To assess the safety and effectiveness of phacoemulsification combined with XEN45 implant surgery in patients with cataract and open-angle glaucoma, with 12-month follow-up.
MethodsA prospective study conducted on 30 eyes requiring phacoemulsification with, at least, 2 medications to control intraocular pressure (IOP). Phacoemulsification combined with XEN45 implant surgery was performed within 15min of administering subconjunctival mitomycin C. Surgery was performed through 2 temporal incisions, separated by 90°, using the inferior to enter the XEN45 and to implant it in the superior nasal region. A record was made of the best corrected visual acuity, IOP before and 1 day, 1 month, 3 months, 6 months, 9 months, and 12 months after surgery, the number of antiglaucomatous medications, and complications.
ResultsThe best corrected visual acuity was 0.37±0.2 and 0.72±0.15 before and 12 months after surgery, respectively. The pre-operative IOP was 21.2±3.4mmHg, with 3.07 drugs, decreasing by 61.65% on the first day, 37.26% at 1 month, 35.05% at 3 months, 31% at 6 months, 30.6% at 9 months, and 29.34% at 12 months. The number of medications decreased by 94.57%. Complications occurred in 3 eyes, 2 of them were excluded because we could not complete the implantation (280° subconjunctival hemorrhage and XEN extrusion when trying to reposition). In a third case, the bleb was encapsulated at 5 months after surgery.
ConclusionsThe phacoemulsification combined with XEN45 implant surgery can effectively reduce IOP and the number of drugs in mild-moderate open-angle glaucoma, as they rehabilitate the VA. The use of only 2 micro-invasive incisions makes it simple, quick and safe, with few complications at 12 months follow-up from surgery.
Analizar la eficacia y seguridad de la técnica combinada de facoemulsificación e implante XEN45 empleando acceso temporal y 2 únicas incisiones, en casos de catarata y glaucoma crónico de ángulo abierto, con seguimiento de 12 meses.
MétodosEstudio prospectivo de 30 ojos que requerían facoemulsificación y que precisaban, al menos, dos medicamentos para controlar presión intraocular (PIO). Se efectuó cirugía combinada de facoemulsificación e implante XEN45 a los 15min de administrar mitomicina C subconjuntival. El procedimiento se realizó a través de 2 incisiones temporales, separadas por 90°, utilizando la inferior para introducir el XEN45 e implantarlo en región nasal superior. Se registró agudeza visual mejor corregida, PIO previa y en días 1-30-90-180-270 y 365 poscirugía, número de medicamentos hipotensores y complicaciones.
ResultadosLa agudeza visual mejor corregida preoperatoria fue 0,37±0,2 y 0,72±0,15 a los 12 meses. La PIO previa fue 21,2±3,4mmHg con 3,07 fármacos, descendiendo un 61,65% el primer día, 37,26% al mes, 35,05% al tercer mes, 31% al sexto mes, 30,6% al noveno mes y 29,34% a los 12 meses. El número de fármacos disminuyó un 94,57%. Solo hubo complicaciones destacables en 3 ojos, de ellos, 2 se excluyeron al no poder completar implantación (uno por hemorragia subconjuntival en 280° y otro, por extrusión del XEN al intentar recolocarlo). En un tercero, la ampolla se encapsuló a los 5 meses poscirugía.
ConclusionesLa cirugía combinada de facoemulsificación e implante XEN45 reduce eficazmente la PIO y el número de medicamentos en el glaucoma crónico de ángulo abierto leve-moderado, al tiempo que rehabilita la AV. El empleo de solo 2 incisiones posibilita una cirugía microinvasiva sencilla, rápida y segura con escasas complicaciones tras 12 meses de seguimiento.
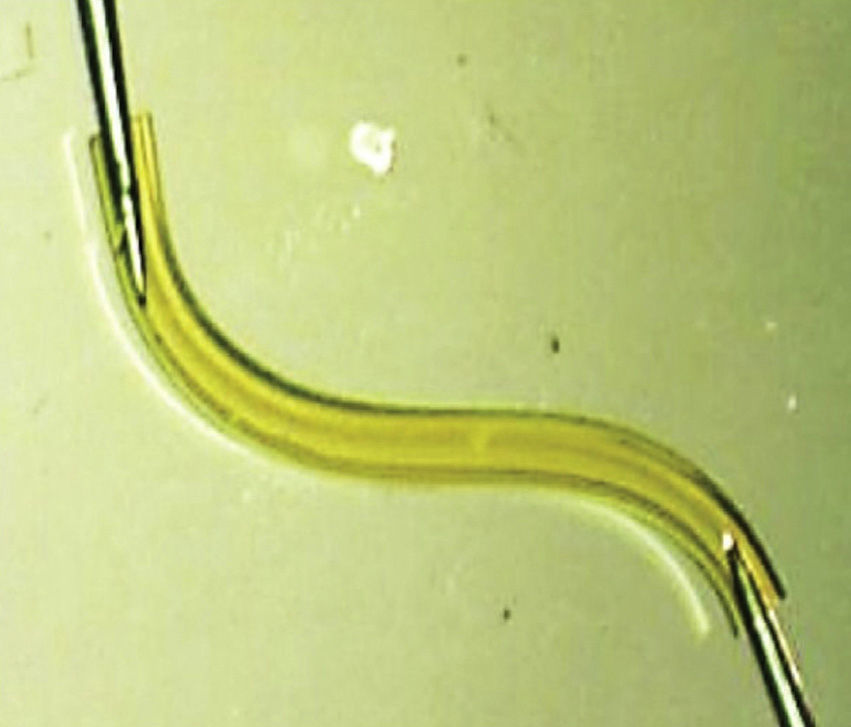
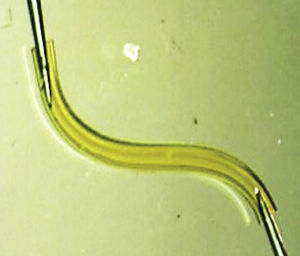
