Bacterial infections frequently cause decompensating events in cirrhotic patients and are also the most common factor identified for the development of acute-on-chronic liver failure (ACLF). The increase in the prevalence of infections caused by multidrug-resistant (MDR) microorganisms has resulted in the reduced effectiveness of empiric antimicrobial treatment. We conducted a PubMed search from the last 20 years using the Keywords cirrhosis; multidrug-resistant; infections; diagnosis; treatment; prophylaxis; monitoring; sepsis; nutrition and antibiotic resistant. We made a review about bacterial infections among cirrhotic patients; we mainly focus on the description of diagnostic tools; biomarkers; clinical scores for diagnosis and prognosis also; we made an analysis concerning the monitoring of cirrhotic patients with sepsis and finally made some recommendations about the treatment; prophylaxis and prevention.
Patients with cirrhosis are at a higher risk for developing bacterial infections (BI). Patients that present with advanced cirrhosis, ascites, variceal bleeding (VB), reduced protein concentration in ascites and a history of spontaneous bacterial peritonitis (SBP) are particularly susceptible [1]. BI frequently cause decompensating events in the cirrhotic patient, such as VB, hepatorenal syndrome (HRS) and hepatic encephalopathy (HE) and are also the most common factor identified for the development of acute-on-chronic liver failure (ACLF) [2]. The increase in the prevalence of infections caused by multidrug-resistant (MDR) microorganisms (bacteria that are not susceptible to at least one agent in three or more antimicrobial categories) [2] has resulted in the reduced effectiveness of empiric antimicrobial treatment, one of the measures considered to greatly decrease the mortality rates in patients with sepsis [3]. The aim of the present review is to analyze and establish new recommendations for diagnosis, monitoring, treatment and prevention.
2Predisposing factors for infections in cirrhosisNumerous factors are associated with an increased risk of infections in cirrhotic patients. We briefly expose these factors.
2.1ImmunodeficiencyCirrhosis is a state of immune dysfunction and also a state of excessive activation of pro-inflammatory cytokines, this is called as cirrhosis-associated immune dysfunction syndrome, which increases the risk of infections [4]. Monocyte spreading, chemotaxis, bacterial phagocytosis, neutrophil mobilization, phagocytic activity and intracellular killing are impaired in cirrhosis [4]. As a result of hypersplenism, cirrhotic patients may have neutropenia. They also have lower levels of immunoglobulins IgM, IgG and IgA. In both serum and ascites fluid, C3, C4 and CH50 concentrations are inferior leading to diminished bactericidal activity [4]. Genetic polymorphisms of toll-like receptors and nucleotide-binding oligomerization domain 2 genes could be responsible for bacterial translocation [4].
2.2Bacterial translocationBacterial translocation is the migration of bacteria or bacterial products from the intestinal lumen to the mesenteric lymph nodes. Changes in the intestinal mucosa like vascular congestion, edema, oxidative stress and local inflammation are factors associated with an increased intestinal permeability, additionally, autonomic dysfunction, increased nitric oxide synthesis and oxidative stress retard intestinal motility, which leads to intestinal bacterial overgrowth. The conjunction of increased intestinal permeability, bacterial overgrowth, dysbiosis and immunodeficiency facilitate the spread of intestinal bacterial to extra intestinal sites and predispose patients with cirrhosis to infections [4,5].
3Implications of infections in cirrhosisBacterial infections increase mortality four-fold in patients with cirrhosis. Thirty percent of cirrhotic patients with sepsis die within the first month after infection and another 30% within a year [10–12]. BI has been related to reduced 5-year survival in patients with cirrhosis due to HCV (60.2% versus 90.4%) and HBV (69.2% versus 97.6%) [6].
