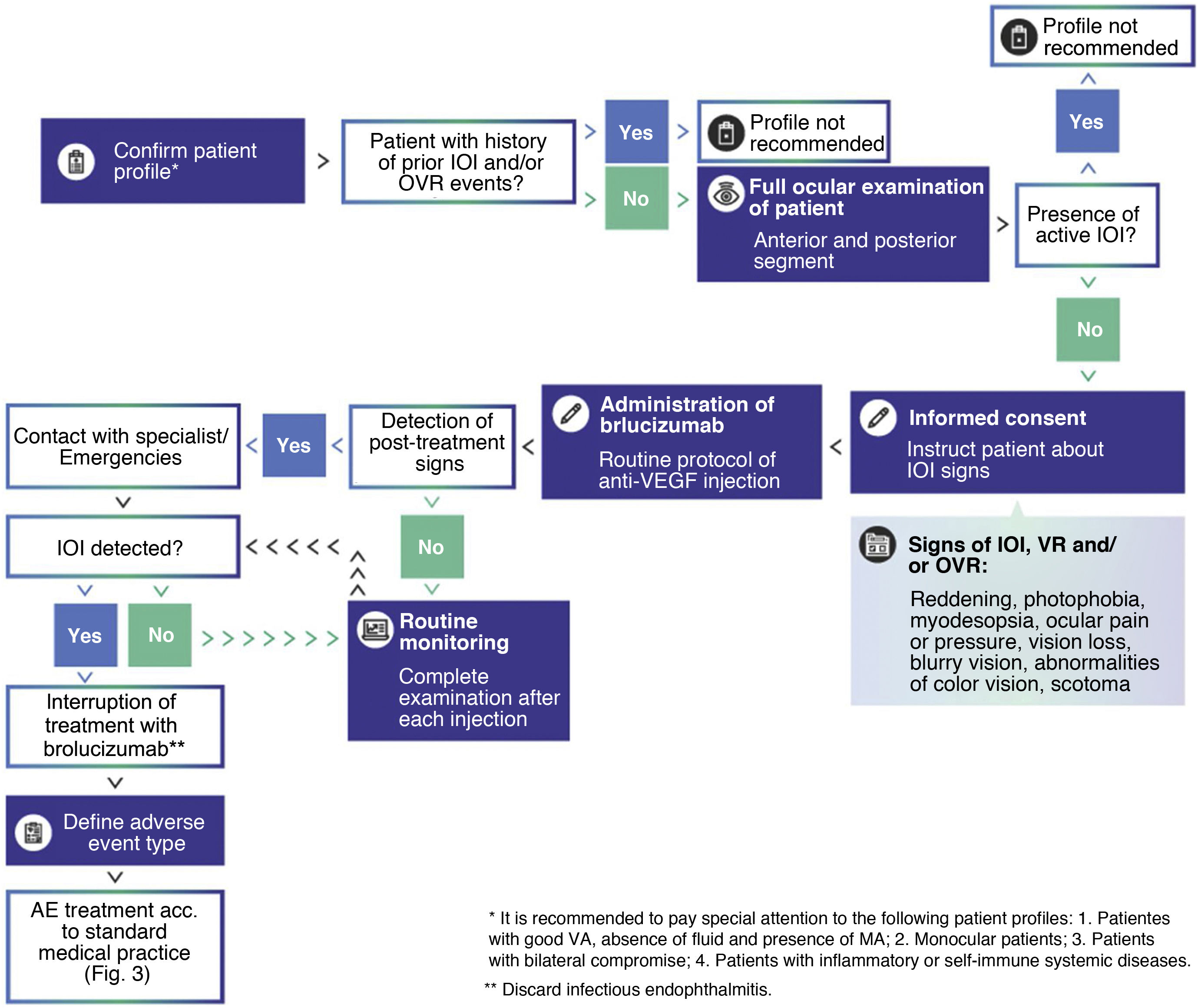
Medical decision algorithm for brolucizumab treatment. It is recommended to confirm the patient's appropriate profile for brolucizumab treatment, perform a thorough examination of the patient prior to each injection, and routinely monitor the patient for IOI events. In the presence of active intraocular inflammation, brolucizumab treatment is contraindicated and should be discontinued.
