Background and rationale of the study. Hepatitis C infection is very common among injection drug users(IDUs). In clinical practice there is reluctance to treat IDUs, because considered difficult-to-treat. Aim of this study was to evaluate the response to antiviral treatment in IDUs compared to non-IDUs.
Main results. In this observational retrospective study, 204 non cirrhotic patients(112 IDUs, 92 non-IDUs) with chronic hepatitis C, treated with PEG-IFN and ribavirin in a tertiary centre for IDUs of Southern Italy from 2008 to 2011 were analyzed. Age, sex, genotype, steatosis, response to previous therapy, rapid(RVR), early(EVR), end-of-treatment(ETR), sustained(SVR) virological response were evaluated. IDUs were mainly young and males, with prevalence of genotype 3. A higher SVR rate in IDUs group compared to non-IDUs only in PerProtocol(PP) analysis (90% vs. 78,9% ;p = 0.04). On the contrary, in IntentionToTreat(ITT) analysis, no significant difference was relieved. A higher SVR rate at ITT analyses in naïve non-IDUs patients was found (76,13% vs. 90%, p = 0.021), but at PP analysis wasn’t confirmed. Treatment was well tolerated; a higher dropout rate was reported in IDUs (24 patients) compared to non-IDUs (2 patients). In order to exclude the effect of viral genotypes on SVR a genotype matched statistical analysis was done and no difference was found.
Conclusions. IDUs naïve patients, due to young age and high prevalence of genotype 3, appear good candidates to dual antiviral therapy with high SVR rates. Dropout is the main non-response cause among these subjects, but through an optimal monitoring program with a multidisciplinary setting, their “difficult to treat” characteristics can be overcome.
Hepatitis C virus (HCV) infection is a major healthcare problem and the leading cause of chronic liver disease worldwide. According to recent World Health Organization (WHO) data, approximately 170-200 million people throughout the world are infected with HCV.1
The primary objective of anti-HCV therapy is the complete elimination of the virus, which is termed a sustained virological response (SVR). SVR, defined as undetectable serum HCV RNA at least 6 months after completion of antiviral therapy, has become the best indication of successful therapy for HCV infection.2
Therapy-induced SVR is a clinically meaningful end point and a durable marker of viral eradication.3 Once achieved, an SVR is considered to be a cure of HCV infection, because the rate of late relapse (defined as reappearance of serum HCV RNA) is extremely low (< 1%). Achievement of SVR has been associated with improvement in liver histology (reduced inflammation and fibrosis) and health-related quality of life, as well as reduced risk of hepatocellular carcinoma (HCC) and liver-related morbidity and mortality.2–4 The survival of patients who achieved SVR was reported to be comparable to that of general population, matched for age and sex.4
Until 2011, the standard of care (SOC) for chronic HCV genotype 1 infection was the combination of pegylated interferon-α (PegIFN) and ribavirin (RBV).5 This regimen achieves SVR rates of 40% to 50% in patients with genotype 1, and up to about 80% in those with genotype 2, 3, 5 and 6.2,6,7 In 2011 first-generation direct-antivirals (DAAs), namely boceprevir (BOC) and telaprevir (TVR), were licensed for use in HCV genotype 1 in combination with PegIFN/RBV(PR). These triple drug regimen, administered on response-guided based therapy, have proven to be effective for previously untreated (naïve) and for treatment-experienced patients achieving SVR rates of 65-80%8 or other genotype PR is still represented by dual therapy combination of PegIFN/RBV. New agents will be soon available for interferon free regimens that should provide a response rate as high as 100%.9,10 At present, injection drug users (IDUs) constitute the largest proportion of HCV patients in industrial countries.1 In Italy, the IDU population is estimated to range from 200,000 to 300,000 individuals and HCV infection prevalence among this population ranges between 42.4% and 89.7%.1
Although international guidelines no longer regard ongoing illicit drug use as a contraindication to antiviral therapy for chronic hepatitis C (CHC), in routine clinical practice there is a continuing reluctance to treat IDUs: they are considered difficult to treat, because of the poor treatment adherence, the high dropout rate, the increased likelihood of reinfection, the high rates of concomitant alcohol abuse or mental health issues, all potentially impacting treatment compliance and effectiveness;11,12 therefore, HCV infection is a complex and challenging medical condition in this population of patients.13 A debated issue concerns the health costs of drug-related HCV, which is accounting for nearly 40% of expenses in IDUs between hepatitis B and HIV.14 These patients are considered at low priority for the high risk of dropout and relapse. Nevertheless they are often young and naïve patients in whom treatment would be appropriate to avoid hepatic and extrahepatic HCV related complications. Furthermore, in approaching the era of high cost interferon-free antiviral therapy, the previous cheaper standard of care with PR might still be considered a real strategic option.
