Hepatocellular adenomas (HCAs) are benign liver tumors recently characterized into 4 different types according to genetic, pathological and clinical features. The prognosis is not well established yet and malignant transformation has been recently associated with β-catenin activation. We aimed to describe a case of a pigmented HCA with β-catenin nuclear expression and inflammatory features and to review the cases of pigmented HCAs in the literature. We report a case of a young female patient without contraceptive use, with a liver tumor diagnosis. Liver biopsy revealed diffuse expression of β-catenin and a partial hepatic resection was performed. The histologic analysis revealed a hepatocellular tumor composed of uniform trabeculae of hepatocytes and solid areas, the later with a significant amount of black pigment highlighted by Masson-Fontana stain. Immunohistochemistry showed co-expression of C-reactive protein and serum amyloid A in the tumor. Literature review revealed that pigmented HCAs, previously reported as dark adenomas, are rare tumors. In HCAs, the presence of β-catenin activation should be searched for due to the higher risk of malignant transformation in hepatocarcinoma. We describe a pigmented HCA with β-catenin nuclear expression and inflammatory features being the fifth case reported so far.
Hepatocellular adenomas (HCAs) are benign epithelial liver tumors predominantly seen in young women (between 20 and 44 years of age). They are usually located in the right hepatic lobe and 70 to 80% of the cases present as solitary lesions.1 Nonetheless, multiple adenomas have been described in patients with prolonged contraceptive use, glycogen storage diseases and hepatic adenomatosis (nosologic entity defined when more than 10 HCAs are present).2 The prognosis of these tumors is not well established, and malignant transformation has been reported in previous studies, associated with β-catenin activation.3–5 Recently, four different types of HCAs have been identified according to the genotype/phenotype features.5,6 In all subgroups, deposition of Dubin-Johnson-like pigment is rarely observed. The authors describe a case of a pigmented HCA with β-catenin activation and inflammatory features, developed in a young female patient.
Case ReportA 27-year-old caucasian female patient was referred as an outpatient to our Hospital due to a large mass (8 cm of maximum diameter), in the right hepatic lobe (segment VI and VII) diagnosed in an abdominopelvic computed tomography (CT) performed to evaluate endometriosis. She had a past history of laparoscopic ovarian cystectomy due to ovarian endometrioma after which a lenogorgestrel contraceptive device was placed in the uterus.
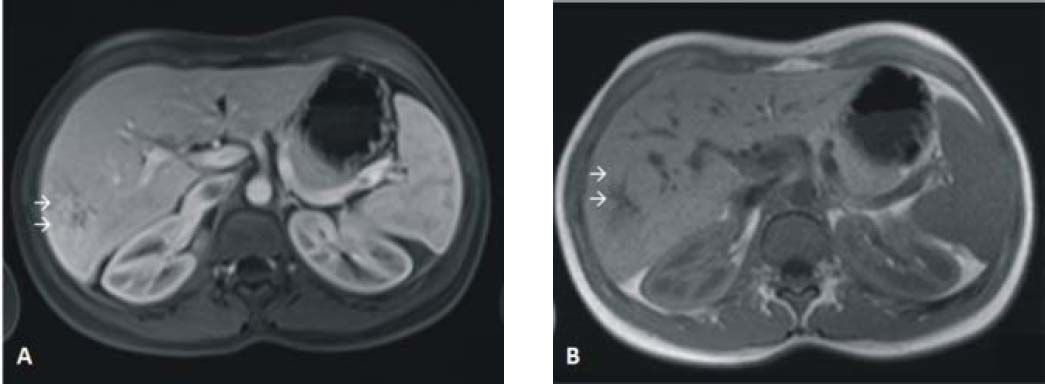
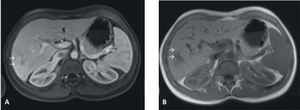
She was not taking any medications and there was no family history of liver disease. Laboratory workup was unremarkable with normal liver tests. Hepatitis B and C markers were negative and serum levels of α-fetoprotein were within normal range. The liver mass was well demarcated and had low attenuation; in the contrast-enhanced scans it did not show peripheral enhancement during the early phase. During the late phase the lesion became isodense compared to the hepatic parenchyma. At this point, these features were not typical for HCA or focal nodular hyperplasia. A magnetic resonance imaging revealed a tumor with 8.0 × 7.7 cm that was hyperintense in T1 and moderately isointense in T2. This heterogeneous liver mass was hyperintense in the arterial phase that equalized the remaining hepatic parenchyma in the portal and late phases (Figure 1).
