Background & rationale. Limited information related to Liver Transplantation (LT) costs in South America exists. Additionally, costs analysis from developed countries may not provide comparable models for those in emerging economies. We sought to evaluate a predictive model of Early Discharge from Hospital after LT (ERDALT = length of hospital stay ≤ 8 days). A predictive model was assessed based on the odds ratios (OR) from a multivariate regression analysis in a cohort of consecutively transplanted adult patients in a single center from Argentina and internally validated with bootstrapping technique.
Results. ERDALT was applicable in 34 of 289 patients (11.8%). Variables independently associated with ERDALT were MELD exception points OR 1.9 (P = 0.04), surgery time < 4 h OR 3.8 (P = 0.013), < 5 units of blood products consumption (BPC) OR 3.5 (P = 0.001) and early weaning from mechanical intubation OR 6.3 (P = 0.006). Points in the predictive scoring model were allocated as follows: MELD exception points (absence = 0 points, presence = 1 point), surgery time < 4 h (0-2 points), < 5 units of BPC (0-2 points), and early weaning (0-3 points). Final scores ranged from 0 to 8 points with a c-statistic of 0.83 (95% CI 0.77-0.90; P < 0.0001). Transplant costs were significantly lower in patients with ERDALT (median $23,078 vs. $28,986; P < 0.0001). Neither lower patient and graft survival, nor higher rates of short-term re-hospitalization and acute rejection events after discharge were observed in patients with ERDALT. In conclusion, the ERDALT score identifies patients suitable for early discharge with excellent outcomes after transplantation. This score may provide applicable models particularly for emerging economies.
Liver transplantation (LT) has become an acceptable medical therapy and the gold standard for treating patients with end-stage liver disease.1 Short and long-term survival, as well as quality of life after LT have improved over the last 20 years.2–5 However, LT is still one of the most complex and costly medical therapies.6,7 During the last years, special attention has been focused on the unbalanced and escalating costs of health care concomitantly with limited financial resources in South America. However, there is scarce information related to LT costs in these countries.8 Additionally, costs analysis from LT programs in developed countries may not provide comparable or applicable models for those in emerging economies.7,9–11
As shown by several authors, transplant outcomes depend on pre and post-transplantation factors, which include both recipient and donor fitness, surgeon’s skills and transplant unit team’s experience.9,12,13 Recently published series from developed countries have postulated predictors of prolonged length of hospital stay (LOS) and discharge destination following LT.9,11,13 Early discharge after LT can be feasible; however, to our knowledge, no studies on economic impact of this strategy have been published in South America.
Different common problems are seen in most South American countries, such as overpopulation, unorganized urbanization, inflation, poverty and lack of elementary health and social services. Ultimately, this economic burden may impact on the health care of the population. Liver transplantation has been performed since 1968 in South America;14 still, there are some countries without LT programs in this region. In most of these nations, lack of financial support, as well as lack of educational policies on organ donation exist, ultimately barring access to transplantation. It is in this economic scenario that strategies aimed at reducing medical costs are needed.
We believe identifying predictors of Early Discharge after LT (ERDALT) is feasible. In an attempt identify immediate pre and post-LT predictors of ERDALT; we have recently reviewed our clinical care pathways and discharge planning processes following LT. The aim of this study was to propose a clinical predictive score of ERDALT that would help to identify those patients who might be discharge earlier, prepare them better for the post-transplant period and reduce transplant costs.
Material and MethodsProspectively collected data was retrospectively reviewed including all consecutive adult (≥ 18 years) liver transplant recipients between October 2001 and December 2013 in order to identify predictive factors for ERDALT. This study was conducted at the Austral University Hospital, from Argentina. Pediatric patients were excluded from the analysis. Information was obtained from medical records and from an electronic database. Patients who died during the immediate post transplant period and were not discharged from hospital were also included in the analysis.
Pre-transplant variables included in the analysis were: recipient’s age, gender, etiology of liver disease, history of previous abdominal surgery and presence of clinically severe ascites. Additionally, patient hospitalization status in Intensive Care Unit (ICU) and renal replacement therapy (RRT) immediately prior to transplant were recorded. Laboratory values registered at the time of transplantation were serum creatinine, prothrombine time, International Normalized Ratio (INR), total bilirubin, serum sodium and serum albumin. Immediate pre-transplant Model for End Stage Liver Disease (MELD) score was calculated and patients receiving a MELD exception allocation policy were registered, namely: Hepatocellular carcinoma (HCC) within Milan criteria, and non-HCC reasons such as hepatopulmonary syndrome, familial amiloidotic polyneuropathy, biliary cholangitis, vascular disorders, post-transplant acute artery thrombosis and refractory ascites.
Donor characteristics, age and body mass index (BMI), donor and fluid transport microbial cultures, as well as cold ischemia time (CIT) were registered. Duration of LT surgery (hours); surgical technique, use of Piggy Back technique, T tube placement, type of biliary anastomosis and total blood product consumption (BPC) during organ implant were also included. Total BPC estimation was based on the sum of red blood cells, platelets, cryoprecipitates and plasma units consumed during surgery. Finally, need for RRT and in-hospital infection events after LT were analyzed. The latter was defined as any infectious event requiring antimicrobial treatment during transplant hospitalization.