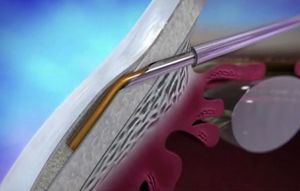
Cataracts and glaucoma are among the main causes of visual loss worldwide.1 The development of these 2 diseases, which frequently occurs simultaneously with aging, means that over 20% of patients who undergo cataract surgery also have glaucoma.2 Traditionally, in combined surgery, trabeculectomy was associated to phacoemulsification. However, even though trabeculectomy is the gold standard for lowering intraocular pressure (IOP), it involves potentially severe complications.3 For this reason, to minimize said complications micro-invasive surgery techniques have emerged, such as microinvasive glaucoma surgery – (MIGS).3–5 One of the new devices is XEN (AqueSys Inc., California, USA). The third generation of this implant, XEN45, is a valve-free collagen tube measuring 6mm long, 150μm thick and 45μm inner diameter (Fig. 1) that is implanted from the anterior chamber (AC) into the subconjunctival space (SC), creating a scleral channel which facilitates aqueous humor drainage.6–9 Even though several multicenter studies are being developed in Europe and Canada, their results have not yet been published in impact journals even though the preliminary studies indicate that XEN45 is efficient and safe for diminishing IOP and the number of medicaments in glaucomatous patients.10–15
The purpose of this study is to assess the safety and efficacy of combined surgery comprising phacoemulsification and XEN45 implant (FACO-XEN) through temporal access and 2 single incisions. To this end, the procedure was completed in 30 cases referred for cataract surgery with slight or moderate chronic open angle glaucoma (COAG), with a follow-up period of 12 months.
Materials and methodsStudy designA prospective, non-controlled and non-randomized study to assess the efficacy and safety of combined FACO-XEN surgery with temporal access and 2 single incisions. Selected patients presented a diagnostic of cataracts and COAG. Informed consents in writing were obtained from said patients for performing said combined surgery and for the present study. The protocol was approved by the Ethics Committee of the authors’ institution in compliance with the Helsinki Declaration guidelines as well as the stipulations of Spanish laws in force.
SubjectsPatients were recruited in the Glaucoma Section of the Ophthalmology Dept. of the University Clinic Hospital of Valencia (Spain). Each patient underwent a full ophthalmological examination comprising clinical history and anamnesis, Snellen scale best corrected visual acuity (BCVA), slitlamp biomicroscopy, Goldmann applanation tonometry (AT 900, Haag Strait, Bern, Germany), gonioscopy, ocular fundus examination with dilatation, biometry IOL Master 500 (Carl Zeiss Meditec, Jena, Germany), central corneal pachymetry (OcuScan RxP®, Alcon Laboratories, Fort Worth, TX, USA), Humphrey automatic perimetry (HFA II 740i®, Carl Zeiss Meditec, Jena, Germany) and retinal nerve fiber layer optical coherence tomography (Cirrus HD-OCT 500®, Carl Zeiss Meditec, Jena, Germany). The inclusion criteria comprised: previous slight or moderate COAG diagnostic (determined by a mean deviation between 0 and −12dB in the 24-2 Humphrey campimetry strategy)16; IOP under 30mmHg with at least 2 hypotensor pharmacological principles, associated cataracts diagnostic with BCVA not above 0.6, healthy and mobile conjunctival area in the superior nasal quadrant, Shaffer angle equal to or above 3 in gonioscopy and age 18 or over. The exclusion criteria comprised any condition other than the above or associated pathology that could hinder follow-up or the surgical procedure.