Ultrasound-guided biopsy was performed and showed a well-differentiated hepatocellular tumor composed of hepatic cells without atypia, with the deposition of abundant ceroid pigment. Immunohistochemical studies revealed nuclear and cytoplamic expression of β-catenin, focal expression of C-reactive protein (CRP), serum amyloid A2 (SAA) and glutamine synthetase (GS) in the absence of glypican-3 expression.
Partial hepatic resection was performed and the surgical specimen measured 90 × 67 × 72 mm, weighted 205.3 g and was almost totally occupied by a dark green tumor.
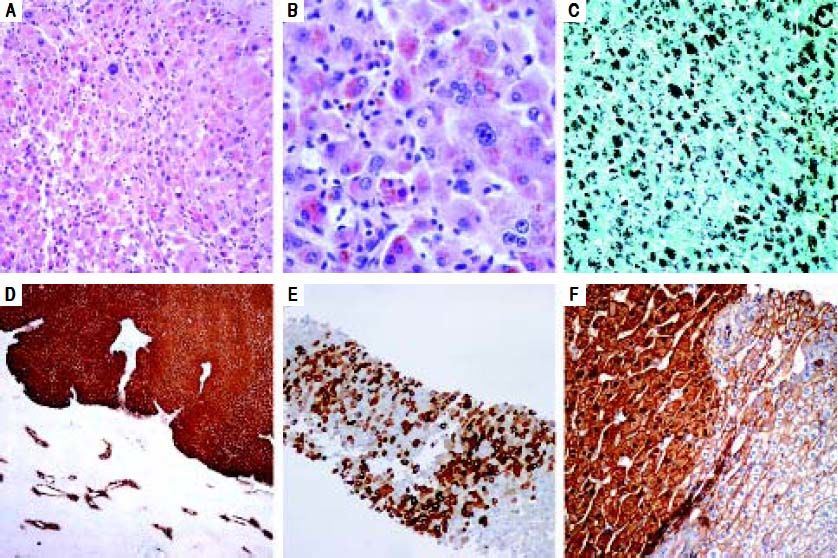
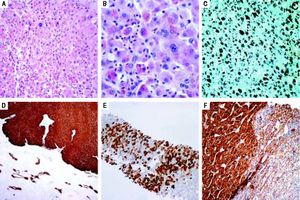
Histologic analysis revealed features similar to those observed in the preoperative biopsy and, in addition, showed solid areas composed of hepatocytes with atypia, with large nuclei and prominent nucleoli (Figures 2A and 2B). In these areas, there was deposition of brown pigment (Figure 2B), negative for Perls’ Prussian blue and Hall stains, that strongly stained as black granules by Masson-Fontana stain (Figure 2C). Immunohistochemical study showed diffuse expression of GS (Figure 2D), as well as expression of CRP (Figure 2E). Furthermore, some areas were identified with nuclear expression of β-catenin (Figure 2F). The patient remains without tumor recurrence one year post-operation.
A and B. Solid areas displaying some atypical hepatocytes with large nuclei and prominent nucleoli (H&E; original magnification: 200x; 400x). C. Deposition of brown pigment strongly stained as black granules by Masson-Fontana stain (original magnification: 200x). D. Immunoexpression of glutamine synthetase (IHC; original magnification: 40x). E. Immunoexpression of CRP (IHC; original magnification: 100x). F. Nuclear and cytoplasmic immunoexpression of β-catenin coexisting with areas with expression of β-catenin only at the cell membrane (IHC; original magnification: 200x).