As mentioned above, BI are the most common precipitating factor for HRS and ACLF [2], the latter considered the main cause of death in patients with cirrhosis. ACLF is a syndrome characterized by acute decompensation of chronic liver disease (even without cirrhosis) associated with organ failure and high short-term mortality [7]. The pathophysiology is unclear, but an excessive systemic inflammatory response is a hallmark of ACLF [7]. In the CANONIC study, the most comprehensive registry of ACLF, BI were the major identifiable trigger (30%) [7]. The diagnosis and grade of ACLF is stablished according to the presence, type and number of organ failures calculated with the CLIF-C ACLF score, this is based on the CANONIC study population and has a higher prognostic accuracy than the previous diagnose score system; CLIF-SOFA (CLIF-C ACLF can be calculated in the website: http://www.efclif.com) [8,9]. The severity is graded according to the number of organ failures in grade 1–3, mortality correlates with ACLF severity 22%, 32% and 73% respectively [7]. The resolution rate depends on the initial ACLF grade, 55% in ACLF grade 1 and 15% in grade 3, but the clinical course is the most important determinant of short-term mortality [9], the most of the patients reach their final grade of ACLF in the first week after diagnosis, therefore, the reassessment of ACLF should be done between the 3rd and 7th after diagnosis, this reassessment predicted 28-day and 90-day mortality more accurately than the calculated at diagnosis [8,9]. Patients with ACLF should be admitted to the ICU and ideally in a transplant center, the treatment is based on life support as well as management of the associated complications and precipitating factors. Liver transplant (LT) is the definitive treatment for patients with ACLF [10], in patients with ACLF grade 2 or 3 survival without liver transplant is<20% and increases to 80% when LT is performed, as comparable with transplanted patients without ACLF [7].
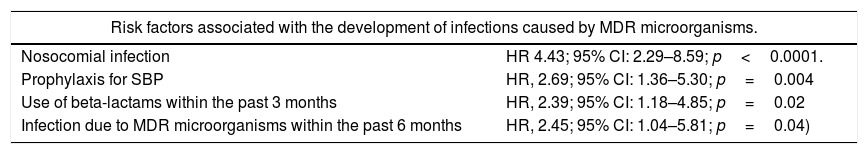
4Epidemiology, types of infection and bacterial resistance in cirrhosisBacterial infections are present in 32–34% of hospitalized cirrhotic patients, which is 4–5 times more frequent compared with patients hospitalized for other causes and occur more often in patients hospitalized for gastrointestinal bleeding [11]. According to infection site, BI present as: spontaneous bacteremia (5.4–21%), urinary tract infection (21–25%), pneumonia (8–19%), soft tissue infection (8–13%) and spontaneous bacterial peritonitis (23–27%) [12–14]. As in other populations, an increase in the frequency of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E), methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcus faecium has been found in patients with cirrhosis [1,2,15], with a global prevalence of 34% in hospitalized patients [14]. Table 1 shows the risk factors that have been identified for infections by those microorganisms.
Risk factors associated with the development of infections caused by MDR microorganisms according to a multivariate analysis at a single center. MDR: multidrug-resistant.
| Risk factors associated with the development of infections caused by MDR microorganisms. | |
|---|---|
| Nosocomial infection | HR 4.43; 95% CI: 2.29–8.59; p<0.0001. |
| Prophylaxis for SBP | HR, 2.69; 95% CI: 1.36–5.30; p=0.004 |
| Use of beta-lactams within the past 3 months | HR, 2.39; 95% CI: 1.18–4.85; p=0.02 |
| Infection due to MDR microorganisms within the past 6 months | HR, 2.45; 95% CI: 1.04–5.81; p=0.04) |
The importance of the increased prevalence of those infections lies in the choice of antibiotic empiric therapy and the consequences of its failure. Patients with MDR bacterial isolates have been found to have a lower rate of infection resolution [70% versus 92% (p≤0.0001)], a greater probability of sepsis [26% versus 10% (p≤0.0001)] and a higher mortality rate [25% versus 12% (p≤0.001)] [3,14].
Nosocomial infections (those diagnosed after 48h of hospitalization) in cirrhotic patients cause greater mortality, compared to healthcare-associated infections (patients with hospitalization or short term admission for at least 2 days in the previous 90 days, resident in nursing home or a long-term care facility or chronic hemodialysis) and community-acquired infections (25–58% vs 9–23% vs 7–21%, respectively) [3]. In hospital-acquired and healthcare-associated infections, MDR bacteria are more frequently isolated (35% and 14%, respectively) than in patients with community-acquired infections (4%, p<0.001) [3,15].