Length of hospital stay after liver transplantationWe define LOS as the number of consecutive days from the date of transplantation until hospital discharge, either home or to a special care facility. If a patient was re-transplanted during the first LT hospitalization, LOS was considered from the date of the first LT until discharge. Applying a preselected cutoff value, those patients who were discharged from hospital before the 8th day post transplant were considered ERDALT. This cut-off was previously selected considering recently published data with a mean LOS of 14.9 ± 13.9 days,11 16.3 ± 18.2 days,9 and 13.7 ± 17.5 days.13 Additionally, length of ICU stay after transplantation until transfer to general ward and days of assisted mechanical ventilation (AMV) after LT were recorded in all patients. Early extubation or early weaning form AMV was considered in cases where AMV lasted < 12 h.
Economic analysisLiver transplant costs were calculated for each transplant event. Only costs directly attributable to the procedure, during hospitalization and its immediate complications were applied. Cost data were obtained from the costs of BPC during surgery, use of cell saver during surgery, induction and maintenance immunosuppression, antimicrobial prophylaxis, rejection episodes, ICU stay and LOS. In addition, costs related to in-hospital infection events were also calculated. Professional fees of hepatologists, transplant surgeons and anesthesiologists were included, although the corresponding fees of the rest of the physicians with fixed salary were not included. Liver transplantation costs were retrospectively calculated applying actual financial values prevailing in Argentina during 2014 and were expressed in US dollars. Average hospital stay costs per-day included expenses related to hospital bed, medications, blood tests and imaging studies. Costs of readmissions were not considered.
Endpoints/DefinitionsPrimary outcomes analyzed were early discharge from hospital, and patient and graft survival; while secondary outcomes were rate of biopsy-proven acute cellular rejection events (ACR);15 rate of shortterm re-hospitalization (during the first 30 days after discharge from hospital) and overall LT costs. The Institutional Review Board approved the study protocol and written informed consent was obtained from all patients (or a family member). This study was conducted in conformance with the 2008 Helsinki Declaration.
Statistical analysisCategorical data were compared applying Fisher’s exact test (2-tailed) or χ2-Square test with Yates’ correction. Continuous variables were compared with Student’s T test (mean ± standard deviation) or Mann-Whitney U test (median and interquartile ranges, IQR) according to their distribution. Continuous variables were transformed into qualitative or ordinal variables based on either cut-offs values based on receiver-operating characteristic curve (ROC) analysis or clinical relevance or the median value of the parameter. Univariate and multivariate analysis, using logistic regression were performed in order to identify significant variables related to ERDALT. Those variables with a P value < 0.1 in the univariate analysis were included in the multivariate regression model, generated by stepwise backward elimination (Wald test), P <0.05. Odds Ratios (OR) and 95% Confidence Intervals (CI) derived for each factor in the multivariate analysis were subsequently used as weights (rounded OR’s values divided by the lowest OR observed) to construct a simple clinical prediction rule. Final scores corresponded to sum of points for each individual patient. Calibration and validation of the model was performed by Hosmer-Lemeshow test and bootstrapping technique (1000 samples), respectively. Model goodness of fit and discrimination power was assessed by ROC analysis and concordance statistic calculated. Finally, Kaplan Meier survival curves were performed and compared using log-rank test. Collected data was analyzed with R software (version 3.0.1 for Mac).
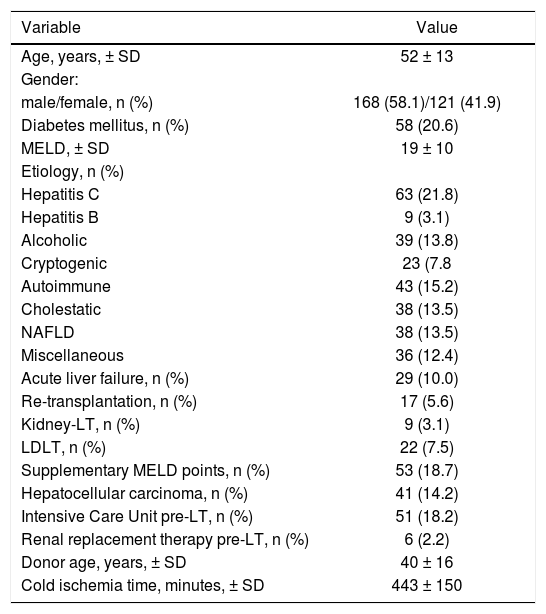
ResultsDemographic characteristics and clinical dataA total of 289 patients were included in the analysis. Mean recipients’ age was 52 ± 13 years, 168 were male (58.1%), 58 patients had pre transplant diabetes mellitus (20.6%). Principal etiologies of liver disease were hepatitis C virus infection 21.8% (n = 63), alcoholic liver disease 13.8% (n = 39) and non-alcoholic fatty liver disease 13.5% (n = 38). Overall, 29 patients had acute liver failure (10.0%), 17 patients re-transplantation (5.6%), 9 patients with simultaneous liver-kidney transplantation (3.1%), 22 patients received a living donor LT (7.5%) and 41 patients had HCC (14.2%). MELD supplementary points were allocated in 53 patients (18.7%), of whom 26 (49.1%) and 27 patients (50.9%) were because of HCC within Milan criteria and other non-HCC reasons, respectively. Fifty-one patients were located at ICU immediately before transplant (18.2%), and 6 patients were under RRT. Mean transplant surgery time was 6.6 ± 2.2 hs, with a median BPC of 16 units (IQR 13 units) and cell saver was used in 61 patients (22.9%). Early extubation from AVM was performed in 157 patients (54.4%). Positive microbial cultures on donor or graft transport fluids were observed in 21 (7.3%) and 24 patients (8.3%), respectively (Table 1).