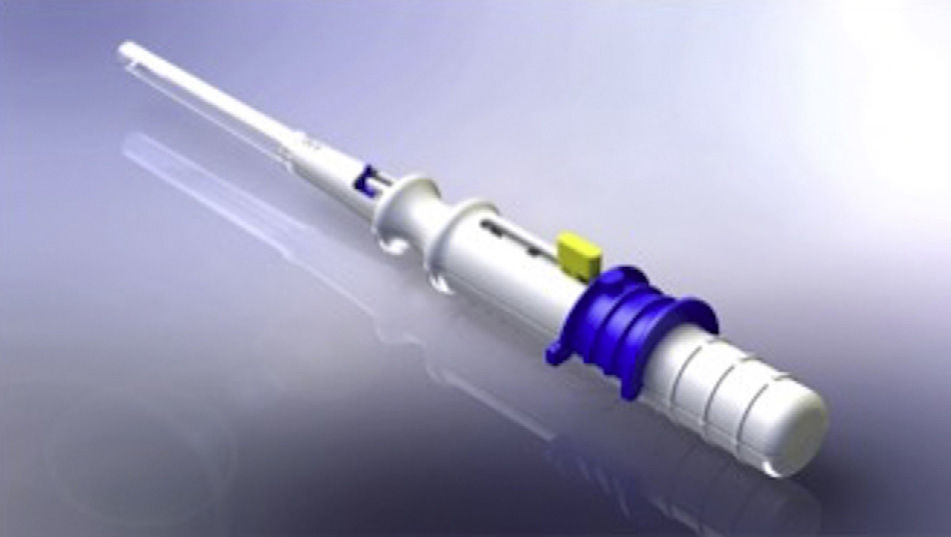
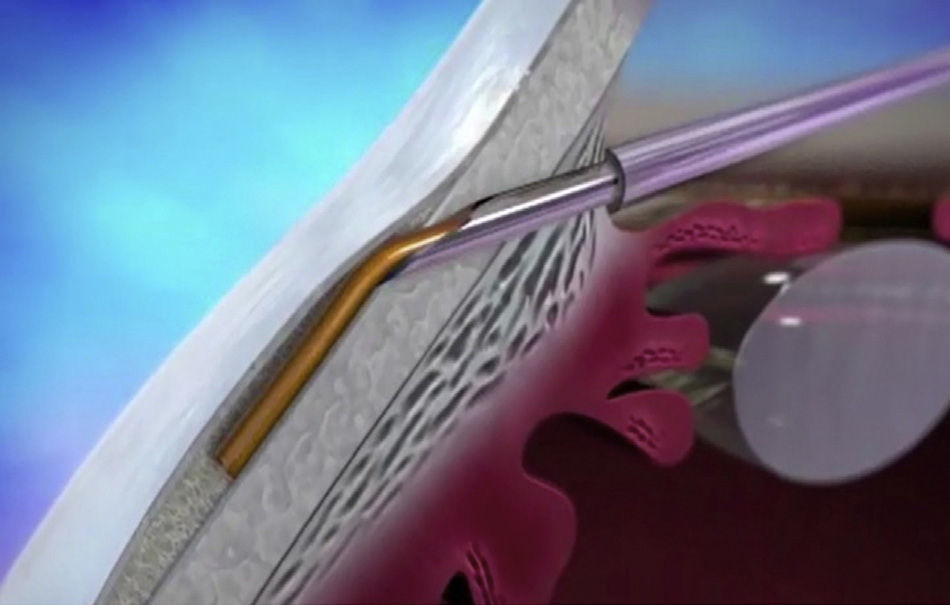
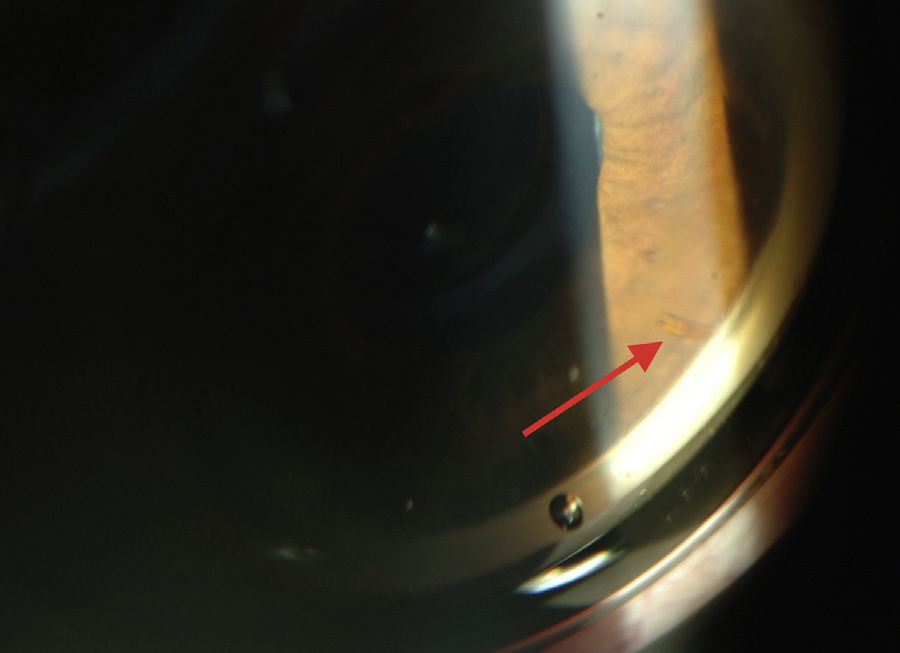
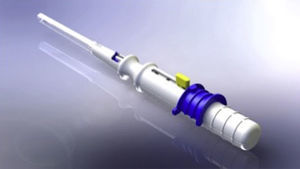
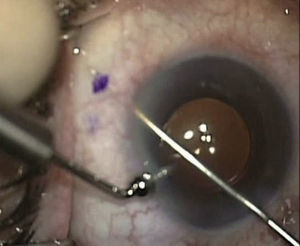
XEN45The XEN endo-prosthesis is a hydrophilic collagen tube made up of gelatin derived from porcine dermis and mixed with glutaraldehyde.7–9 In the dry state it is a hard material but when it becomes hydrated after implantation it expands, becoming softer and more flexible. This assists to avoid the migration of the implant postop.9 The third generation of this device, XEN45, features adjusted and reduced dimensions to avoid excessive drainage.6 For surgical implant, The XEN device is preloaded in an injector for implanting it during surgery. The injector consists in a 27-Gauss injection needle (through which the XEN is injected) and a sleeve with a slider which enables the application of controlled pressure for implanting the device (Fig. 2). The XEN device is inserted ab interno, in the angle, and emerges at 3mm from the limbus, creating an intra-scleral channel that drains the aqueous humor from the AC toward the SC space (Fig. 3).