AM: macular atrophy; VA: visual acuity; IOI: intraocular inflammation; VEGF: vascular endothelial growth factor; VR: retinal vasculitis; RVO: retinal vascular occlusion; VEGF: retinal vasculitis.
Major efforts are being made to investigate the root cause of these events. Although there are several hypotheses that are still being worked on, the underlying cause for the development of these inflammatory events is currently unknown. More information on the pathogenesis of OII will help to better support recommendations on patient profiling and risk-benefit stratification of brolucizumab.60
Brolucizumab treatment regimenThe approved dosing schedule for brolucizumab in patients with ONAMD based on the phaseIII HAWK and HARRIER clinical trials17,18 is one interval every 8−12weeks after the loading phase (initial 3monthly injections).28 Other brolucizumab treatment regimens are still under study. Given the preliminary results of the phase IIIa MERLIN study,65 it is recommended that brolucizumab should not be given at intervals of less than 8weeks after the initial loading phase.28,66 The ongoing phase IIIb TALON67 and TALON-EXT68 clinical trials are evaluating the efficacy and safety of brolucizumab 6mg compared to aflibercept 2mg using a flexible treat-to-control (treat-and-extend) regimen in patients with AMD. These studies will provide new data on the use of brolucizumab at longer treatment intervals of up to 16 and 20weeks, respectively. Evidence from actual long-term clinical practice in other countries will provide additional information on the management and scheduling of brolucizumab in clinical practice. Injection every 8−12 weeks after the loading phase remains an important and effective treatment option for patients with ONAMD28
Mitigation of AEOIs and monitoring of brolucizumab treatmentIn HAWK and HARRIER, approximately 50% of AIAEs occurred during the first 3months of brolucizumab treatment, 75% during the first 6months, and about 70% of cases occurred during the first 4injections of the drug. However, some AEOIs were reported after 18 months from the start of treatment, after 8–10 injections of brolucizumab, and, in some cases, up to 72 days after the last injection.26 More than 50% of AEOIs were classified as mild, and the vast majority resolved without complications.25 Early detection and early treatment have been shown to be key to minimising harm. Data from HAWK and HARRIER showed that signs of IOI preceded RV or RVO events in some patients,25,26 suggesting that these IOIs can be mitigated by performing basic clinical procedures. If a mild adverse event of IIO is not detected early and the patient continues to be treated with brolucizumab, the adverse event may worsen and the patient may eventually develop RV and/or RVO (Fig. 2). Therefore, the medical community and scientific societies recommend that patients receiving brolucizumab during the first months of treatment should be monitored for possible symptoms associated with OVI at every scheduled visit (Fig. 1).