Patients with advanced cirrhosis are highly susceptible to the development of MDR BI, these patients require frequent hospitalizations and are frequently exposed to antibiotic use. Fernandez and cols, in a single center surveillance epidemiological study found an alarming increase of the prevalence of MDR microorganisms from<10% in 1998–2000 to 23% in 2010–2011 [3]. There is a marked difference among geographic regions of the prevalence of MDR organisms [16], in the CANONIC study, the largest analysis of MDR BI in patients with decompensated cirrhosis and ACLF in Europe, the overall prevalence of MDR infection was 29.2% in 9 of the 12 countries incorporated in the study. In the GLOBAL study, a worldwide report of hospitalized patients with cirrhosis in 46 centers from Europe, Asia and America the global prevalence of MDR bacteria was 34% [14]. Infections caused by MDR bacteria were associated with a more severe course, poorer infection resolution and a higher 28-day mortality rate, especially if empiric treatment was inadequately administered [13].
5Diagnosis of bacterial infection in patients with cirrhosisInfections in cirrhotic patients have a wide range of clinical presentations: asymptomatic, classical presentation according to the infection site, sepsis, hepatic decompensation (hepatic encephalopathy or VB) and ACLF. Thus, it is important to always rule out an infection in patients with a recently decompensating event (jaundice, HE, VB and ascites) and have a low threshold of suspicion, in order to avoid a delay in the proper treatment.
It is well-known that BI can induce systemic inflammatory response syndrome (SIRS), which presents in 57–70% of infected cirrhotic patients [19]. However, the diagnostic criteria for SIRS has a low sensitivity and specificity for diagnosing BI in cirrhotic patients (10–30% of the patients with decompensated cirrhosis present with SIRS without BI) [19,20]. Therefore, other markers that suggest or ratify the presence of infection in patients with cirrhosis must be considered. Both C-reactive protein (CRP) and procalcitonin are biomarkers that have been shown to be useful auxiliaries in the diagnosis of BI in cirrhosis and they have a higher sensitivity and a better negative predictive value when used together [21]. There is a direct relation between serum CRP levels and the severity and speed of progression of sepsis [16].
Culture samples in all patients are recommended in accordance with clinical suspicion of the infection site. When organ failure is present, blood cultures should be taken, ideally before antibiotic administration. Ascites fluid cultures must be collected in blood culture bottles, thrombocytopenia and prolonged prothrombin times should not hinder the performance of paracentesis, given that it has been shown to be a safe procedure in such settings [17].
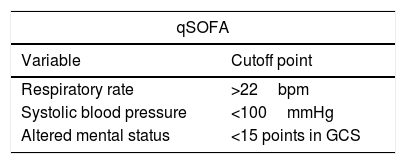
6The initial evaluationThe first evaluation maneuver is correct patient stratification. Historically, SIRS criteria have been used for that purpose. However, their poor discriminatory value has been shown and their use is no longer recommended [18]. The qSOFA score (Table 2) is the suggested replacement for the SIRS criteria. It evaluates the patient's mental status, respiratory rate and systolic blood pressure and is a clinical tool that is easy and rapid to perform. Its usefulness has been demonstrated to identify the patients with the most severe cases of BI [19]. The Sepsis-3 diagnostic criteria mention that patients with a qSOFA≥2 or a change in SOFA≥2 are likely to have sepsis [20]. The criteria for organ failure, acute kidney injury in the cirrhotic patient and acute-on-chronic liver failure should be established through SOFA, the criteria of the International Club of Ascites and CLIF-C ACLF score system respectively [1,21–23].
The stratification of patients according to the prognosis is useful, in order to monitor treatment response and decide admission to the ICU. Clinical scores such as MELD-Na, SOFA and CLIF-SOFA scores have been demonstrated to be useful for this purposes [24–27].