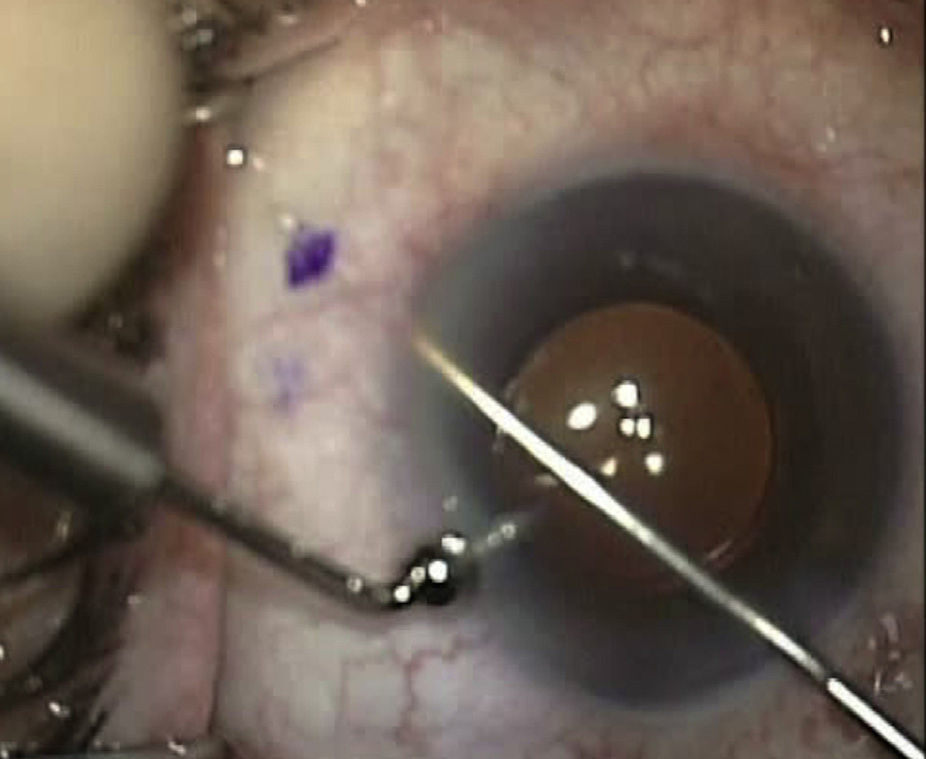
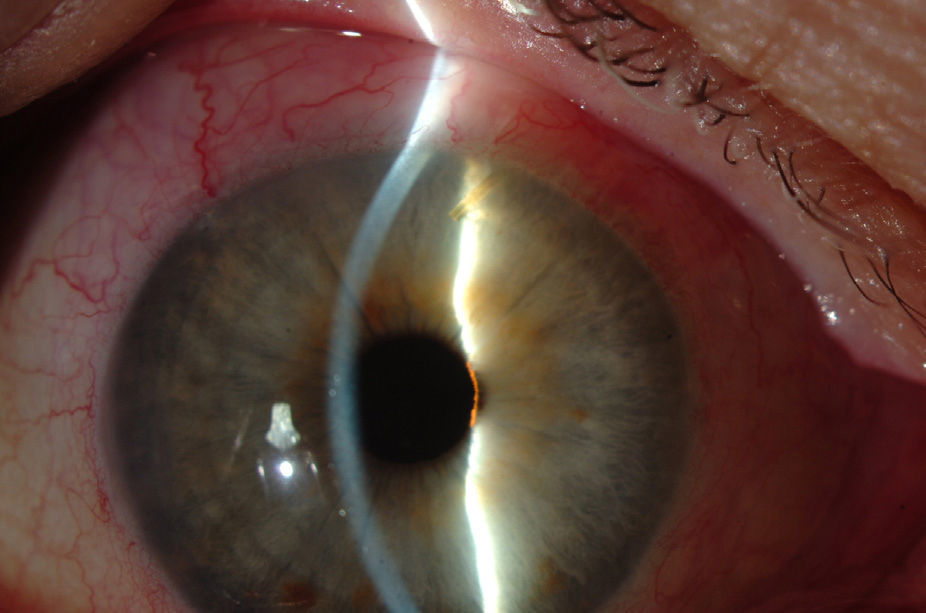
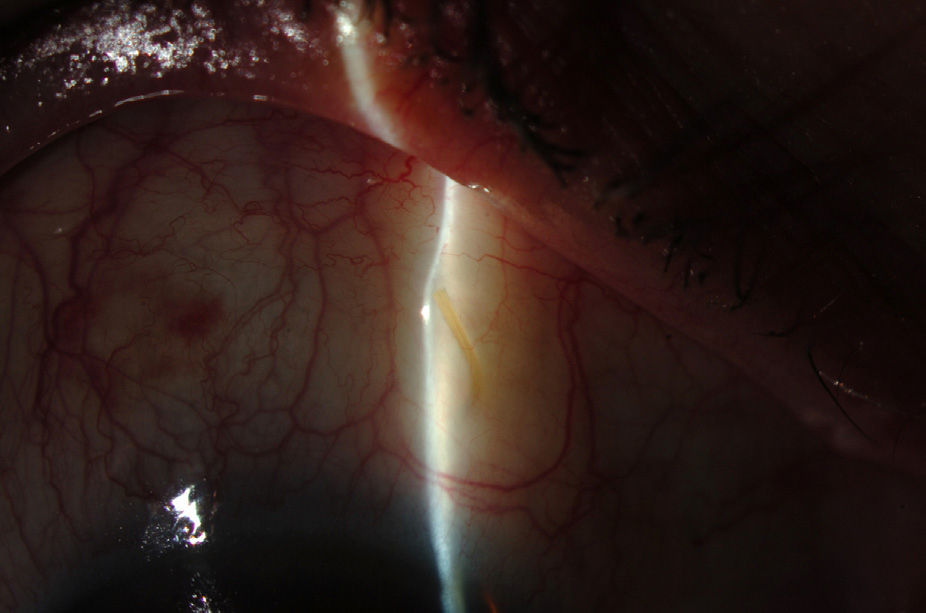
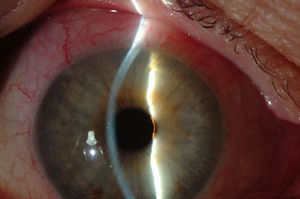
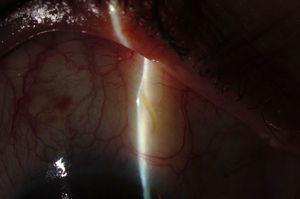
Surgical techniqueAll the operations were performed by the same surgeon (VTP-T) and with the same surgical technique. Peribulbar anesthesia was applied (with 5mL mepivacaine and 5mL bupivacaine) and posterior application of the Honan balloon. Fifteen minutes before the surgical procedure, 1mL of 0.01% mitomycin C (MMC) was injected with a gauge 13 needle SC at 5mm of the limbus at 12 o’clock, subsequently displaced and distributed with a surgical sponge to the superior nasal sector. All patients underwent standard phacoemulsification, with the surgeon standing in temporal position from the eye as this was the usual technique. For implanting the XEN device the 2 previously performed incisions were utilized without requiring new ones. Accordingly, in right eyes a 1.8mm inferior temporal incision was made and a 1mm paracentesis at 90°, superior temporal position. In the left eyes, the main incision was temporal superior and paracentesis at 90° temporal inferior. After implanting an acrylic intraocular lens in the capsular sac, 1% acetylcholine was injected (Acetilcolina 1%®, Alcon Cusí, El Masnou, Barcelona, Spain) in AC in order to contract the pupil, followed by high density viscoelastic (Healon®, Abbott Medical Optics, Uppsala, Sweden) for greater angle opening. Reference points were marked at 3mm from the limbus and at 12 o’clock nasal superior, the area in which the implant would be placed. Thereafter the XEN45 injector needle was introduced into the AC through the inferior temporal incision, which was in the main incision for the right eye and the paracentesis of the left eye. At the same time, in order to fix the eye and exert counterpressure, a Vera hook was introduced through the superior temporal incision. The injector penetrated through the opposite side to the implantation site. The needle was introduced and fixed in the angle to carry out a 3mm intra-scleral pathway (Fig. 4). The SC needle was withdrawn. When the entire bezel is seen, it is rotated 90°. After moving down the implantation sleeve, the implant is released. Subsequently the needle is withdrawn. The SC pathway of the XEN device is verified. Viscoelastic is aspired and, by means of gonioscopy, the adequate implementation of the device