HCAs are relatively rare benign tumors, more common in female patients in association with prolonged contraceptive therapy. Bioulac-Sag, et al.5 described four subgroups of HCAs according to genetic, pathological and clinical features. Type 1 HCA (HNF-1α-inactivated HCA) is caused by mutations in hepatocyte nuclear factor 1 (HNF-1α), and is almost exclusively found in female patients. Type 2 HCA (β-catenin-activated) is usually solitary and has the highest risk of malignant transformation. This subgroup is characterized by β-catenin mutation and up-regulation of glutamine synthetase. The inflammatory HCA (type 3) is characterized by increased expression of SAA and CRP. The fourth subgroup, unclassified HCA, accounts for less than 10% of all HCAs and does not have specific markers.5
Apart from this classification, Ye, et al.7 described for the first time in 1999 a pigmented hepatic adenoma in a female patient with long-term use of oral contraceptives. This rare variant of HCA is histologically characterized by the presence of Dubin-Johnson-like pigment (that is derived from lipofuscin, atypical bile pigments or porhyrins) and results from a defect in biliary excretion.8 The pigment granules are variably stained with periodic acid-Schiff after diastase digestion and by the long Ziehl-Neelsen technique and Masson-Fontana staining, and are negative with Perls’ Prussian blue and Shikata’s orcein stainings.
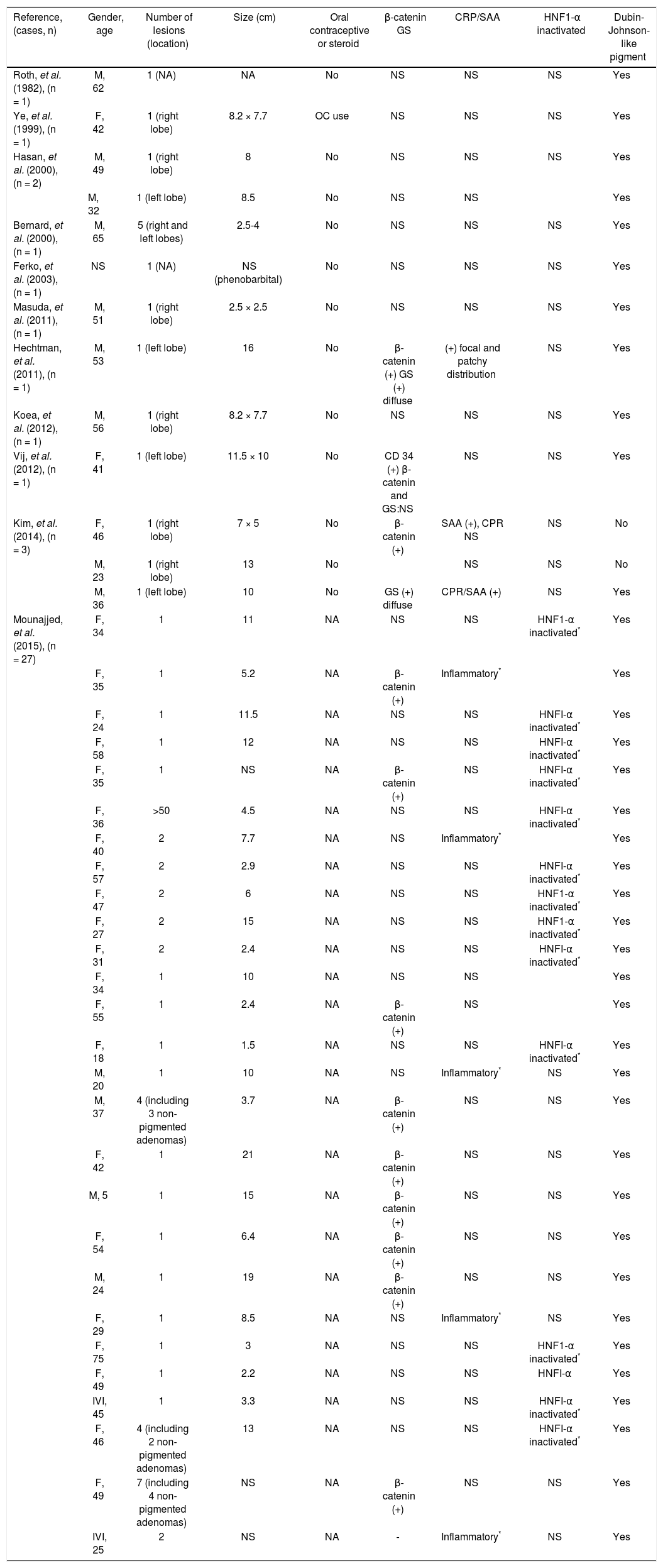
In the literature there are 11 cases of pigmented hepatocellular adenomas reported so far.7,9–17 However, a large case series of 27 pigmented hepatocellular neoplasms was recently described by Mounajjed, et al.18 The epidemiological, macroscopic, microscopic and immunohistochemical features of these pigmented (dark) hepatocellular neoplasms are shown in table 1.