The aim of this retrospective observational study was to evaluate the response to dual antiviral therapy in IDUs, the largest subpopulation infected with HCV, compared to non-IDUs patients in order to better understand how to select patients who can be treated successfully for hepatitis C.
Material and MethodsStudy designThis was an observational, retrospective, singlecentre study performed in an outpatients service for IDUs care in a tertiary structure of Southern Italy.
PatientsAll consecutive non-cirrhotic adult patients with chronic hepatitis C treated with PEG-IFN alpha-2a and ribavirin in a tertiary centre for IDUs of Southern Italy from 2008 to 2011 were analyzed. The selection criteria for the treatment included a multidisciplinary program of initial observation of patients (hepatological and psychiatric counselling). The IDUs stabilized on methadone or buprenorphine substitution treatment were subjected to tox screen every 15 days and carbohydrate-deficient transferrin (CDT) assay every 20 days. Cirrhosis, active chronic hepatitis B, HIV infection, drug and alcohol abuse during the last six months and higher score than 18 at the evaluation Hamilton depression scale were considered exclusion criteria to be enrolled to antiviral therapy. Based on that, 36% (62 out 174 IDUs: 16% cirrhotic, 15% with mental problems, 5% who were using drugs during the last six months) and 45% (74 out 166 of non-IDUs: 40% cirrhotic and 5% with mental problem) of the patients were excluded from the study.
Therefore, 204 patients were enrolled: 112 IDUs (54.9%) and, as a control group, 92 non-IDUs (45.1%), coming from an outpatients Hepatology service of the same structure.
Among the IDUs enrolled 60% were under substitute therapy with oral agents (E.G. Metadone), none of them have concomitant alcohol abuse and other mental problems. Nevertheless all of them underwent to formal psychiatric evaluation as part of patients selection for treatment. Treatment with escitalopram at an initial daily dose of 10 mg was initiated three weeks before HCV treatment in those patients (45%) with a higher score than 14 at the evaluation Hamilton depression scale to the psychiatric counselling. The dose was increased to 20 mg/day after one week and continued throughout antiviral treatment. PEG-IFN alpha-2a was administered subcutaneously at the dose of 180 μgonce weekly, ribavirin was administered orally at the dose of 800-1,200 mg/day, depending on the body weight. The duration of treatment was 12 months for patients with genotype 1 and 6 months for patients with genotype 2 and 3. Treatment durations and dosage were chosen on the basis of current treatment schedules approved in our country. During the treatment helpline was available seven days a week. Monthly patient’s multidisciplinary evaluation and biweekly tox screen to IDUs was performed. Also carbohydrate deficient transferrin (CDT) assay in patients with alcohol abuse was executed every 20 days.
The study was performed in accordance with Good Clinical Practice and complying with the principles laid down in the Declaration of Helsinki.15
The study was approved by the appropriate Ethical Committee of the centre and all patients gave their written informed consent before enrollment.
Assessments and endpointsOf every patient enrolled in the study were evaluated: age, sex, genotype, clinical parameters (presence of hepatic steatosis, assessed clinically and by ultrasound; the ultrasound evaluation was always made by the same operator and with the same equipment, Aloka SSD500 - Aloka Co., Ltd, Tokyo, Japan-), response to previous therapy (naïve or relapsers patients).
The following treatment outcomes were also analyzed: rapid virological response (RVR: undetectable HCV RNA at week 4 of therapy), early virological response (EVR: ≥ 2 log10 decrease in HCV RNA at week 12 compared with baseline), end-of-treatment response (ETR: undetectable HCV RNA at the end of treatment) and sustained virological response (SVR: undetectable HCV RNA at least 6 months following the end of treatment).