in the angle is verified. The incisions are hydrated and bleb formation (caused by filtration of the serum toward the SC space) is also verified. Finally, 0.1mL of 1% cefuroxime is injected in AC and betamethasone acetate inferior subconjunctival (Celestone Cronodose®, MSD, Madrid, Spain). Postop care included antibiotic prophylaxis with 0.3% ciprofloxacin (Oftacilox®, Alcon Cusí, El Masnou, Barcelona, Spain) 4 times a day during 2 weeks, and anti-inflammatory therapy with 0.1% sodium diclofenac (Voltaren®, Thea Laboratoires, Clermont-Ferrand, France) 4 times a day during 4 weeks, in association with 0.1% dexamethasone (Dexametasona, Alcon Cusí) in decreasing dosage during 8 weeks. No antihypertensive medication was administered as part of the post-surgery protocol. Figs. 5–7 show the appearance at 24h post-implant.
The surgical complications and difficulties, as well as the postoperative checkups carried out at 24h and at 30, 90, 180, 270 and 365 days were recorded. All visits comprised slitlamp biomicroscopy, IOP measurement with applanation tonometry (previously adjusted following manufacturer specifications) and registration of the number of hypotensor medicaments. BCVA was also measured in visits at 30, 180 and 365 days post-surgery.
Data analysisThe main efficacy parameter was IOP measured with applanation tonometer. The secondary efficacy parameter was the number of required hypotensor medicaments. Safety measures comprised BCVA and the appearance of complications. All the statistical analyses were performed with the SPSS software (V.21.0 for Mac; SPSS Inc., Chicago, IL, USA). The Student's t-test was utilized for related data for comparing IOP results, and the Wilcoxon non-parametric test for comparing BCVA results in the study group. Statistical significance was established at p≤0.05.
ResultsDemographic dataPrior to the analysis, 2 patients were excluded because it was not possible to adequately implant the XEN device. One exhibited a subconjunctival hemorrhage over 280° after the MMC injection and in the other the device extruded to the SC space when trying to relocate it. Accordingly, the data of 30 eyes were analyzed (16 right eyes and 14 left eyes) of 18 patients: 5 males and 13 females, with ages between 67 and 91 years (mean 76±5.85 years). Bilateral surgery was performed in 12 patients and unilateral surgery in 6. All patients exhibited COAG and cataracts. The follow-up comprised 12 months.