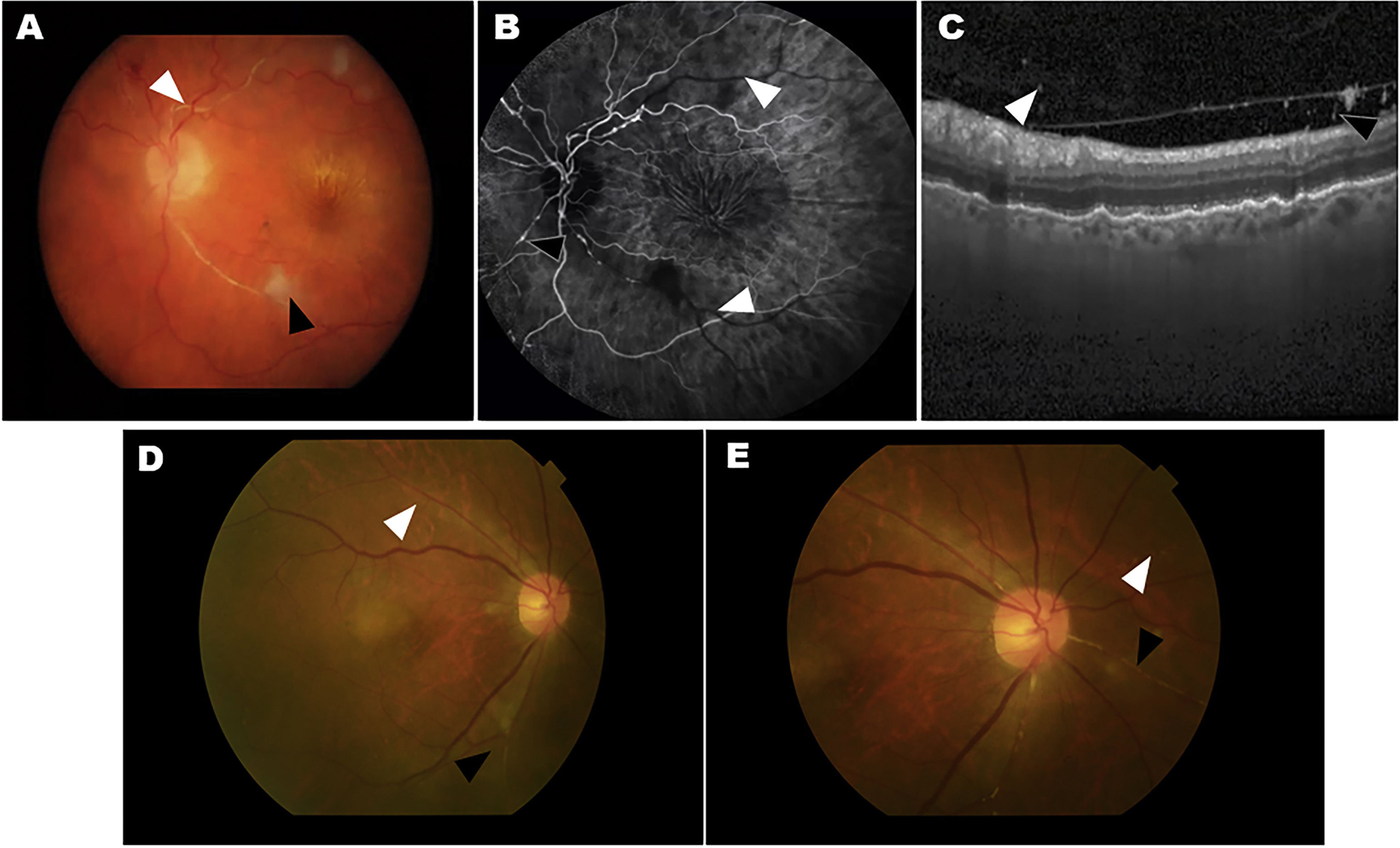
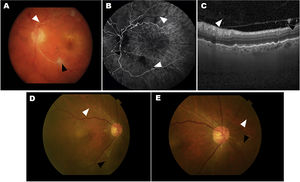
Two cases of eyes with adverse events related to intraocular inflammation (IOI) from the HAWK and HARRIER studies. A–C, case 1: iridocyclitis and RVO. A) Fundus colour photograph shows retinal artery whitening compatible with retinal vascular occlusion (RVO, white arrowhead) and cotton-wool spot (black arrowhead). B) Fluorescein angiography in the venous phase demonstrating lack of retinal arteries perfusion (white arrowheads) and arterial box-carrying (black arrowhead). C) Optical coherence tomography (OCT) image showing the presence of cells in the vitreous in the posterior hyaloid. D–E, case 2: uveitis and RVO. Colour fundus photograph shows small, focal narrowing of retinal arterioles (white arrowhead) and occlusion (black arrowhead).
Figure courtesy of Michael Singer et al.,25 licensed under Creative Commons 4.0 International License.