For safety evaluation, the incidence of adverse events and the dropout rate were assessed in both groups.
Methods- •
RNA Preparation and Quantitative Real-Time PCR Analysis. A real-time nucleic acid amplification assay (RT-PCR), was used for quantitative detection of HCV RNA. Sera were rapidly frozen at -80°C within 2 h of blood drawing. HCV RNA was isolated from 0,85 ml aliquots of controls and clinical specimens using the automated COBAS Ampliprep instrument (Roche Diagnostics, Meylan, France). HCV quantification standard was added to the sample in order to achieve full process control. Amplification and detection were performed according to the manufacturer’s instructions. After COBAS Ampliprep-based extraction of nucleic acids, samples and controls were processed for amplification and detection by an automated RT-PCR using the COBAS Taq-Man 48 Analyzer (Roche Diagnostics, Meylan, France) according to the instructions of the manufacturer.16 The lower limit of detection is 15 IU/mL, with ≥ 95% probability, using 1 mL of serum. Carryover PCR contamination was avoided by the application of the measures suggested by Kwok and Higuchi.17
- •
HCV Genotyping. To classify the HCV genotypes, serum polymerase chain reaction (PCR) products were hybridized to type-specific and subtype-specific probes 1a, 1b, 2a, 2b and 3a. The probes had to fulfill two main criteria: there could be no more than 2 mismatches in comparison with the corresponding published sequences of the same subtype, and they had to differ by 3 or more mismatches in comparison with published sequences of other types and subtypes. The only exception was probe 2b, which had only 2 mismatches in comparison with the corresponding sequence of type 3a.18
- •
Statistical analysis. Chi-Square test with YATES correction or Fisher-exact test was used to compare categorical variables (sex, presence of steatosis and cirrhosis, response to previous therapy [naïve, relapsers and non-responders], genotype, viral load at the start of treatment and duration of infection). Response to current therapy, as a categorical variable, even among patients matched for genotype, was evaluated by Chi-Square Test with yates correction. A binary logistic regression was performed to evaluate the independent factors associated with SVR. All statistical analyzes were performed with Statistical Program for Social Sciences (SPSS®) ver.20.0 for Macintosh® (SPSS Inc., Chicago, Ill.). P-value < 0.05 was considered significant. An Intention-To-Treat (ITT) and a Per-Protocol (PP) analysis were performed.
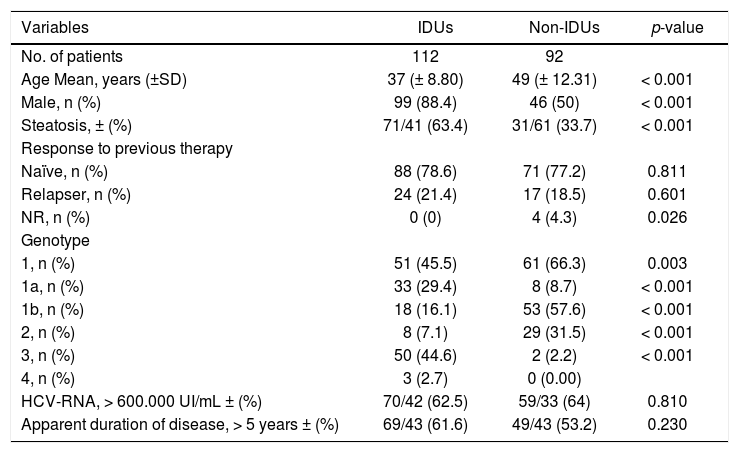
112 patients (54.9%) were IDUs and 92 were non-IDUs (45.1%). The demographic characteristics of the study population were the following (Table 1): the IDUs were mainly male (88.4% vs. 50%; p < 0.001), young (mean ± SD age: 37 ± 8.8 vs. 49 ± 12.3, p < 0.001) and had more frequently steatosis (63.4% vs. 33.7%; p < 0.001) compared to non-IDUs. However, in both groups, most patients were naïve(88 [78.6%] IDUs vs. 71 [77.2%] non-IDUs), than relapsers (24 [21.4%] IDUs vs. 17 [18.5%] non-IDUs), without significant differences.