Intra-surgery difficulties and complicationsDifficulties were observed in the introduction of the XEN injector needle in 8 eyes (26.6%) because it encountered the orbit edge or the blepharostat. This was resolved by rotating the head and relocating the blepharostat, even reaching the nasal position. After MMC injection, 11 eyes (36.6%) experienced subconjunctival hemorrhage. After introducing the needle in the angle, slight intra-chamber hemorrhage occurred in 26 eyes (86.6%), although it was attenuated by the application of dense viscoelastic and resolved in all cases with mechanical irritation-aspiration. Discrete hemorrhage in the scleral exit points of the XEN device was observed in 27 eyes (90%), without additional consequences. In 6 eyes (20%) it was necessary to relocate the implant due to short subconjunctival pathway (under 2mm), by means of scleral approach with blunt tweezers. In one eye (3.3%), the XEN device was extracted from the AC due to an excessively long intrachamber pathway, although it was reimplanted with the same injector. None of the eyes exhibited intra-surgery complications related to cataract surgery (Table 1).
Surgical difficulties and complications of FACO-XEN surgery.
| Difficulties and complications of FACO-XEN45 surgery | ||
|---|---|---|
| Number (%) | ||
| Difficulties/complications | (n=30) | |
| Cataract surgery | 0 | (0) |
| Mitomycin injection | ||
| Subconjuntival hemorrhage | 11 | (36.6) |
| XEN45 implantation | ||
| Slight intracameral hemorrhage | 26 | (86.6) |
| Hemorrhage at scleral exit point | 27 | (90) |
| XEN relocation required | 6 | (20) |
| XEN re-implant required | 1 | (3.3) |
| Post-surgery follow-up | ||
| Athalamia or hypotonia <5mmHg | 0 | (0) |
| Encapsulation of filtration bleb | 1 | (3.3) |
None of the eyes exhibited athalamia, hypotony below 5mmHg or other severe complications. None of the cases that exhibited intrachamber hemorrhage during surgery evidenced hyphema over 1mm at 24h. In one case (3.3%) the bleb encapsulated at follow-up month 5, requiring topical hypotensor treatment with 3 pharmacological principles for controlling IOP at 12 months (Table 1).
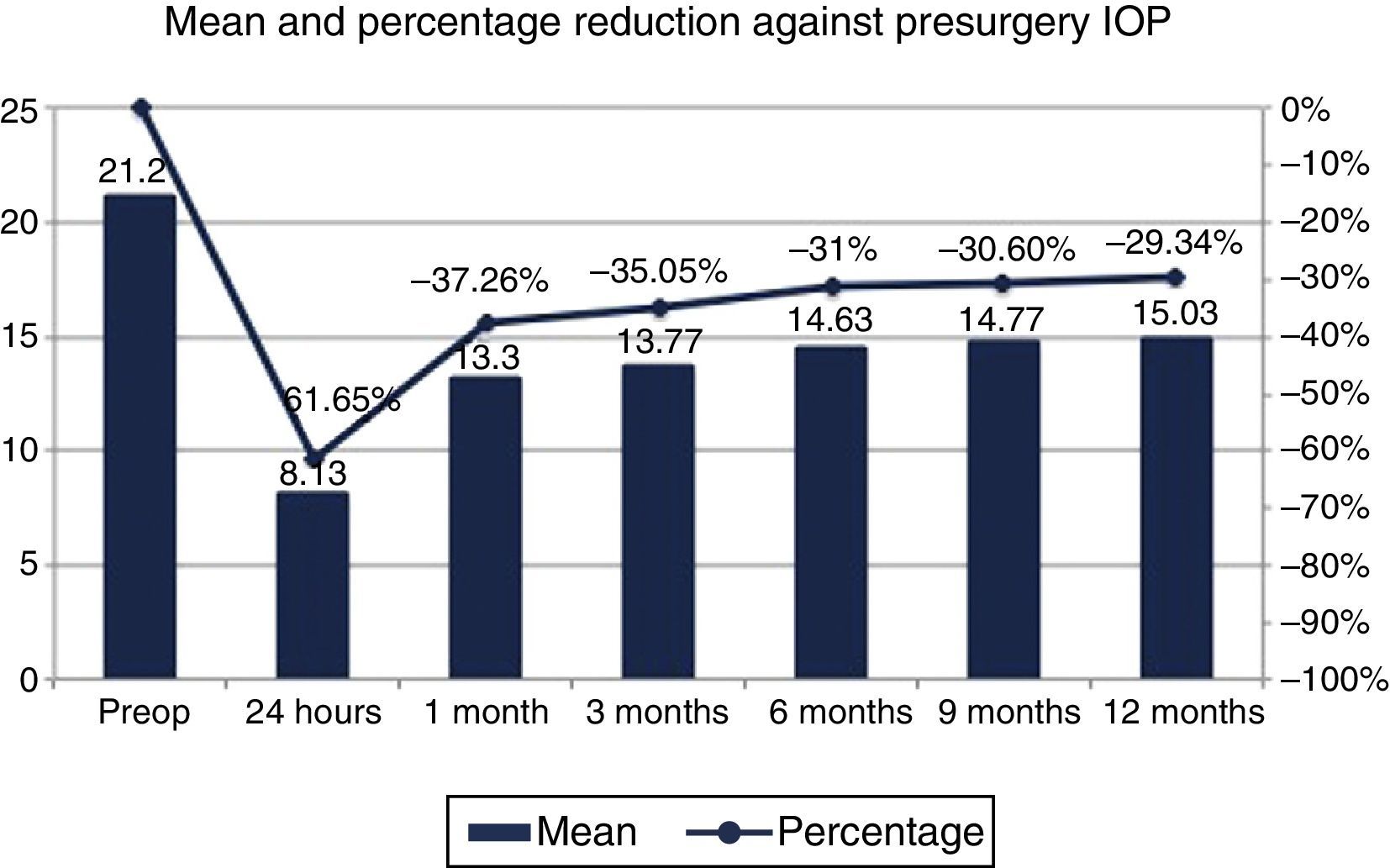
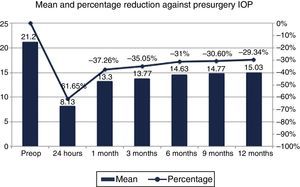
IOP resultsMean presurgery IOP with medication was 21.2±3.4mmHg. In postoperative follow-up, mean IOP was 8.13±3mmHg at 24h, 13.30±2.25mmHg at month 1, 13.77±2.06mmHg at 3 months, 14.63±1.81mmHg at 6 months, 14.77±2.22 at 9 months and 15.03±2.47mmHg, involving an IOP reduction of 61.65% at 24h, 37.26% at month 1, 35.05% at 3 months, 31% at 6 months, 30.6% at 9 months and 29.34% at 12 months (p<0.001) (Fig. 8).