7MonitoringHemodynamic status assessment is a challenge in the patient with cirrhosis. The commonly utilized hemodynamic variables (lactate, ventricular filling pressures, SvcO2, etc.) do not adequately correlate in the patient with cirrhosis. Mean arterial pressure is usually lower in patients with cirrhosis and often does not respond to fluids. We do not recommend making decisions that rely only on that parameter. Therapy based on goals related to mean arterial pressure outside the scenario of hepatorenal syndrome is not advised. There is a decrease in hepatic lactate clearance in patients with cirrhosis, thus a single elevated value and even discrete elevations in patients with initial resuscitation, may not be directly related to worsening. Different determinations and correlation with the rest of the clinical and biochemical parameters are required for its interpretation. Echocardiography has been shown to be a useful and noninvasive tool in hemodynamic status monitoring. Likewise, some patients may require the use of pulmonary catheterization [22].
We should bear in mind that patients with cirrhosis can present with hypoxemia, with no apparent cause identified in imaging studies, possibly due to the decrease in chest distensibility from ascites and thoracic wall edema or from hepatopulmonary syndrome.
As in patients that do not have cirrhosis, renal function monitoring is carried out through urinary volume quantification and serum creatine level determinations every 24–48h, however serum creatinine is not an ideal biomarker for kidney function and it overestimates the glomerular filtration rate in patients with cirrhosis. Cystatin C determination provides a more exact glomerular filtration rate determination than creatinine, but it is not widely available [28]. Acute kidney injury results in an important increase in mortality in patients. In the context of hospitalized patients, a daily review of drugs is convenient, removing those with a risk for nephrotoxicity, if possible.
8General treatmentCirrhotic patients with sepsis frequently require admission to ICU or monitored units as well as thorough and continuous evaluation. In such complex settings, it is not surprising that important aspects of medical care can be omitted. To prevent that from occurring, we recommend the use of the FAST HUG checklist created by Vincent [29]. Originally fashioned for the management of ICU patients in order to prevent the omission of critical aspects in medical care. The components of this mnemotechnic are: F: food and fluids, A: analgesia, S: sedation, T: thromboprophylaxis, H: head position, U: ulcer prophylaxis and G: glucose control. This list is a patient safety and quality initiative, it has no cost and it could be used by any member of the medical team and their use could improve overall critical care deliver [30–32].
Here we address some modifications of this check list for the cirrhotic patient. Regarding solutions, crystalloids are the mostly recommended for resuscitation and maintenance. The dose for maintenance is lower than that for other patients (10–20mL/kg/h) [22]. The administration of solutions should be carried out judiciously, given that there may be a decrease in the effective arterial pressure in a hypervolemic state. Crystalloids with albumin in a 4–5% proportion can be used as a fluid for reanimation, especially in patients with high fluid input requirements [22,33]. We recommend against the use of hydroxyethyl starch in fluid resuscitation [22,33].
Periods of fasting should be reduced to the minimum. Enteral nutrition has been proven to reduce complications and increase survival in cirrhosis [34]. Nasogastric tube feeding is reserved for patients with encephalopathy, because of the risk of bronchoaspiration. In those cases, formulas based on branched-chain amino acids are recommended. Patients with cirrhosis have a greater energy demand so nutritional requirements are calculated by 35–40kcal/kg/day (dry weight), with no protein restriction (1.2–1.5g/kg/day) [34].
Concerning analgesia, nonsteroidal anti-inflammatory drugs should not be used as pain-relieving treatment as they have numerous side effects, especially their association with acute kidney injury.
The recommendations and contraindications of thromboprophylaxis and glycemic control do not differ from those for other patients.
Beta-blockers can be use with caution, in a sepsis scenario, although discontinuation may be considered, especially in patients with SBP [22,35].
If a vasopressor is necessary, beginning with norepinephrine is recommended because it has fewer adverse effects [22,33]. Terlipressin and vasopressin can be used as second-line drugs [22]. A therapeutic trial of 200mg/day of hydrocortisone can be used in patients with persistent hypotension [22,36].
In patients with suspected SBP with a high risk of acute kidney injury (Table 3), human alb [37,38]. That indication cannot be extrapolated to any other sepsis scenario [39]. In patients that develop a grade ≥2 of acute kidney injury as a consequence of HRS, the recommendation is to suspend diuretics and administer 1g/kg/day (maximum 100g/day) of human albumin for 2 days and continue with 20–40g/day until acute kidney injury is resolved, if there is no response within the first 48h, vasopressor administration (terlipressin/norepinephrine) is recommended [21].