In HAWK and HARRIER, almost 25% of AEOIs were reported at unscheduled visits.25 Therefore, it is important that physicians also educate patients to detect early the characteristic symptoms and signs of IOI. Patients should watch for signs such as redness, photophobia, eye pain or pressure, myodesopsia, visual field defects, blurred vision, colour vision abnormalities, metamorphopsia, scotoma or loss of vision61,69,70 (Fig. 1). Reaction time is key to avoid severe RV or RVO conditions that ultimately affect vision. The patient should immediately inform the specialist or healthcare facility so that appropriate mitigation measures can be taken. In the presence of active IOI, brolucizumab treatment is contraindicated and should be discontinued (Fig. 1).
Intraocular inflammationPrior to the initiation of brolucizumab treatment, the presence of active inflammation in the patient should be ruled out.28 For this purpose, a complete examination of the anterior and posterior segment of the patient's eye should be performed in detail71 (Fig. 1) to detect: 1) cells in the aqueous humour by slit-lamp examination of the anterior segment; 2) vitreous clouding by slit-lamp fundoscopy in mydriasis (colour fundus photography should also be considered), and 3) the presence of cells in the posterior hyaloid on OCT (Fig. 3). It is important not to confuse the presence of cells in the hyaloid with a posterior vitreous detachment. It is possible not to detect the presence of cells in the posterior hyaloid, although a certain turbidity preventing correct imaging.72,73 In these cases, the ophthalmologist plays an important role in the early detection of IOI. In this regard, a recent post hoc analysis of OCT images of HAWK patients has found the presence of hyperreflective pre-retinal deposits prior to AEOI.74 On imaging, these signs are similar to pre-retinal deposits found in other inflammatory retinal pathologies, although of different aetiology.75–78