MedicamentsThe mean anti-glaucomatous medicaments prior to surgery were 3.07±0.69 with a range of 2–4 pharmacological active principles. At follow-up month 12, the mean amount of medicaments diminished to 0.17±0.65 (p<0.001), a reduction of 94.57%. At the end of the follow-up period, 3 patients (3 eyes) required anti-glaucomatous treatment.
Success ratePrior to the beginning of the study, treatment success rate was defined as IOP reduction of ≤18mmHg without medicaments, in accordance with other clinical studies.17,18 According to said definition, after 12 months follow-up, the success rate was of 90%, being achieved in 27/30 patients. Two of the remaining patients required hypotensor medication to maintain IOP ≤18mmHg and the other required 3 medicaments to maintain IOP ≤21mmHg (encapsulated bleb case).
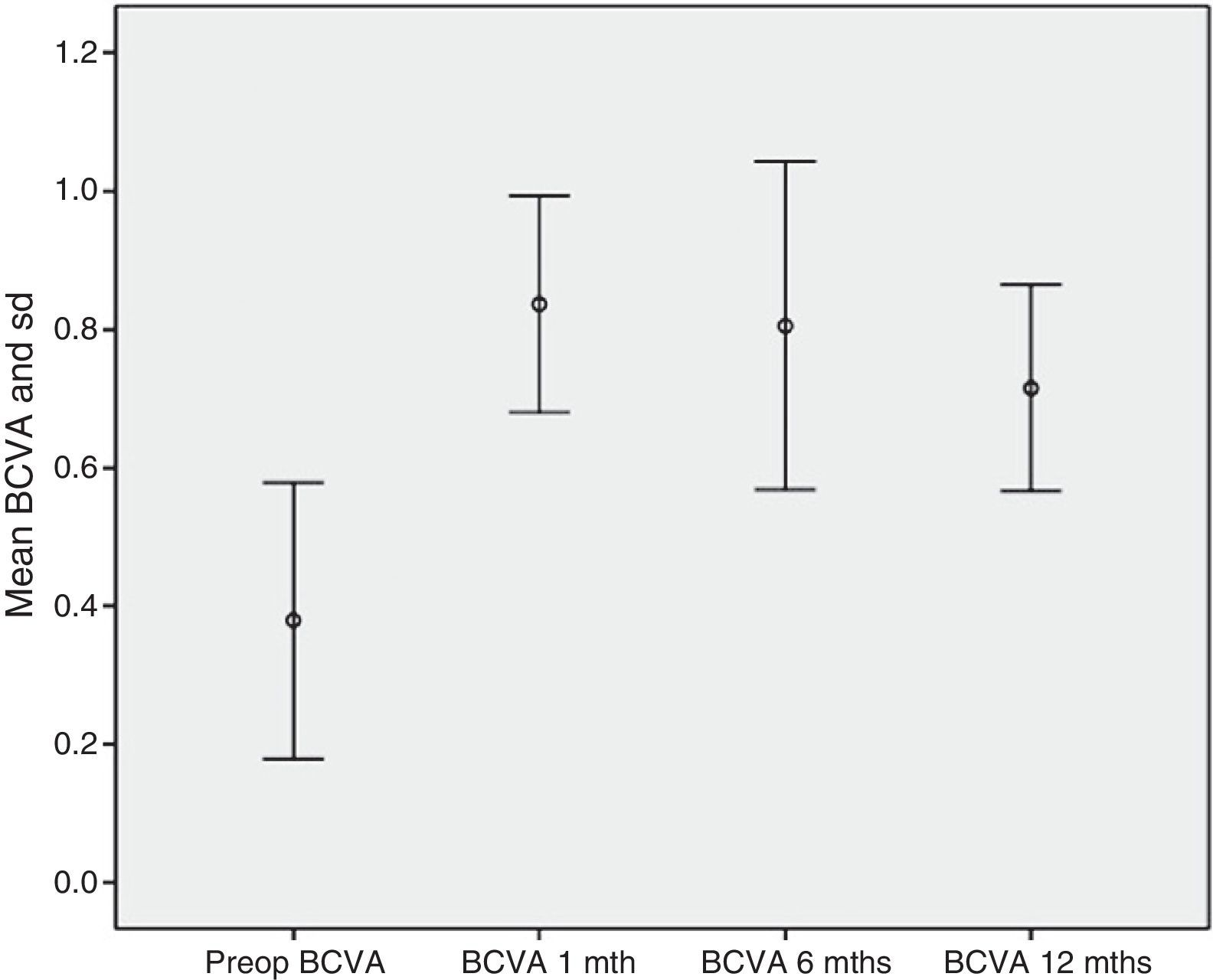
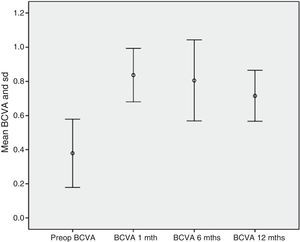
Safety resultsNone of the patients exhibited diminished VA when compared to pre-op values. BCVA increased from 0.37±0.2 pre-op to 0.84±0.16 at month 1, 0.81±0.24 at 6 months and 0.72±0.15 at 12 months follow-up (p<0.001) (Fig. 9). In addition, none of the patients exhibited severe intra- or post-surgery complications.