Patients’ demographic and baseline characteristics.
| Variable | Value |
|---|---|
| Age, years, ± SD | 52 ± 13 |
| Gender: | |
| male/female, n (%) | 168 (58.1)/121 (41.9) |
| Diabetes mellitus, n (%) | 58 (20.6) |
| MELD, ± SD | 19 ± 10 |
| Etiology, n (%) | |
| Hepatitis C | 63 (21.8) |
| Hepatitis B | 9 (3.1) |
| Alcoholic | 39 (13.8) |
| Cryptogenic | 23 (7.8 |
| Autoimmune | 43 (15.2) |
| Cholestatic | 38 (13.5) |
| NAFLD | 38 (13.5) |
| Miscellaneous | 36 (12.4) |
| Acute liver failure, n (%) | 29 (10.0) |
| Re-transplantation, n (%) | 17 (5.6) |
| Kidney-LT, n (%) | 9 (3.1) |
| LDLT, n (%) | 22 (7.5) |
| Supplementary MELD points, n (%) | 53 (18.7) |
| Hepatocellular carcinoma, n (%) | 41 (14.2) |
| Intensive Care Unit pre-LT, n (%) | 51 (18.2) |
| Renal replacement therapy pre-LT, n (%) | 6 (2.2) |
| Donor age, years, ± SD | 40 ± 16 |
| Cold ischemia time, minutes, ± SD | 443 ± 150 |
LT: liver transplantation. LDLT: living donor liver transplantation. MELD: Model for End Stage Liver Disease. NAFLD: non-alcoholic fatty liver disease.
Overall median LOS was 14.5 days (IQR 13 days), with a median stay in ICU and duration of AMV of 5 and 0 days, respectively. In-hospital mortality rate was 9.9% (n = 30), whereas in-hospital infections and short-term re-hospitalization rates were 38.8% (n = 112) and 23.3% (n = 60), respectively. One hundred and twelve patients (38.9%) had at least 1 ACR event, 29% (n = 32) of these events occurred during LT hospitalization and 71% (n = 80) after discharge from hospital. The median LT cost among the entire cohort was $28,206 (IQR 8,536). For a median follow-up time of 35 months, overall patient and graft survival rates at 1 and 5 years after LT were 86.8 and 76.8%, and 76.7 and 75.4%, respectively.
Patients with early discharge from hospital after liver transplantationThirty-four patients (11.8%) were discharged within the first 8 days post LT. Patients who were discharged early had lower pre transplant MELD score (15 ± 11 vs. 19 ± 10, P = 0.021), and 47.1% of these were granted with MELD exception points (n = 16/34; P < 0.0001). No significant differences related to proportion of patients with HCC and MELD exception points between groups were observed. Significant differences were observed between patients with or without ERALDT among the following baseline parameters: presence of severe pre-LT ascites (23.5 vs. 43.3%; P = 0.02) and pre-LT serum creatinine (0.81 ± 0.35 mg/dL vs. 1.13 ± 0.89 mg/dL; P = 0.04). No statistically significant differences were observed regarding quality of donors, namely: age (41 ± 17 vs: 40 ± 16 years; P = 0.67), donor BMI (27.7 ± 5.5 vs. 25.8 ± 3.4 kg/m2, P = 0.08), positive microbial cultures on donor or graft transport fluids (20.6 vs. 13.7%; P = 0.20), or CIT duration (6.6 ± 1.8 vs. 7.7 ± 2.1 h, P = 0.49).
LT surgery time was shorter for ERDALT patients (4.8 ± 2.2 vs. 6.8 ± 2.1 h; P < 0.0001), no T tube placement was required (0 vs. 30.6%; P = 0.004) and less median BPC during LT surgery was observed (5.0 vs. 18.0 units; P < 0.0001). Median LOS of patients with and without ERDALT was significantly different (7.0 vs. 15.5 days; P < 0.0001), as well as ICU stay (4.0 vs. 5.0 days; P = 0.014). ERDALT patients were more frequently early extubated from AMV (91.2 vs. 49.6%; P < 0.0001) and presented less in-hospital infections events (0 vs. 43.9%; P = 0.0001).
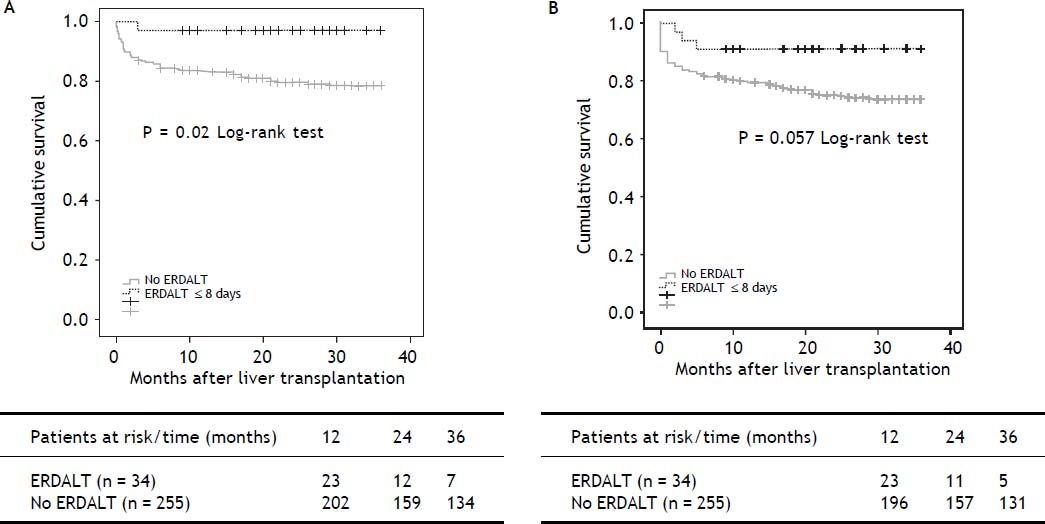
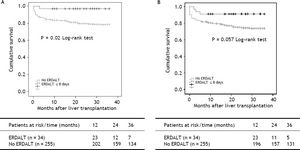
Survival analysis, liver transplant costs and re-hospitalization ratesKaplan Meier analysis revealed significant differences with respect to patient and graft survival at 1 and 5 years between patients with and without ERDALT. Kaplan Meier curve revealed significant difference on 3-year patient survival rate and a trend for improved graft survival between patients with and without ERDALT (97.1 vs. 79.2%, respectively; P = 0.02, and 91.2% vs. 74.5%, P = 0.05, log rank test, respectively) (Figure 1).