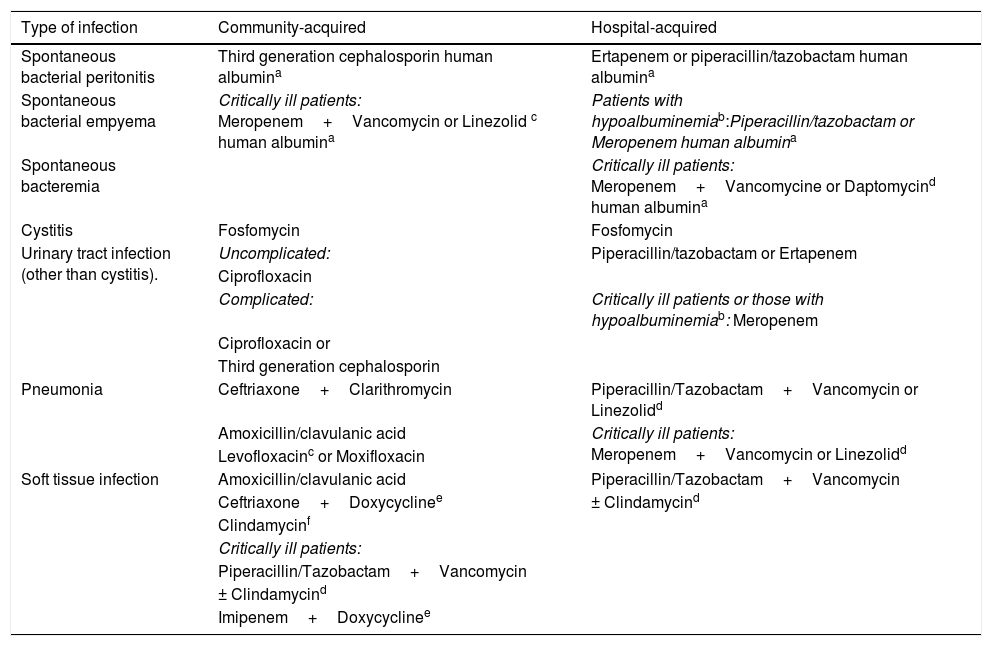
Empiric treatment recommendations for the most common infections in cirrhotic patients in Mexico. MRSA: methicillin-resistant Staphylococcus aureus.
| Type of infection | Community-acquired | Hospital-acquired |
|---|---|---|
| Spontaneous bacterial peritonitis | Third generation cephalosporin human albumina | Ertapenem or piperacillin/tazobactam human albumina |
| Spontaneous bacterial empyema | Critically ill patients: Meropenem+Vancomycin or Linezolid c human albumina | Patients with hypoalbuminemiab:Piperacillin/tazobactam or Meropenem human albumina |
| Spontaneous bacteremia | Critically ill patients: Meropenem+Vancomycine or Daptomycind human albumina | |
| Cystitis | Fosfomycin | Fosfomycin |
| Urinary tract infection (other than cystitis). | Uncomplicated: | Piperacillin/tazobactam or Ertapenem |
| Ciprofloxacin | ||
| Complicated: | Critically ill patients or those with hypoalbuminemiab: Meropenem | |
| Ciprofloxacin or | ||
| Third generation cephalosporin | ||
| Pneumonia | Ceftriaxone+Clarithromycin | Piperacillin/Tazobactam+Vancomycin or Linezolidd |
| Amoxicillin/clavulanic acid | Critically ill patients: Meropenem+Vancomycin or Linezolidd | |
| Levofloxacinc or Moxifloxacin | ||
| Soft tissue infection | Amoxicillin/clavulanic acid | Piperacillin/Tazobactam+Vancomycin |
| Ceftriaxone+Doxycyclinee | ± Clindamycind | |
| Clindamycinf | ||
| Critically ill patients: | ||
| Piperacillin/Tazobactam+Vancomycin | ||
| ± Clindamycind | ||
| Imipenem+Doxycyclinee |
In patients with spontaneous bacterial peritonitis at high risk for developing acute kidney injury (serum creatinine>1mg/dL, BUN>30mg/dL or total bilirubin>4mg/dL), administer human albumin at 1.5g/kg on day 1 and 1g/kg on day 3.