Epidemiological, macroscopic, microscopic and immunohistochemical features of the cases of pigmented hepatocellular neoplasms.
| Reference, (cases, n) | Gender, age | Number of lesions (location) | Size (cm) | Oral contraceptive or steroid | β-catenin GS | CRP/SAA | HNF1-α inactivated | Dubin-Johnson-like pigment |
|---|---|---|---|---|---|---|---|---|
| Roth, et al. (1982), (n = 1) | M, 62 | 1 (NA) | NA | No | NS | NS | NS | Yes |
| Ye, et al. (1999), (n = 1) | F, 42 | 1 (right lobe) | 8.2 × 7.7 | OC use | NS | NS | NS | Yes |
| Hasan, et al. (2000), (n = 2) | M, 49 | 1 (right lobe) | 8 | No | NS | NS | NS | Yes |
| M, 32 | 1 (left lobe) | 8.5 | No | NS | NS | Yes | ||
| Bernard, et al. (2000), (n = 1) | M, 65 | 5 (right and left lobes) | 2.5-4 | No | NS | NS | NS | Yes |
| Ferko, et al. (2003), (n = 1) | NS | 1 (NA) | NS (phenobarbital) | No | NS | NS | NS | Yes |
| Masuda, et al. (2011), (n = 1) | M, 51 | 1 (right lobe) | 2.5 × 2.5 | No | NS | NS | NS | Yes |
| Hechtman, et al. (2011), (n = 1) | M, 53 | 1 (left lobe) | 16 | No | β-catenin (+) GS (+) diffuse | (+) focal and patchy distribution | NS | Yes |
| Koea, et al. (2012), (n = 1) | M, 56 | 1 (right lobe) | 8.2 × 7.7 | No | NS | NS | NS | Yes |
| Vij, et al. (2012), (n = 1) | F, 41 | 1 (left lobe) | 11.5 × 10 | No | CD 34 (+) β-catenin and GS:NS | NS | NS | Yes |
| Kim, et al. (2014), (n = 3) | F, 46 | 1 (right lobe) | 7 × 5 | No | β-catenin (+) | SAA (+), CPR NS | NS | No |
| M, 23 | 1 (right lobe) | 13 | No | NS | NS | No | ||
| M, 36 | 1 (left lobe) | 10 | No | GS (+) diffuse | CPR/SAA (+) | NS | Yes | |
| Mounajjed, et al. (2015), (n = 27) | F, 34 | 1 | 11 | NA | NS | NS | HNF1-α inactivated* | Yes |
| F, 35 | 1 | 5.2 | NA | β-catenin (+) | Inflammatory* | Yes | ||
| F, 24 | 1 | 11.5 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 58 | 1 | 12 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 35 | 1 | NS | NA | β-catenin (+) | NS | HNFI-α inactivated* | Yes | |
| F, 36 | >50 | 4.5 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 40 | 2 | 7.7 | NA | NS | Inflammatory* | Yes | ||
| F, 57 | 2 | 2.9 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 47 | 2 | 6 | NA | NS | NS | HNF1-α inactivated* | Yes | |
| F, 27 | 2 | 15 | NA | NS | NS | HNF1-α inactivated* | Yes | |
| F, 31 | 2 | 2.4 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 34 | 1 | 10 | NA | NS | NS | Yes | ||
| F, 55 | 1 | 2.4 | NA | β-catenin (+) | NS | Yes | ||
| F, 18 | 1 | 1.5 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| M, 20 | 1 | 10 | NA | NS | Inflammatory* | NS | Yes | |
| M, 37 | 4 (including 3 non-pigmented adenomas) | 3.7 | NA | β-catenin (+) | NS | NS | Yes | |
| F, 42 | 1 | 21 | NA | β-catenin (+) | NS | NS | Yes | |
| M, 5 | 1 | 15 | NA | β-catenin (+) | NS | NS | Yes | |
| F, 54 | 1 | 6.4 | NA | β-catenin (+) | NS | NS | Yes | |
| M, 24 | 1 | 19 | NA | β-catenin (+) | NS | NS | Yes | |
| F, 29 | 1 | 8.5 | NA | NS | Inflammatory* | NS | Yes | |
| F, 75 | 1 | 3 | NA | NS | NS | HNF1-α inactivated* | Yes | |
| F, 49 | 1 | 2.2 | NA | NS | NS | HNFI-α | Yes | |
| IVI, 45 | 1 | 3.3 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 46 | 4 (including 2 non-pigmented adenomas) | 13 | NA | NS | NS | HNFI-α inactivated* | Yes | |
| F, 49 | 7 (including 4 non-pigmented adenomas) | NS | NA | β-catenin (+) | NS | NS | Yes | |
| IVI, 25 | 2 | NS | NA | - | Inflammatory* | NS | Yes |
M: male, F: female, cm: centimeters, OC: oral contraceptive, GS: glutamine synthetase, SAA: serum amyloid A, CRP: C-reactlve protein, NA: not available, NS: not specified. Symbols: negative (−); positive (+)