Kaplan Meier patient (A) and graft (B) 3-year survival comparative analysis between patients with and without early discharge from hospital after liver transplantation. Kaplan Meier curves revealed significant differences on 3-year patient and graft survival rates between patients with and without ERDALT (97.1 vs. 79.2%, respectively; P = 0.021, and 91.2% vs. 74.5%, P = 0.05, log rank test, respectively). ERDALT: early discharge from hospital after liver transplantation. LT: liver transplantation.
There were no significant differences between both groups regarding early re-hospitalization rate (ERDALT 20.6%, n = 7/34 vs. no ERDALT 23.8%, n = 53/223; P = 0.43). Patients with ERDALT had lower rate of ACR during the first 3 months after LT than patients discharged from hospital later (18.2%, n = 6/34 vs. 42.0%, n = 94/ 224; P = 0.006), and similar rate of ACR after hospital discharge (14.7%, n = 5/34 vs. 10.7%, n = 24/224; P = 0.32).
Analysis of expenditure comparison between patients with or without ERDALT revealed that the median overall LT costs were lower for early discharge patients ($23,078, IQR 6,866 vs. $28,986, IQR 118,036; P < 0.0001, respectively). This difference between subgroups were due to a significant difference in costs of hospital stay ($1,113, IQR 1,013 vs. $823, IQR 19,664; P < 0.0001), cost of duration of the transplant surgery ($929, IQR 709 vs. $1,229, IQR 2,930; P < 0.0001), BPC ($713, IQR 1,718 vs. $1,396, IQR 12,175; P < 0.0001), ICU stay ($2,261, IQR 1,130 vs. $3,391, IQR 105,138; P < 0.0001) and immunosuppression during hospitalization ($459, IQR 262 vs. $1,050, IQR 12,216; P < 0.0001).
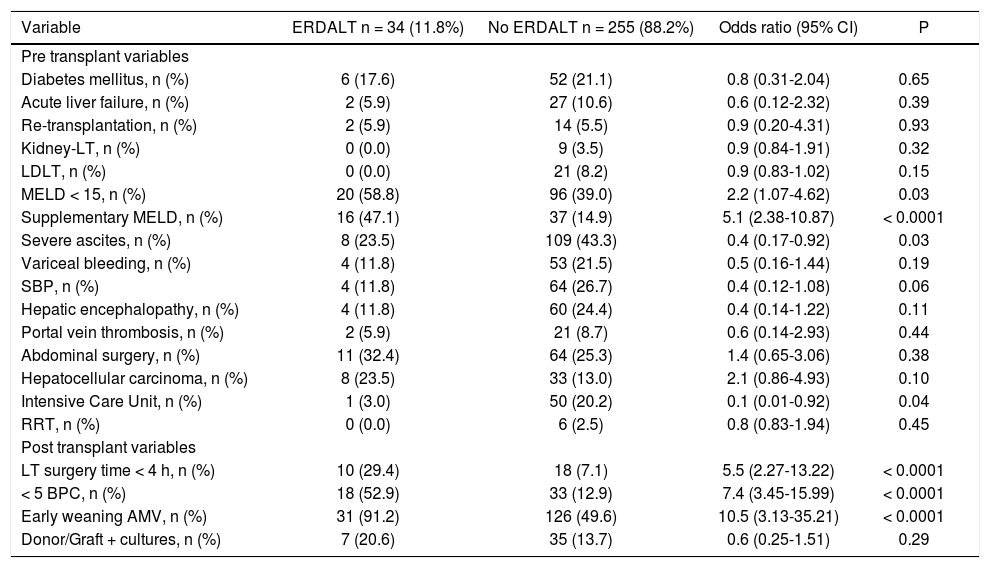
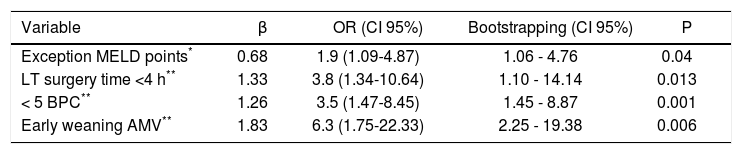
Predictive variables for early discharge from hospital after liver transplantationTable 2 shows immediate pre and post-LT associated variables with ERDALT in the univariate analysis. Pre-LT variables associated with ERDALT were as follows: MELD score < 15 (P = 0.03), supplementary MELD points (P < 0.0001), severe ascites (P = 0.03) and ICU stay immediately before transplant (P = 0.04). Among post transplant variables, LT surgery duration < 4 h (P < 0.0001), requirement of < 5 units of BPC (P < 0.0001) and early weaning from AMV (P < 0.0001) were related with ERDALT. Variables independently associated with ERDALT after multivariate stepwise regression analysis were MELD exception points OR 1.9 (1.09-4.87, P = 0.04), surgery time < 4 h OR 3.8 (1.34-10.64, P = 0.013), < 5 units of BPC OR 3.5 (1.47-8.45, P = 0.001) and early weaning from AMV OR 6.3 (1.75-22.33, P = 0.006). Model calibration with Hosmer-Lemeshow test showed no significant differences between observed and expected events (P = 0.58). Bootstrap validation showed overfitting was negligible and therefore no adjustment of logistic regression model estimates was required (Table 3).
