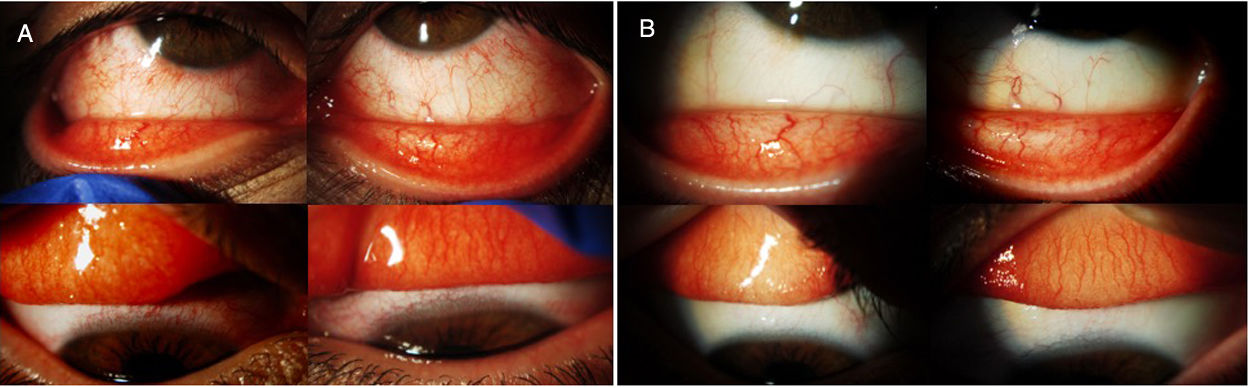
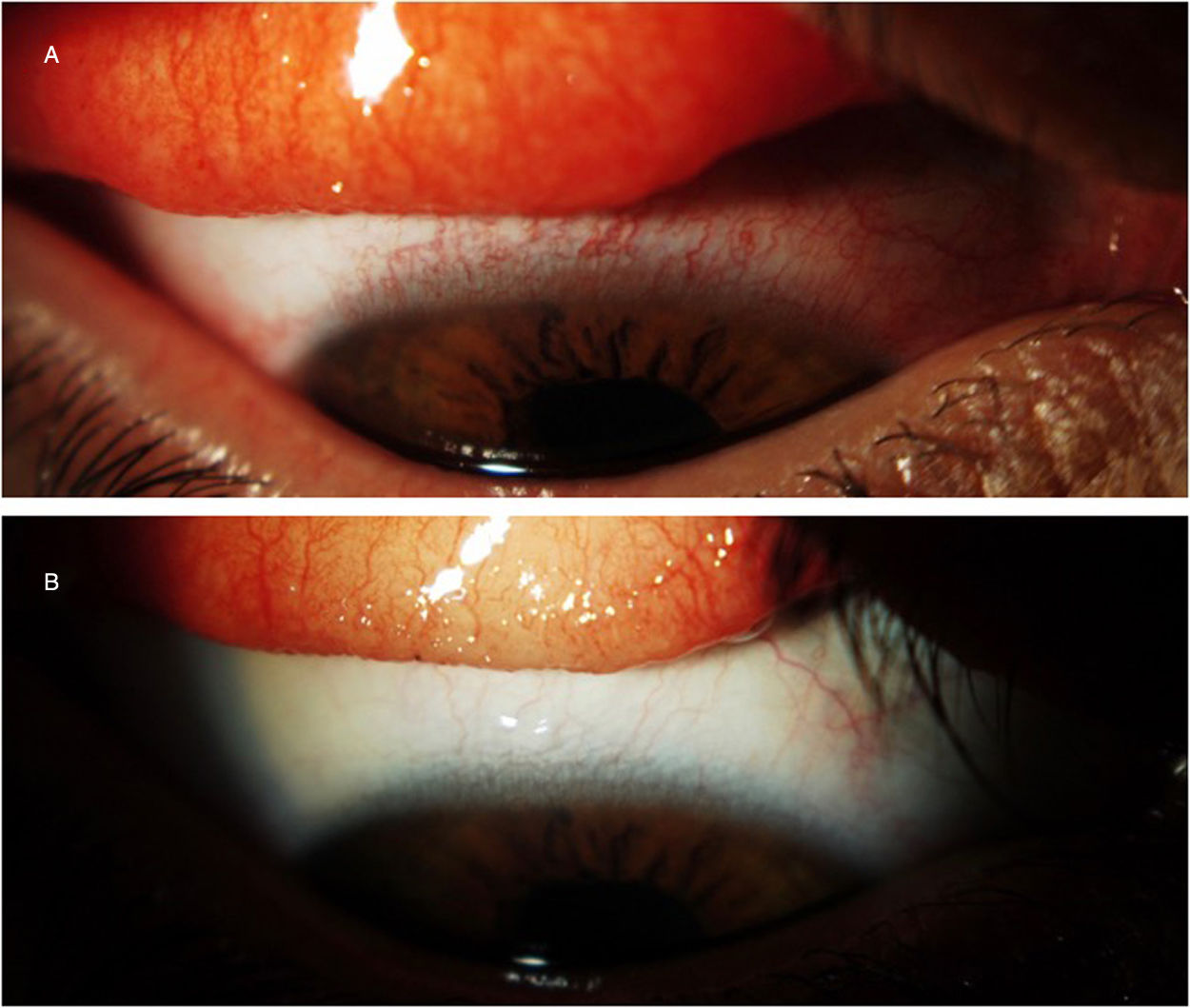
Dupilumab is a drug that has recently been approved by the Food and Drug Administration (FDA) and European Medical Agency (EMA) for the treatment of moderate-to-severe atopic dermatitis in adults. An increase in frequency of conjunctivitis related to dupilumab treatment has been reported in recent publications and clinical trials. We report two steroid-dependent cases satisfactorily treated with cyclosporine 0.1% (Ikervis®). To our knowledge there are no reported cases of dupilumab-associated conjunctivitis treated with cyclosporine 0.1% (Ikervis®).
El dupilumab es un fármaco de reciente aprobación por la Food and Drug Administration (FDA) y la European Medical Agency (EMA) para el tratamiento de la dermatitis atópica moderada-severa en adultos. El incremento de la frecuencia de conjuntivitis asociadas a dupilumab ha sido expuesto en publicaciones y ensayos recientes. Presentamos 2 casos de conjuntivitis corticodependiente tratados satisfactoriamente con ciclosporina al 0,1% (Ikervis®). No hay casos previos descritos de conjuntivitis asociada a dupilumab tratados con ciclosporina al 0,1% (Ikervis®).