Patient demographic and baseline characteristics.
| Variables | IDUs | Non-IDUs | p-value |
|---|---|---|---|
| No. of patients | 112 | 92 | |
| Age Mean, years (±SD) | 37 (± 8.80) | 49 (± 12.31) | < 0.001 |
| Male, n (%) | 99 (88.4) | 46 (50) | < 0.001 |
| Steatosis, ± (%) | 71/41 (63.4) | 31/61 (33.7) | < 0.001 |
| Response to previous therapy | |||
| Naïve, n (%) | 88 (78.6) | 71 (77.2) | 0.811 |
| Relapser, n (%) | 24 (21.4) | 17 (18.5) | 0.601 |
| NR, n (%) | 0 (0) | 4 (4.3) | 0.026 |
| Genotype | |||
| 1, n (%) | 51 (45.5) | 61 (66.3) | 0.003 |
| 1a, n (%) | 33 (29.4) | 8 (8.7) | < 0.001 |
| 1b, n (%) | 18 (16.1) | 53 (57.6) | < 0.001 |
| 2, n (%) | 8 (7.1) | 29 (31.5) | < 0.001 |
| 3, n (%) | 50 (44.6) | 2 (2.2) | < 0.001 |
| 4, n (%) | 3 (2.7) | 0 (0.00) | |
| HCV-RNA, > 600.000 UI/mL ± (%) | 70/42 (62.5) | 59/33 (64) | 0.810 |
| Apparent duration of disease, > 5 years ± (%) | 69/43 (61.6) | 49/43 (53.2) | 0.230 |
IDUs: injection drug users. NR: non-responder. HCV: hepatitis C virus.
Genotype 3 was significantly higher in the IDUs group compared to controls (44,6% vs. 2,22%; p<0.001), while genotype 1 and 2 was higher in non-IDUs group. Genotype 1 was found in 45,5% of IDUs and 66.3% (p = 0.003) of non-IDUs, genotype 2 was found in 7.1% of IDUs and 31.5% of non-IDUs (p < 0.001).
There were no significant differences in the other parameters evaluated (basal viral load and apparent duration of infection).
EfficacyThe prevalence of virological response was evaluated by means of ITT and PP analysis. ITT analysis included 204 patients (112 IDUs and 92 non-IDUs), while PP analysis included 180 patients (90 IDUs and 90 non-IDUs).
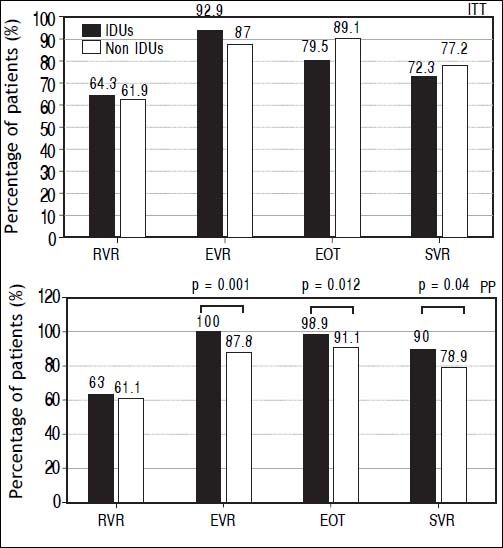
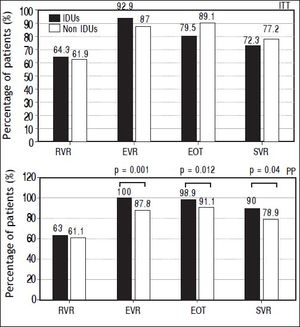
Results show a higher SVR rate in IDUs group compared to non-IDUs group only in PP analysis: 90% vs. 78.9%; p = 0.04. Higher EVR (100% vs. 87.7%; p = 0.001) and EOT (98.9% vs. 91.9; p = 0.012) rate was found in IDUs group as well, while in ITT analysis, no significant difference was relieved (Figure 1).