Univariate comparative analysis between patients with and without early discharge from hospital after liver transplantation.
| Variable | ERDALT n = 34 (11.8%) | No ERDALT n = 255 (88.2%) | Odds ratio (95% CI) | P |
|---|---|---|---|---|
| Pre transplant variables | ||||
| Diabetes mellitus, n (%) | 6 (17.6) | 52 (21.1) | 0.8 (0.31-2.04) | 0.65 |
| Acute liver failure, n (%) | 2 (5.9) | 27 (10.6) | 0.6 (0.12-2.32) | 0.39 |
| Re-transplantation, n (%) | 2 (5.9) | 14 (5.5) | 0.9 (0.20-4.31) | 0.93 |
| Kidney-LT, n (%) | 0 (0.0) | 9 (3.5) | 0.9 (0.84-1.91) | 0.32 |
| LDLT, n (%) | 0 (0.0) | 21 (8.2) | 0.9 (0.83-1.02) | 0.15 |
| MELD < 15, n (%) | 20 (58.8) | 96 (39.0) | 2.2 (1.07-4.62) | 0.03 |
| Supplementary MELD, n (%) | 16 (47.1) | 37 (14.9) | 5.1 (2.38-10.87) | < 0.0001 |
| Severe ascites, n (%) | 8 (23.5) | 109 (43.3) | 0.4 (0.17-0.92) | 0.03 |
| Variceal bleeding, n (%) | 4 (11.8) | 53 (21.5) | 0.5 (0.16-1.44) | 0.19 |
| SBP, n (%) | 4 (11.8) | 64 (26.7) | 0.4 (0.12-1.08) | 0.06 |
| Hepatic encephalopathy, n (%) | 4 (11.8) | 60 (24.4) | 0.4 (0.14-1.22) | 0.11 |
| Portal vein thrombosis, n (%) | 2 (5.9) | 21 (8.7) | 0.6 (0.14-2.93) | 0.44 |
| Abdominal surgery, n (%) | 11 (32.4) | 64 (25.3) | 1.4 (0.65-3.06) | 0.38 |
| Hepatocellular carcinoma, n (%) | 8 (23.5) | 33 (13.0) | 2.1 (0.86-4.93) | 0.10 |
| Intensive Care Unit, n (%) | 1 (3.0) | 50 (20.2) | 0.1 (0.01-0.92) | 0.04 |
| RRT, n (%) | 0 (0.0) | 6 (2.5) | 0.8 (0.83-1.94) | 0.45 |
| Post transplant variables | ||||
| LT surgery time < 4 h, n (%) | 10 (29.4) | 18 (7.1) | 5.5 (2.27-13.22) | < 0.0001 |
| < 5 BPC, n (%) | 18 (52.9) | 33 (12.9) | 7.4 (3.45-15.99) | < 0.0001 |
| Early weaning AMV, n (%) | 31 (91.2) | 126 (49.6) | 10.5 (3.13-35.21) | < 0.0001 |
| Donor/Graft + cultures, n (%) | 7 (20.6) | 35 (13.7) | 0.6 (0.25-1.51) | 0.29 |
ERDALT: early discharge from hospital after liver transplantation. LT: liver transplantation. LDLT: living donor liver transplantation. MELD: Model for End Stage Liver Disease. SBP: spontaneous bacterial peritonitis.
Multivariate logistic regression analysis of risk factors associated with early discharge from hospital after liver transplantation and bootstrapped bias corrected-confidence intervals.