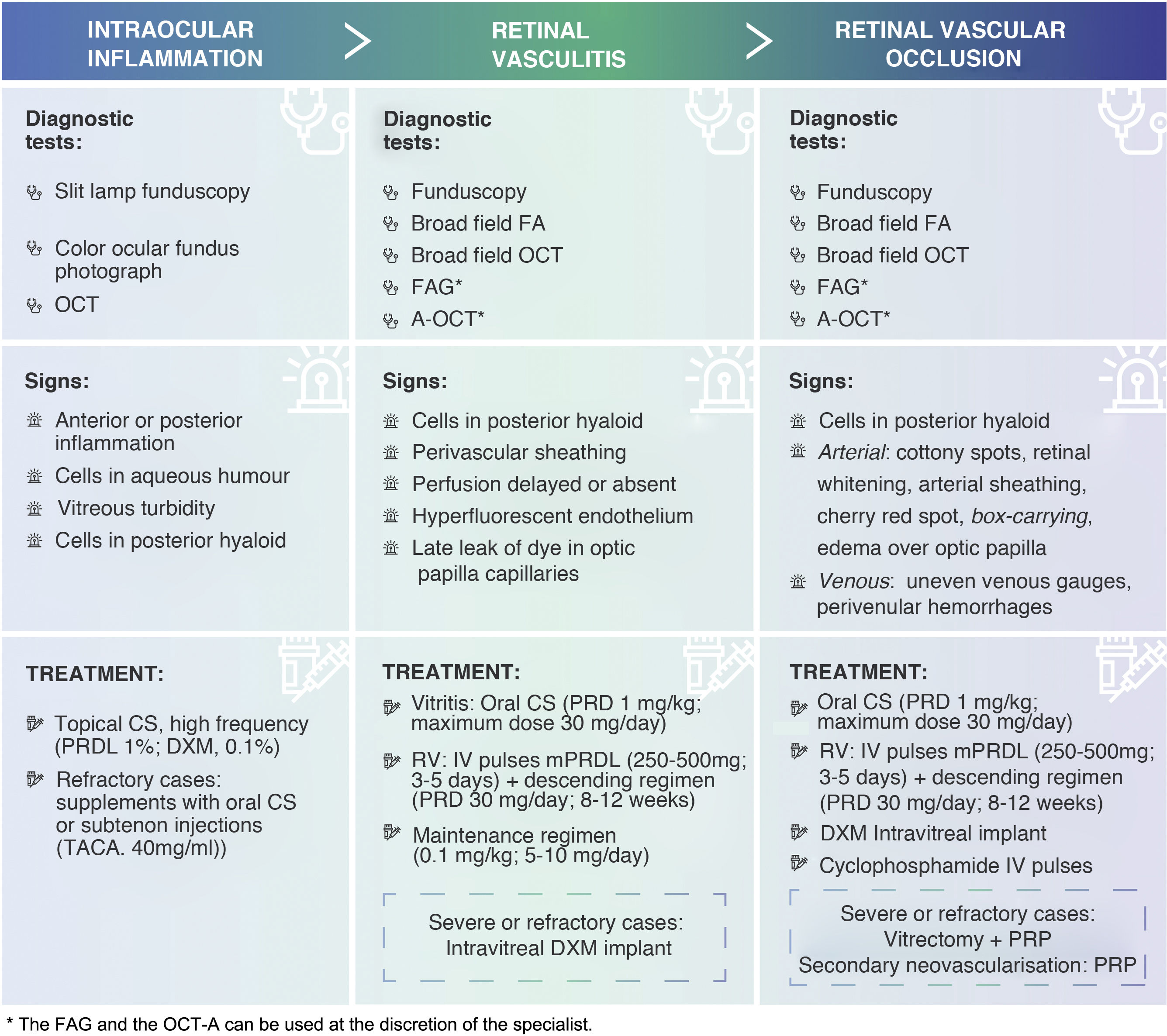
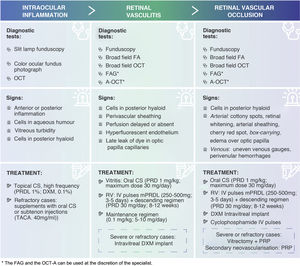
Summary table of signs and treatments of adverse events associated with IIO (EAOI). Corticosteroids are the first-line therapeutic option for the treatment of OII. Depending on the severity of the inflammation, its course and location, clinicians should establish the route of administration, dose and dosage of treatment.
FA: fluorescein angiography; FAG: angiography with indocyanine green; PRP: panretinal photocoagulation; DXM: dexamethasone; IOI: intraocular inflammation; IV: intravenous; IVP: intravenous; mPRDL: methylprednisolone; OCT: optical coherence tomography; OCT-A: angio-OCT; RVO: retinal vascular occlusion; PRD: prednisone; PRDL: prednisolone; TACA: triamcinolone acetonide; RV: retinal vasculitis.
It is essential to distinguish non-infectious IOI from infectious endophthalmitis, as these two conditions may have common clinical presentations, but have different management courses71,79 (Fig. 1). Unlike non-infectious IOI, infectious endophthalmitis develops early, typically within the first week after intravitreal injection of anti-VEGF agent, and is characterised by acute or subacute onset of pain, discomfort, reduced visual acuity, epiphora, conjunctival hyperemia, chemosis, palpebral edema, hypopyon and anterior segment cellular and vitreous or fibrin reaction.71,80 Samples of aqueous and vitreous humour can be taken to ascertain the nature of the infection, and this should be treated according to standard medical practice.71,81 In contrast, IOI presents later after anti-VEGF injection (in the case of brolucizumab, on average 20days after the last injection) and usually does not present with hypopyon or the other acute signs. It is important to rule out any underlying infectious or systemic disease that may contribute to or aggravate these AEAIIs.