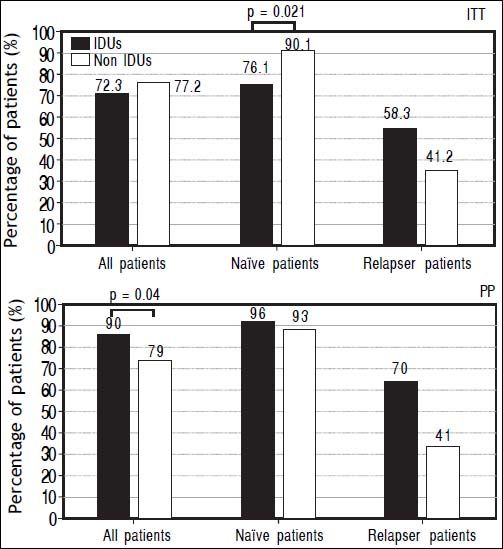
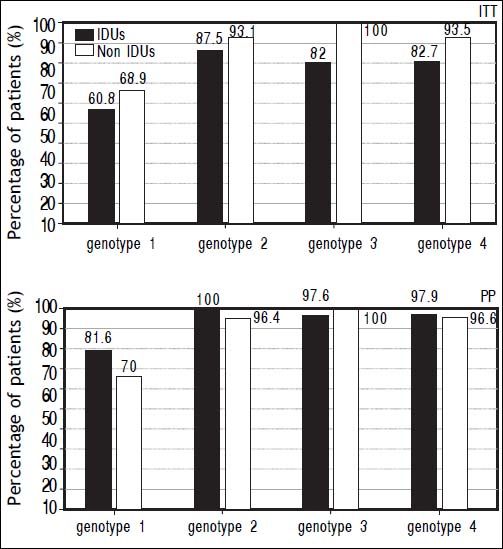
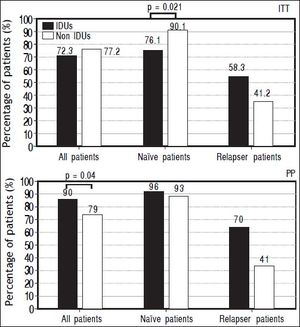
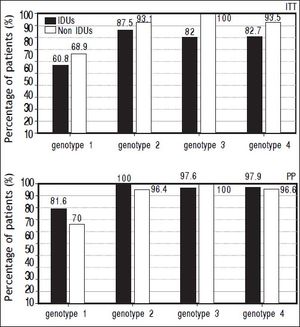
The prevalence of SVR at both ITT and PP analyses was evaluated in different subgroups of the population: naïve and relapser patients. A SVR higher rate at ITT analyses in naïve non-IDUs patients was found (76,13 vs. 90%, p = 0.021) that was not confirmed at PP analysis (Figure 2). In order to exclude the effect of virus genotype variable on SVR a genotype matched statistical analysis was done and no difference was found among the HCV genotypes (Figure 3).
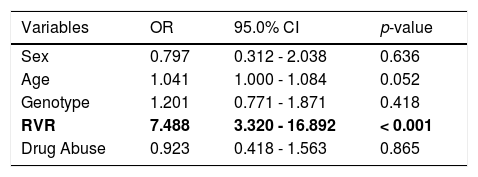
A multiple logistic-regression model was used to explore the effects of various demographic and virological characteristics at baseline and on treatment on the probability of an SVR (Table 2). Factors considered in this analysis were: sex, age (considered as a continuous variable), HCV genotype (assessed by comparing the frequency between IDUs and non-IDUs group for each genotype), RVR and drug abuse. In the multiple logistic-regression model, all these factors were included simultaneously. The results demonstrated that the only independent factor associated with SVR for successful HCV therapy was RVR (O.R.: 7.488, C.I.95%: 3.32-16.982, p < 0.001): a higher RVR independently and significantly increased the odds ratio of a SVR.
multiple logistic-regression model for successful HCV therapy. The only independent factor of sustained virological response was RVR.
| Variables | OR | 95.0% CI | p-value |
|---|---|---|---|
| Sex | 0.797 | 0.312 - 2.038 | 0.636 |
| Age | 1.041 | 1.000 - 1.084 | 0.052 |
| Genotype | 1.201 | 0.771 - 1.871 | 0.418 |
| RVR | 7.488 | 3.320 - 16.892 | < 0.001 |
| Drug Abuse | 0.923 | 0.418 - 1.563 | 0.865 |
HCV: hepatitis C virus. OR: odds ratio. CI: confidence interval. RVR: rapid virological response.