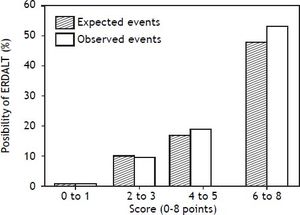
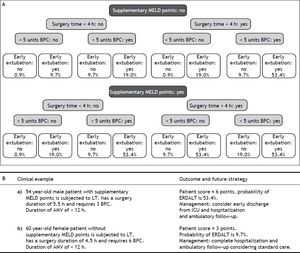
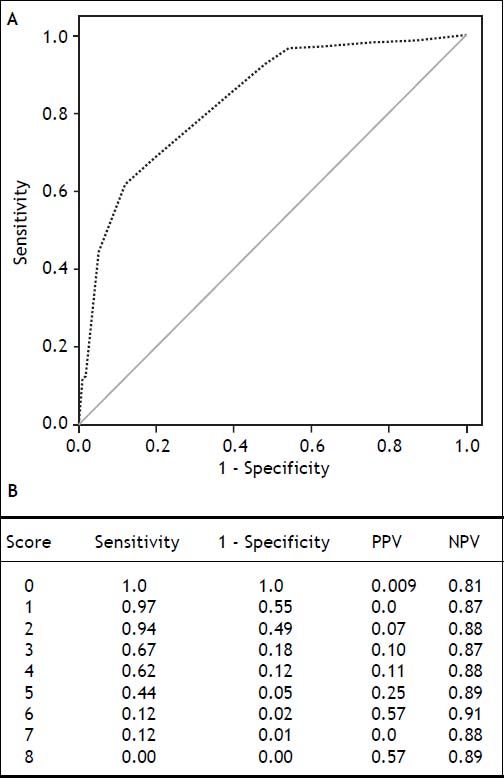
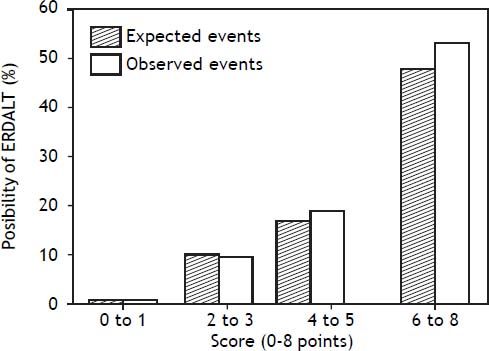
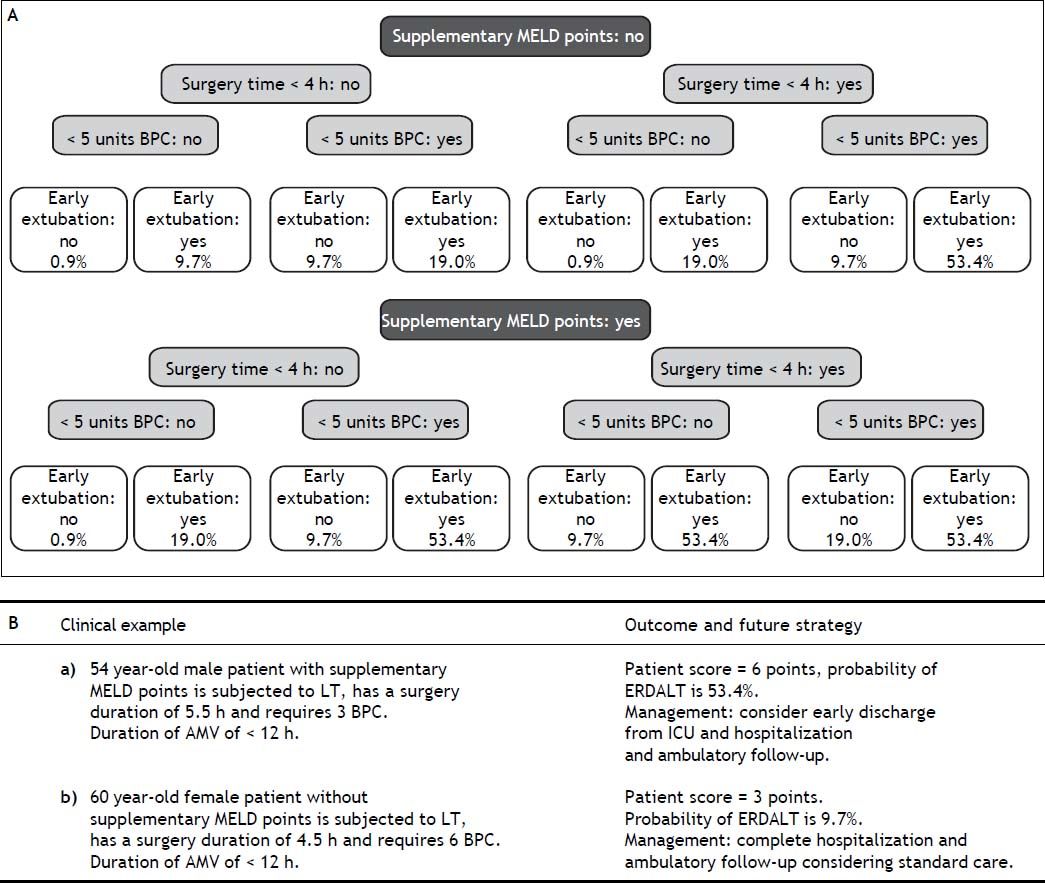
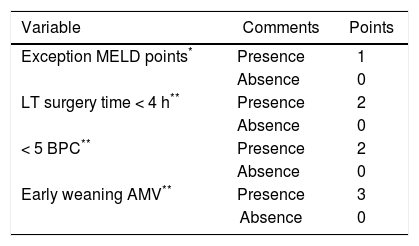
Using these variables, a predictive score for ERDALT was constructed. Each of the predictors was assigned with points depending on its OR from logistic regression multivariate analysis divided by 1.9, which was the lowest OR observed. Points in the predictive scoring system for ERDALT were allocated as follows: MELD exception points (absence = 0 points, presence = 1 point), surgery time < 4 h (absence = 0 points, presence = 2 points), < 5 units of BPC (absence = 0 points, presence = 2 points), and early weaning from AMV (absence = 0 points, presence = 3 points)(Table 4). Final scores ranged from 0 to 8 points and ROC curve revealed good discriminatory power for the model with a c-statistic of 0.83 (95% CI 0.77-0.90; P < 0.0001), and best performance for a cut-off ≥ 3 points (67% sensitivity, 82% specificity) (Figure 2). Positive and negative predictive values were 0.20 and 0.99, respectively. Probability of ERDALT increased progressively as scores went from 0-1 to 2-3, 4-5 to 6-8 points in the following percentages: 0.9% (n = 1/116), 9.7% (n = 10/ 103), 19.0% (n = 8/42), and 53.4% (n = 15/28), respectively (Goodman & Kruskall Gamma P = 0.0001) (Figure 3). A clinical nomogram and possible clinical scenarios and application are shown in figure 4.