In general, the correction of prolonged prothrombin time and thrombocytopenia should not be carried out. In the presence of active bleeding or high risk bleeding procedures the following transfusion thresholds may optimize clot formation: hematocrit>25%, platelet count>50,000 and fibrinogen>120mg/dL [40]. The thresholds for international normalized ratio correction are not supported by evidence [40]. Currently there is a lack of validated target levels of global test of clot formation but may eventually have a role in the evaluation of clotting in patients with cirrhosis [40].
9Antibiotic treatmentAntibiotic therapy should be considered a 2-phase treatment: empiric antibiotic therapy (EAT) and isolate-adjusted antibiotic treatment (IAAT). The delay in the administration of the adequate antibiotic is associated with an increase up to 7.6% in mortality per hour in the first 6h [3], making EAT the best short-term mortality predictor [1,41,42]. The inappropriate use of EAT is associated with an increased mortality rate, with an adjusted odds ratio of 1.1–1.9 for every hour of delay in administering the appropriate antibiotic therapy [41,42].
Likewise, the instauration of an effective EAT has been associated with shorter hospital stay and a lower rate of treatment failure [6,43]. Therefore, the choice of an effective EAT is the most important maneuver that the clinician must dominate completely. There are 4 essential points for choosing the effective EAT: (a) type of infection (soft tissue infection, pneumonia, SBP, etc.); (b) risk of MDR bacterial infection (long-term prophylaxis with norfloxacin, recent beta-lactam antibiotic use (3 months) and previous MDR infections within the past 6 months [13,17,40]); (c) severity of the infection (according to the SOFA, MELD-Na, and CLIF-SOFA) and (d) local epidemiology (Table 3). The IAAT, as its name indicates, has the advantage of being a directed therapy against the causal agents and allows an early de-escalation strategy that must be mandatory. Its benefits include reducing costs and a narrowing of antibiotic therapy. For IAAT to be carried out, it is essential to obtain cultures of the suspected infectious site, blood cultures if merited and ascitic fluid cultures if present, ideally prior to EAT commencement, except in severe cases.
In patients with risk of ESBLs infections, carbapenems are the antibiotic of choice [44], we suggest ertapenem in the majority of scenarios. It has shown activity against ESBL-producing microorganisms and exhibits high protein bonding, however in patients with hypoalbuminemia, the adequate minimum inhibitory concentration is maintained for a shorter period [45], thus reducing its efficacy [46]. Meropenem is the carbapenem recommended in patients with hypoalbuminemia. The addition of a drug with activity against oxacillin-resistant cocci is recommended in patients presenting with organ failure.
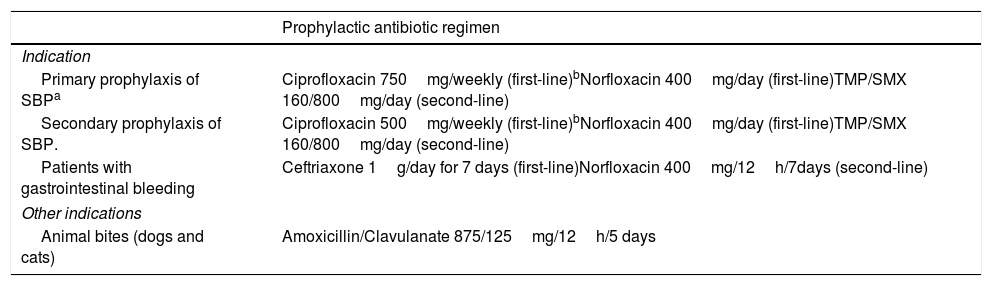
10Prophylactic treatment in cirrhosisProphylaxis of BI is currently recommended in three settings: a) patients with VB, b) primary prophylaxis of SBP and c) secondary prophylaxis of SBP. Table 4 shows the prophylaxis regimens that have demonstrated effectiveness in reducing the incidence of BI. In a recent multicenter randomized controlled non-inferiority trial with 1-year follow-up, 400mg/day of norfloxacin was equally effective for preventing episodes of SBP, compared to 750mg/week of ciprofloxacin (7.3% vs 5.3%, p=0.712). The transplant-free survival rates were comparable (72.7% vs 73.7%, p=0.97) and there were no significant differences in complications related to infections or in HRS, HE, or VB [47]. Flora selection occurs earlier with ciprofloxacin than with norfloxacin, this has not been associated with worse clinical outcomes in any trial within 1 year of follow-up, but we have to bear in mind the occurance of this fact. Therefore, we regard weekly ciprofloxacin as first-line primary or secondary prophylactic treatment for SBP, especially in patients in whom adherence to daily norfloxacin treatment cannot be ensured.