Treatment was well tolerated and no unexpected side effects were seen. The adverse events reported in the two groups were typical of those previously reported with this standard therapy: drug addiction relapse following discontinuation of therapy was found in 12 (13.4%) of IDUs, depression was reported in 16 (17.92%) of IDUs and 8 (7.3%) of non-IDUs, anemia in 10 (11.2%) of IDUs and 7 (6.44%) of non-IDUs, asthenia in 8 (8.69%) of IDUs and 5 (5.4%) of non-IDUs, psoriasis occurred in none of IDUs and in 3.9% of non-IDUs. Excluding drug addiction relapse, there were not significative difference.
On the contrary, dropout rate was significantly higher in the IDUs group: 22 (19.6%) dropouts were reported among IDUs patients and only one (1%) among non-IDUs patients. The reasons for premature termination were the following: in IDUs group, 4 patients dropped out for depression, 6 patients for their own decision to stop treatment earlier, 12 patients for drug addiction relapse; in non-IDUs group, there was only one patient dropped out for depression.
DiscussionGlobally, about ninety per cent of new hepatitis C infections are contracted through injection drug use and the majority of infections, particularly in developed countries, are attributed to injection drug use.11 Because HCV transmission occurs primarily through direct percutaneous exposure to infected blood from various sources, injection drug use is at greatest risk of acquiring HCV infection, and is now responsible for the majority of new and existing cases of hepatitis C. Blood-to-blood contact transmits HCV from person to person very efficiently; thus, people can acquire it through sharing not only needles and syringes, but also other injection equipment, including cottons, cookers and rinse water.
A growing body of data suggests that drug users, even those with multiple potential barriers, can attain successful treatment outcomes in hepatitis C therapy, showing no relevant direct influence of intravenous drugs on the efficiency of anti-HCV therapy among adherent patients.19,20
Based on data from 16 prospective clinical studies of CHC treatment in IDUs published in the past 10 years, findings on effectiveness and tolerability are comparable to those in the general population.21
A retrospective study performed in Greece in a large cohort of former IDUs (with prevalent genotype 3) followed-up from 1994 to 2008 showed that IDUs patients had SVR rates similar to those without drug-dependence.22
Interestingly, some evidence suggests that hepatitis C may follow a more benign course when contracted via injection drug use, despite the potential risks of ongoing injecting behaviours and alcohol consumption.23
A review by Hellard, et al. identified 22 studies reporting on SVR attainment by IDUs with chronic hepatitis C: of the 22 studies included, 10 enrolled a control group of non-IDUs and the remaining 12 studies recruited IDUs only (former or current). The available evidence suggests that IDUs can be successfully treated for hepatitis C: there is evidence that a sizeable proportion of IDUs who begin hepatitis C treatment achieve a SVR. There was a considerable variation in SVR rates among IDUs in different trials, ranging from 15.8% to 94.1% for chronic hepatitis C. The median SVR rate among IDUs (40.6%) suggests that a substantial proportion of treated IDUs achieved a SVR. These data suggest that injection drug use should not preclude treatment. On the same ground, different studies have demonstrated that properly selected HCV-in-fected IDUs can achieve SVR rates comparable to those of non-IDU populations. In studies in which IDUs were compared with non-IDUs, the SVR among IDUs was often similar to and, at times, higher than among non-IDUs. None of the studies that included non-IDU comparison groups reported a statistically significant difference between the rate of SVR among IDUs and that among non-IDUs.11
Several factors predicting non-response to interferon therapy have been investigated since it became available. There is large evidence in the literature that HCV genotype 2 and 3 are associated with higher SVR rates in respect to the others (i.e. genotype 1).24 Moreover, in many studies, a proportion > 50% of unselected IDUs patients has been reported to be infected with HCV genotype 3.1 Also patients related non response predictive factors were largely demonstrated and they are generally divided into two main subgroups: non-modifiable factors (age, gender, ethnicity, degree of liver disease and disease duration) and modifiable (diabetes and insulin resistance, obesity and steatosis, alcohol consumption and drug abuse).24 Based on that and