Predictive model for early discharge from hospital after liver transplantation: points assigned to each variable (0-8 points).
Concordance statistic of predictive score for early discharge after liver transplantation (ERDALT). A predictive ERDALT score was calculated [AUROC of 0.83 (CI 0.77-0.90, P < 0.0001), 67% sensitivity and 82%% specificity for a cut-off ≥ 3 points]. Parameters assigned points included: pre-LT supplementary exception MELD points 1 point, surgery time < 4 h 2 points, < 5 units of BPC 2 points, and early weaning from AMV 3 points. AMV: assisted mechanical ventilation. AUROC: area under receiver operating characteristic. BPC: total blood product consumption. CI: confidence interval. LT: liver transplantation. PPV: positive predictive value. OR: odds ratio. NVP: negative predictive value.
Correlation between points allotted in the predictive model and possibility of early discharge from hospital after liver transplantation. Probability of ERDALT increased progressively as scores went from 0-1 to 2-3, 4-5 to 6-8 points in the following percentages: 0.9% (n = 1/116), 9.7% (n = 10/103), 19.0% (n = 8/42), and 53.4% (n = 15/28), respectively (Goodman & Kruskall Gamma P = 0.0001).
Our results provide relevant information in the field of health care costs of LT. We have evaluated the feasibility of early discharge from hospital after LT and constructed a predictive model. Our data shows that the following immediate pre and post-LT variables can identify patients suitable for ERDALT: exception MELD points, < 5 units of BPC, surgery time ≤ 4 h and early weaning from AMV. We believe that using these factors might be an additional tool to identify patients suitable for a short hospitalization and help reducing LT hospitalization costs. This cost opportunity should also be viewed in light of the fact that early discharge from hospital may allow more procedures, operations for other patients as well as less occupation of hospital beds.
Recently published data from the United Network for Organ Sharing (UNOS) has shown that mean LOS is 14.9 ± 13.9 days.11,16 Other series have reported LOS of 16.3 ± 18.2 days,9,17 and 13.7 ± 17.5 days.13,18–21 Overall median LOS in our series was 14.5 days, 12% of the patients were discharged from hospital during the first week after surgery with a short-term re-hospitalization rate of 23%. Predictors of LOS after LT have already been described, such as recipient and donor age, two or more previous liver transplants, ICU status prior to LT, recipients’ body mass index (BMI), RRT before transplantation9,11,13 and early extubation from AMV after surgery.22 We chose a cut-off for ERDALT of 8 days based on these aforementioned data and considering that our median LOS was 14.5 days. We considered that an 8-day cut off was clinically relevant, and statistically comparable between groups.
We focused our analysis on immediate pre and post-LT variables related with LOS after LT. Neither recipient or donor age, nor re-transplantation or kidney-liver transplantation showed any significant relationship. Although pre-transplant MELD score did show a link, the only variable included in the MELD score that remained relevant in relation to ERDALT after multivariate analysis was serum creatinine. Controversy however persists in previously reported series regarding MELD score and LOS.9,12,13,23 However, those patients granted with supplementary MELD points, particularly those with HCC, had the highest probability of early discharge from hospital. Immediate post-transplant variables, such as < 5 units of BPC, early weaning from AMV and LT surgery duration < 4 h were considered factors affecting LOS and were included in the final model. It seems that the influence of pre-transplant variables on the ERDALT predictive model, including those related with portal hypertension and its complications, appear to be lower than those events occurring during the transplant procedure or immediate post-LT period. It may be that these data might be surrogate markers of a short and uncomplicated transplant surgery.
Previously reported data indicates liberation from mechanical ventilation shortly after LT is feasible. Some authors have even recommended immediate extubation after surgery, thus circumventing ICU stay entirely.24,25 This strategy has not been applied at our center. All patients are transferred to the ICU following transplantation. In the article of reference, LT operative time and intraoperative transfusion requirements were described as predictors for need of ICU admission.24,25 These authors proposed a scoring model for fast tracking and avoiding costly ICU stay after LT. Nine variables were identified, including BPC, operative time, MELD score, body mass index and age at time of transplant. Our model included only 4 variables, including 1 pre LT and 3 immediate post LT factors.
Conflicting data has been reported regarding the impact of donor variables on LOS and LT costs.9,11,13,23,26,27 No significant differences in CIT or rate of positive donor microbial cultures between patients with and without ERDALT were observed. It could be argued that patients with ERDALT had shorter LOS because this group also presented lower ACR events and in-hospital infections events.11 Again, the variables included in the multivariate model might be surrogate markers of lower ACR and in-hospital infection events. In hospital infection was considered present not only in patient manifesting symptoms of infection (bacterial, viral or fungi), but also in cases with positive microbial cultures in samples from on donor tissue or graft transport fluid (13.7%). Although inhospital infection rates seem high in this series, exclusion of the latter group would cause the total to drop to 29.3%. Nevertheless, because some patients with positive donor/ transport fluid cultures required intravenous antibiotic treatment, these events were recorded as inhospital infection cases for the purpose of our database. We observed that patients not discharge early had a significantly higher inhospital infection rates, probably because surrogate markers of infection such as prolonged surgery, higher BPC, ACR events were also present in these patients. Moreover, applying the ERDALT scoring might identify patients less prone to develop ACR events or hospital re-admissions.