Retinal vasculitis and retinal vascular occlusionDuring the first year of treatment, a complete eye examination is recommended prior to repeat treatment with brolucizumab to rule out any signs of OII (Fig. 1). If any of these signs are present (except in cases of anterior uveitis only), fluorescein angiography (FA) is recommended to confirm the diagnosis, better describe the nature of the adverse event and rule out the presence of RV or RVO22,26 (Fig. 2). RV and RVO have varied clinical presentations and may be central, affecting the optic nerve and macula, peripheral or multifocal, affecting large and small vessels. RV usually first affects retinal arteries and later retinal veins with perivenular haemorrhages, with or without occlusive events.82 Wide-field imaging techniques are recommended to review the central and peripheral retina, document the full extent of affected areas and detect any disease activity or progression that might be missed using conventional imaging.26,62,64 In the absence of widefield imaging techniques, standard procedures should be used, imaging the peripheral sectors. Indocyanine green angiography (IGA) or angio-OCT (OCT-A) can be used at the discretion of the specialist to assess changes in vascular flow in the retinal vascular plexuses and choriocapillary vasculature22,82–85 (Fig. 3).
In cases of retinal vasculitis, fundoscopy may be useful to reveal typical signs, such as perivascular sheathing (focal or multifocal),69,86 but must be confirmed by FA. Delayed or absent perfusion in retinal arteries, hyperfluorescence of retinal vascular endothelium or late leakage of dye in dilated capillaries of the optic papilla on FA are signs of RV69,87 (Fig. 3).
In cases of retinal vascular occlusion, fundus examination may be useful to reveal signs of ischemia, such as the presence of cotton-wool spots (typical in precapillary retinal arteriolar occlusions), retinal whitening, arterial sheathing and the cherry red spot in the macula seen in cases of acute central retinal artery occlusion. Other signs may include attenuation of retinal arteries, segmentation of retinal vessel blood flow (box-carrying), edema over the optic papilla, irregular venous gauges and intraretinal perivenular hemorrhages.70,87 OCT imaging is recommended to detect the presence of cells in the posterior vitreous and to explore the thickness of the macula (presence of edema).69 FA is recommended to confirm vascular occlusion, assess the degree of ischemia and define the affected area69,70,88 (Fig. 3).
Treatment of adverse events associated with inflammationRecommendations for the treatment of AEOIs will be guided by standard medical practice and the severity of the adverse event. The duration and intensity of treatment administered will depend on the response of the individual patient. Ideally, such events should be treated by a retina or uveitis specialist.
In HAWK and HARRIER, the investigators reported more than 50% of IRI-related adverse events as mild, with a median duration of 54days, 40% as moderate, with a median duration of 88days, and less than 6% as severe, with a median duration of 237days. More than 80% of EAOIs resolved without sequelae25; some without the need for treatment.22,25 There was no correlation between the number of brolucizumab injections received and the severity of AIAE.25 In some patients, signs of IIO preceded VR or OVR events,25,26 so early detection and early treatment are key to prevent worsening of AEOIs and minimise damage to the patient's vision.
Corticosteroids are the first-line therapeutic option for the treatment of IOI.89,90 Therefore, once the diagnosis of IOI is confirmed, initiation of corticosteroid therapy is recommended. Depending on the severity of the inflammation, its course and location, physicians should establish the route of administration, dose and dosage of treatment (Fig. 3). If symptoms worsen with the use of corticosteroids, the infectious aetiology should be reconsidered62,63 (Fig. 1).
Corticosteroid treatments can increase intraocular pressure (IOP)91 and induce cataract formation.92 IOP should be checked to rule out corticosteroid-induced ocular hypertension or inflammation. If necessary, IOP lowering medication (beta-blockers or carbonic anhydrase inhibitors) can be prescribed. For this reason, special attention should be paid to the patient's medical history to ensure that he or she is not at increased risk for a corticosteroid-induced side effect. In cases of intolerance, significant side effects or contraindications to corticosteroids, other treatment options such as immunomodulatory agents or non-steroidal agents can be considered.93 However, at present, there is no evidence of the effectiveness of these therapies in these conditions62,63; more information on the aetiology of brolucizumab-induced AEOIs will be needed to determine their therapeutic potential.