According to the WHO Classification of Tumors of the Digestive System, our case shares the features of β-catenin activated (type 2) and inflammatory HCA as the immunohistochemistry showed diffuse positivity for GS and aberrant cytoplasmic and nuclear staining for β-catenin as well as increased expression of SAA and CRP.6
The HCAs with β-catenin activation are usually associated with male gender and specific predisposing conditions, such as glycogenosis or male hormone use, and account for 10-15% of all HCAs. Kim, et al.,17 recently reported 3 cases of HCAs in Korean patients, all exhibiting nuclear expression of β-catenin and diffuse cytoplasmic expression of GS. In one of the cases there was cytoplasmic accumulation of a dark-brown granular pigment mimicking Dubin-Johnson-like pigment. Similarly to our case, this HCA displayed immunoexpression of SAA, CRP, β-catenin and GS. However, Mounajjed, et al.18 demonstrated that the immunophenotype in pigmented adenomas could be heterogeneous, being the HNF-1α inactivation type the most common genotype.18
The potential of malignant transformation of β-catenin activated HCAs is well known. In fact, β-catenin activation was the most frequent phenotype in cases classified as hepatocellular carcinoma in the largest case series of dark HCAs recently published.18
As imaging techniques do not distinguish between different types of HCAs, biopsy or complete surgical removal of the tumors and a careful pathological examination are necessary for a correct diagnosis.14 Dubin-Johnson-like pigment has previously been described in hepatocellular carcinomas,9 raising the discussion about the potential of malignant transformation in pigmented HCAs. In fact, it was recently described that pigmented tumors have a high association with atypia and malignancy.18 Mounajjed, et al. suggested that lipofuscin accumulation HCAs serves as a marker for increased risk of progression towards malignancy.18 Previously, lipofuscin was linked indirectly to carcinogenesis as its accumulation is due to cell aging and damage.19–21 However, the exact pathogenetic role remains uncertain.18
Nonetheless, the cases reported so far support the view that β-catenin activation might be more important for malignant transformation than the presence of Dubin-Johnson-like pigment. In fact, considering all pigmented (dark) adenomas described in the literature7,9–18 ten hepatocellular carcinomas were reported, arising within a pig-mented HCA and, in 70% of them, nuclear β-catenin immunoexpression was present.14,18
ConclusionIn conclusion, the authors describe a pigmented (dark) adenoma with histological and immunohistochemical features of type 2 (β-catenin activation) and type 3 (inflammatory) HCA. Similar to this, there are four other cases reported in the literature, the one herein described corresponding to the fifth. Pigmented adenomas are rare and the presence of β-catenin activation should be searched for due to the higher risk of malignant transformation in hepatocarcinoma.
Acknowledgments- •
Rosa Coelho: drafting of the manuscript.
- •
Regina Gonçalves: drafting of the manuscript.
- •
Fátima Carneiro: drafting of the manuscript and critical revision of the manuscript.
- •
Margarida Fernandes: acquisition of data.
- •
Joanne Lopes: acquisition of data.
- •
Susana Guimaråes: acquisition of data.
- •
Guilherme Macedo: critical revision of the manuscript.
The authors confirm that informed, written consent has been obtained. All rules of local ethics committee were followed, preserving patient identity and confidentiality.