Almost all previously published reports have focused on predictors of prolonged LOS and not on early discharge from hospital after LT. In a recent report from UNOS data, short LOS definition was set at < 11 days;11 authors referred ROC predictive value was poor in different models for assessing LOS after LT, including short LOS (ROC 0.56-0.70).11 Our predictive model for ERDALT, on the other hand, showed a good accuracy, with a c-statistic > 0.80, moderate sensitivity (70%) and high specificity (82%) with the best cut-off at ≥ 3 points.
Finally, limited information on LT costs is available for South America since MELD score policy implementation. Argentina was the second country in the world to adopt this organ allocation policy in July 2005. Albeit, waiting list mortality has been reduced in our country,28 it has not been explored if higher MELD score recipients may promote higher LT costs.12,23,29,31 Financial charges for LT in our country are around $30,000-35,000. Liver transplant inpatient costs in the United States have been estimated to range between $80,000 and $114,300, with mean hospital charges of $358,200.7,26 If such prices were applied in Argentina, access to LT would be extremely limited.
Certain limitations to our findings merit mention. First, ERDALT was artificially defined with a cut-off point of 8 days of LOS. We thought that a good definition for early discharge form hospital after LT was the time point of 1 week, as was suggested in the literature,11 considering that median LOS was 14.5 days. Second, overall LT costs calculation is still a complex issue. We tried to reduce any errors and misleading cost analysis including every data, as objective markers of resource utilization.27 Finally, we acknowledge that no scoring system is perfect and an external validation cohort for our model is needed. Therefore, this scoring model should be used along with clinical judgment in deciding whether to early discharge patients after LT.
With respect to future planning processes based on the ERDALT score, we will attempt to fast-track the immunosuppression regimes in LT recipients with ERDALT scores ≥ 3 points, to avoid treatment delays while waiting for therapeutic CNI trough levels, which can be attained during outpatient follow up. Furthermore, for patients presenting ERADLT score ≥ 3 points we hope to limit admission to ICU to less than 24 h.
In summary, our data suggests that early discharge from hospital following LT is feasible. More importantly, it does not have a negative impact on patient or graft survival, nor did increase shortterm re-hospitalization or ACR rates after discharge. We are now implementing our predictive model in order to help us to prepare patients and their families better for the post-transplant period and improve their expectations. We believe the model has already contributed to immediate post-LT early discharge planning, helping fast-track LT recipients to avoid ICU or prolonged hospitalization.
Abbreviations- •
ACR: acute cellular rejection events.
- •
AMV: assisted mechanical ventilation.
- •
AUROC: Area Under Receiver Operating Characteristics.
- •
BMI: body mass index.
- •
BPC: blood product consumption.
- •
CI: confidence interval.
- •
CIT: cold ischemia time.
- •
DRI: donor risk index.
- •
HCC: hepatocellular carcinoma.
- •
HE: hepatic encephalopathy.
- •
ICU: Intensive Care Unit.
- •
INR: international normalized ratio.
- •
IQR: interquartile range.
- •
LDLT: living donor liver transplantation.
- •
LOS: length of hospital stay.
- •
LT: liver transplantation.
- •
MELD: Model for End-stage Liver Disease.
- •
NAFLD: non-alcoholic fatty liver disease.
- •
OR: odds ratio.
- •
ROC: receiving operator curve analysis.
- •
RRT: renal replacement therapy.
- •
SBP: spontaneous bacterial peritonitis.
- •
UNOS: United Network for Organ Sharing.
All authors of this manuscript have not received any grant or funding from agencies in the public, commercial, or not-for-profit sectors and have no conflicts of interest to disclose as described by the Annals of Hepatology Journal.
AcknowledgmentsWe thank C Podesta for her assistance with editing this paper.




![Concordance statistic of predictive score for early discharge after liver transplantation (ERDALT). A predictive ERDALT score was calculated [AUROC of 0.83 (CI 0.77-0.90, P < 0.0001), 67% sensitivity and 82%% specificity for a cut-off ≥ 3 points]. Parameters assigned points included: pre-LT supplementary exception MELD points 1 point, surgery time < 4 h 2 points, < 5 units of BPC 2 points, and early weaning from AMV 3 points. AMV: assisted mechanical ventilation. AUROC: area under receiver operating characteristic. BPC: total blood product consumption. CI: confidence interval. LT: liver transplantation. PPV: positive predictive value. OR: odds ratio. NVP: negative predictive value. Concordance statistic of predictive score for early discharge after liver transplantation (ERDALT). A predictive ERDALT score was calculated [AUROC of 0.83 (CI 0.77-0.90, P < 0.0001), 67% sensitivity and 82%% specificity for a cut-off ≥ 3 points]. Parameters assigned points included: pre-LT supplementary exception MELD points 1 point, surgery time < 4 h 2 points, < 5 units of BPC 2 points, and early weaning from AMV 3 points. AMV: assisted mechanical ventilation. AUROC: area under receiver operating characteristic. BPC: total blood product consumption. CI: confidence interval. LT: liver transplantation. PPV: positive predictive value. OR: odds ratio. NVP: negative predictive value.](https://static.elsevier.es/multimedia/16652681/0000001400000006/v1_201906050954/S1665268119309536/v1_201906050954/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)