Anterior uveitisAnterior chamber swelling without vascular inflammation is a mild adverse event, but may be the initial sign of more serious conditions. Before prescribing treatment for the adverse event, FA is recommended to make an accurate diagnosis and to rule out posterior uveitis or RV.22,26 As a general rule, anterior uveitis can be well managed with topical corticosteroid therapy, preferably with high potency active substances (prednisolone acetate, 1%; dexamethasone, 0.1%) and at a high frequency (up to once an hour in cases of severe inflammation with a SUN score of +3 or higher).90,94,95 If there is no response, topical therapy can be supplemented with oral corticosteroids or subtenon injections (triamcinolone acetonide, 40mg/ml).96 The frequency should be reduced and the dose adjusted (or using less potent active ingredients) as the inflammation subsides. Anterior uveitis may require treatment for several weeks (Fig. 3).
Posterior uveitis, retinal vasculitis and retinal vascular occlusionIf the inflammation involves the vitreous humour and/or the posterior segment of the eye, it is recommended to confirm the presence of retinal vasculitis by FA22,26 and to reinforce treatment with an intensive course of intravitreal and/or systemic corticosteroids. It is important to carefully monitor RV patients early on to assess the extent and severity of vascular leakage and to identify any ischemic complications to allow timely intervention.70 The onset of RV is a key moment, as ischemia and thus irreversible structural damage and visual impairment has not yet occurred.
If IOI consists of vitritis only, oral corticosteroids (approximately 1mg/kg of prednisone equivalents; maximum dose 30mg/day) are recommended. If, in addition, RV is found, intravenous methylprednisolone pulses (250−500mg for 3−5days) are recommended, followed by prednisone 30mg/day in a tapering schedule for a period of 8−12weeks depending on the clinical course of the event.97 The side effects of long-term systemic corticosteroid use are well known.98 Therefore, ideally, steroid treatment should be withdrawn gradually. If necessary, it should be gradually tapered to a maintenance dose (0.1mg/kg; 5−10mg/day)99 (Fig. 3). An alternative to methylprednisolone pulses, or as adjunctive treatment in severe or refractory cases, is intravitreal implantation of dexamethasone100 (Fig. 3). However, it is preferable to avoid intraocular treatment in such cases. Intravitreal implantation of fluocinolone is not recommended because the onset of its effect is late (up to one month in some cases) and its duration is too long for these cases (up to 3years).101 In the case of occlusive vasculitis, higher doses of methylprednisolone (500mg pulses for 3−5days), initial dexamethasone implantation or even intravenous pulses of cyclophosphamide can be considered, in which case collaboration with the internal medicine, rheumatology or immunology departments is recommended. In these cases, vitrectomy associated with panretinal photocoagulation may be an alternative with potential benefit22,23; however, clinical evidence on this option is limited (Fig. 3).
It is very important to closely monitor patients with retinal vascular occlusive events. Severe ischemic conditions may develop secondary neovascularisation. Panretinal photocoagulation can be used in case of secondary neovascularisation and considered in patients with extensive peripheral ischemia before the appearance of new vessels62 (Fig. 3).
ConclusionsSo far, the HAWK and HARRIER studies are the most recent and robust clinical evidence on the efficacy and safety of brolucizumab.17,18 Early data in real clinical practice provide preliminary evidence on the incidence and management of AIAEs; however, these are limited by small sample sizes and short follow-up periods of the studies from which they have been obtained.29–42 In addition to standard safety reporting, a systematic, multi-pronged analysis is currently underway in an effort to better understand the nature of AEOIs following brolucizumab treatment and how to address them.25,26,62,64
Current anti-VEGF treatments have proven to be effective and safe in the management of AMD; however, there are still significant unmet needs for these therapies.7,9,14,15 Brolucizumab is a new generation anti-VEGF capable of providing effective disease control with improved durability.17,18 Although IOIs (VR and OVR) detected during the commercialisation of brolucizumab are generally rare28 and affordable to manage if detected early, they may be associated with a loss of visual acuity.26,62 Treatment with brolucizumab therefore requires: 1) detailed initial assessment to rule out patients with profiles not recommended for brolucizumab; 2) screening of the patient before each injection to rule out the presence of active inflammation; 3) monitoring for warning signs for early detection of possible IOIs, especially during the first 6months of treatment; and 4) immediate treatment of IOIs develop (Fig. 1).