Prophylactic indications and treatment regimens.
| Prophylactic antibiotic regimen | |
|---|---|
| Indication | |
| Primary prophylaxis of SBPa | Ciprofloxacin 750mg/weekly (first-line)bNorfloxacin 400mg/day (first-line)TMP/SMX 160/800mg/day (second-line) |
| Secondary prophylaxis of SBP. | Ciprofloxacin 500mg/weekly (first-line)bNorfloxacin 400mg/day (first-line)TMP/SMX 160/800mg/day (second-line) |
| Patients with gastrointestinal bleeding | Ceftriaxone 1g/day for 7 days (first-line)Norfloxacin 400mg/12h/7days (second-line) |
| Other indications | |
| Animal bites (dogs and cats) | Amoxicillin/Clavulanate 875/125mg/12h/5 days |
In the cases of prophylaxis for SBP, treatment duration should be extended up to transplantation, the presentation of SBP, or death. In patients with VB, prophylaxis with ceftriaxone or norfloxacin has been shown to reduce rebleeding, all-cause mortality, BI mortality and length of hospitalization [48].
For animal bite wounds, the soft tissue infections guidelines of the American Society of Infectious Diseases recommend prophylactic treatment with amoxicillin/clavulanic acid for 3–5 days in patients with cirrhosis [16]. In addition, Vibrio vulnificus infection should be highly suspected, given that conventional EAT is not sufficient [16,49].
11PreventionThe strategies aimed to reduce the infections caused by MDR bacteria include general measures such as hand wash, antibiotic use surveillance and target strategies like scrutiny and isolation of asymptomatic bearers.
The most cost-effective strategy to diminish MDR bacteria including MRSA and ESBL is hand wash [50]. Antibiotic use surveillance has shown to be effective for MRSA but not for ESBL [51]. Target strategies (scrutiny and isolation) had shown controversial results for MRSA, more evidence is needed to know its effectiveness for ESBL [51]. Other kind of strategies such as selective decontamination of the gastro intestinal tract has not enough evidence to draw a conclusion [51]. Some future perspectives are been tested such as fecal transplant [52].
12Conclusions and future researchBacterial infections frequently cause decompensating events in the cirrhotic patient and are also the most common factor identified for the development of ACLF. The early recognition of an infection as well as prompt management with an EAT are the best strategies known to reduce mortality. The increase in the prevalence of infections caused by MDR microorganisms has caused a reduced effectiveness of EAT.
Therefore, as future perspectives, it would be important to develop new biomarkers that could identify in an easy and quick manner the microorganisms and their susceptibilities. It should also be important to rely on new biomarkers that could measure the empirical treatment effectiveness in short term. We should also find new strategies besides selective decontamination of the gastrointestinal tract to diminish bacterial translocation.AbbreviationsACLF acute-on-chronic liver failure multidrug-resistant extended-spectrum beta-lactamase-producing Enterobacteriaceae methicillin-resistant Staphylococcus aureus variceal bleeding spontaneous bacterial peritonitis hepatorenal syndrome hepatic encephalopathy bacterial infection systemic inflammatory response syndrome C-reactive protein empiric antibiotic therapy isolate-adjusted antibiotic treatment
All the authors contributed in the search and selection of articles. All the authors established the recommendations unanimously by consensus.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflicts of interest to declare.
Paulina Moctezuma Velazquez for proofreading and language help. All the authors have approved the final article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.