DiscussionThe XEN45 is one of the MIGS alternatives. These techniques are attracting growing attention in recent years in the endeavor to achieve less traumatic surgical procedures for glaucoma and easier to combine with phacoemulsification, while being safe and efficient to control IOP.3–5,19,20 MIGS are based on creating various drainage pathways for aqueous humor according to the device and share an ab interno approach, without impacting the conjunctiva and with small corneal incisions. In principle, MIGS are safer than trabeculectomy but it is yet to be seen whether they are comparable in efficiency. The XEN implant is the only MIGS procedure that creates a subconjunctival bleb and can benefit from the use of MMC to improve its results and prevent fibrosis.21
The present series of 30 eyes which underwent combined FACO-XEN surgery achieved an IOP reduction of 29.34% against presurgery values. This reduction is greater than the reduction demonstrated by isolated phacoemulsification in patients with COAG.22 The number of hypotensor medicaments diminished 94.57% at month 12 post-surgery. As regards safety, both intra-and post-surgery complications were minor and inherent to the surgical technique, such as bleeding during the implantation, and resolved with relative ease. A critical point is the final placement of the XEN device. Due to the size of the device (6mm), the best pathway would be subconjunctival (2mm), intra-scleral (3mm) and 1mm long in AC, to avoid extrusion or contact with the corneal endothelium, having at the same time a 3mm pathway of intra-scleral resistance to avoid excessive drainage. This led the authors to intra-surgery relocation of 6 implants (20%) and reimplantation of one (3.3%). None of the patients exhibited severe intra-or postoperative complications related to phacoemulsification on the XEN45 implant. In the authors’ experience, implanting the XEN45 device is relatively simple and fast in combination with temporal access to cataract surgery due to the fact that the same 2 small incisions are utilized. This facilitates surgery and saves time, in addition to diminishing added ocular manipulation as well as facilitating easier postoperative periods. In addition, BCVA at the end of the follow-up improved significantly vis-à-vis presurgery values in all patients, as can be expected after cataract surgery.
To date there are no publications about the XEN device in international impact journals but reports in meetings continue to communicate good results. For Reitsamer et al.10 the implantation of the XEN device, in its various inner diameter versions, is safe and efficient, achieving 40% IOP reduction, although only 28 of 567 patients have reached a follow-up of 3 years. Stalmans et al.,11 who carried out a one year follow-up after the implant of XEN45, achieved IOP reduction close to 41%. Sheybani and Ahmed,12 reported numbers very similar to those of the present study with combined surgery comprising XEN45 implant and MMC in 31 eyes, obtaining IOP reduction of 30% and 37% at 6 and 12 months respectively, although they achieved a reduction in the use of medicaments of only 67% at one year post-surgery. In Spain, the Prospective Clinical Trial of Teus et al.13 is the most relevant study, characterized by 3 months follow-up, and the study by Díaz Céspedes et al.14,15 with a one year follow-up, obtaining IOP reductions of 30–37%. The present study achieved IOP reduction of 29.34% at 12 months, although the baseline comprised a higher number of presurgery drugs and no presurgery cleansing period was carried out. In addition, the sample utilized in the present series was homogeneous because FACO-XEN was performed in all cases with the same surgical technique and by the same surgeon. Other authors implanted the XEN device in various conditions: as a single technique, in phakic and pseudophakic patients, as well as in combination with cataract surgery, in addition to carrying out surgery in different hospitals by different surgeons.10,13–15 However, all reports emphasize the efficiency and safety of the device.10–15
The conclusion of the present study is that combined FACO-XEN surgery enables efficient reduction of IOP and number of hypotensor medicaments in eyes with slight or moderate COAG, in addition to recovering visual acuity. Temporal access and 2 unique incisions for both techniques allow simple, fast, efficient and safe micro-invasive surgery with very few complications in 12-month follow-ups. However, randomized and controlled studies with higher numbers of patients and longer terms are necessary to confirm the promising results described above.
Conflict of interestsNone declared.
Please cite this article as: Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, Gargallo-Benedicto A, Osorio-Alayo V, Barreiro-Rego A, et al. Cirugía combinada mediante facoemulsificación e implante XEN45 con acceso temporal y 2 únicas incisiones. Arch Soc Esp Oftalmol. 2016;91:415–421.
This paper has partially been presented as a communication in the 91 Congress of the Ophthalmology Society of Spain, Sevilla, Spain.