Patients treated with brolucizumab should be made aware and take an active role in the monitoring process. They should be educated to identify early symptoms of ocular inflammation and be made aware of the importance of early treatment. These symptoms are warning signs that should not be ignored by the patient, but should be reported immediately to the relevant health centre (Fig. 1). It is advisable for the specialist to share educational information with them, whether linked to the Novartis Risk Management Plan or associated with hospital protocols. In addition, it is desirable to inform and train hospital emergency departments so they know how to care for and explore these patients, and identify and manage this type of adverse event.
NEAMD is a condition that requires strict treatment and monitoring,7 which is very resource-intensive.102 In this sense, brolucizumab is a different and necessary therapeutic alternative that meets important unmet needs. The recommendations proposed in this study are based on the evidence published to date and on the authors' experience in order to implement correct management of brolucizumab by the ophthalmological community, guarantee patient safety and, in the event of an IOI, minimise its impact on vision. Evidence from actual long-term clinical practice in other countries and the results of the research conducted so far will provide additional information on how to manage brolucizumab in clinical practice.
EndorsementsThis work has the scientific endorsement of the Spanish Society of Retina and Vitreous (SERV) and the Spanish Society of Ocular Inflammation (SEIOC).
FundingThe services of BCNscience for the drafting and translation of this manuscript have been contracted in collaboration with Novartis Farmacéutica S.A.
Authors' contributionAll authors have contributed equally to the preparation, drafting and revision of the manuscript. This paper has been developed based on published scientific evidence and the authors' experience. The content of the manuscript has been independently chosen and developed by the authors. Novartis Farmacéutica S.A. has contributed funding for the writing and translation services of the text, but has not participated in the preparation, review and/or approval of the manuscript.
Conflicts of interestAlejandro Fonollosa has consulted for Novartis, Bayer and Brill, and has held paid conferences for Abbvie, Allergan and Brill.
Roberto Gallego-Pinazo has been a consultant for Carl Zeiss Meditec, Novartis, ORA Clinical and Roche, has held paid conferences for Alimera, Allergan, Heidelberg Engineering, Horus Pharma, Novartis, NTC Pharma and Roche, and has worked as a researcher for Celltrion, Ionis, Iveric BioPharma, Novartis and Roche.
Laura Sararols has been a consultant for Novartis, Bayer, Allergan and Roche, and has worked as a researcher for Celltrion, Chengdu Khanghon, Ionis, Iveric BioPharma, Novartis, Bayer and Roche.
Alfredo Adán has been a consultant, paid lecturer and researcher for Novartis, Bayer, Abbvie and Alimera.
Maribel López Gálvez has been a consultant for Novartis, Bayer, Allergan, Roche, Alimera and Ferrer.
Marta S. Figueroa has been a consultant for Novartis, Bayer, Allergan, Roche, Alcon and Zeiss, and has worked as a researcher for Novartis, Roche, Allergan, Chengdu Kanghong Biothechnology, Gyroscope, Celltrion and Opthea Limited.
Other than those mentioned, the authors have no other relevant affiliations or financial holdings with any organisation or entity with a financial interest or conflict with the subject matter or materials discussed in the manuscript.
Please cite this article as: Fonollosa A, Gallego-Pinazo R, Sararols L, Adán A, López-Gálvez M, Figueroa MS. Guía de recomendaciones para el manejo de brolucizumab. Arch Soc Esp Oftalmol. 2022;97:626–638.