to avoid confounding results, among the exclusion criteria we identified liver cirrhosis that might significantly weight on SVR. Nonetheless, some epidemiological and viral non response predictive factors (i.e. age, genotype, etc.) were still significantly different between the two groups and despite that, the SVR rate was still comparable between the IDUs and non-IDU and also comparable to that reported into registered trials.2,6,7 Moreover, SVR rates of both study groups, calculated according to a genotype-matched analysis, were similar in all HCV genotypes. This might be accounted for the different HCV genotypes distribution between groups. In fact, although genotype 1 infected patients, who might account for a worst response to therapy, were more prevalent among non-IDU patients, the easy-to-treat subjects being homogeneously distributed between the two groups (genotype 3 patients higher in IDUs and genotype 2 higher in non IDUs) might balance the overall response and be responsible for no SVR rates difference between IDUs and non-IDUs.
The SVR rates both in naïve and relapser and IDU and non-IDU patients, both at ITT and PP analyses seem higher than that reported in the literature. In our mind, this might be related to the high clinical surveillance applied to our patients during therapy. Therefore such care and surveillance seem of great significance and to be recommended to pursue as high a response rate as possible. Moreover, at the ITT analysis a higher rate of SVR was relieved in naïve non-IDU patients whereas in PP analysis no difference was shown between groups and no differences were found while considering all patients. Looking at the PP analysis a higher SVR rate was shown in IDUs group in the overall population. Trying to explain this apparent discrepancy, we realize that it might be accounted for the higher number of dropout subjects in the group of IDUs. In fact, IDUs patients prematurely terminated treatment more frequently than the control group (22 dropouts in IDUs group and only one in non-IDUs group), confirming a higher dropout rate among IDUs also reported in other studies.25 A lower dropout rate (19.6%) was found at our center compared to those reported by other structures.26,27 A possible explanation accounting for this discrepancy might be that in our center was performed a close monitoring of the IDUs patients by a multidisciplinary approach, including psychiatric and hepatologic counselling, treatment helpline ever available and strict checking of drug addiction relapse by tox screen and CDT assay. Thus IDUs patients with appropriate adherence and compliance have very good chances to reach the SVR and, through an optimal monitoring program with multidisciplinary setting, their “difficult to treat” characteristics can be overcome. Our study demonstrates that in our geographical area, there may be no differences in SVR rates between IDUs and non-IDU patients. This finding is even more significant if we compare it with other literature’s reports, because of the nature of our study that was based on a single-center, and therefore less prone to biases arising from patients selection and characteristics. Moreover, the higher genotype 3 prevalence among IDUs here demonstrated in agreement with other studies, suggests that, despite new therapies, for these patients the dual therapy remains a real option and considering physical and physiological fragility of drug addicts together with the excellent SVR rates obtained in this study, we believe that dual antiviral therapy may still be considered a first choice option also for genotype 1 patients. In fact, the known poor compliance for antiviral therapy together with the high number of drop-outs among IDUs might suggest that it might be still worse for triple therapy with DAAs, which are in general less tolerated than the dual therapy.5
ConclusionThere is no scientific or clinical reason to exclude IDUs from antiviral therapy for CHC. Although no international treatment guidelines are available for management of HCV infection among IDUs, the results of our observational study show that IDUs naïve patients, appear to be good candidates and very good responders to a standard antiviral combination of ribavirin and pegylated inferferon alpha with high prevalence of SVR. This finding suggests that the proportion of IDUs who start treatment for HCV infection should be increased and dual therapy might still be considered a real strategic option for them.
AknowledgementsThe present study did not receive any financial support to be declared.
Conflict of Interest DisclosureAll authors: no conflict.
AbbreviationsBOC: boceprevir.
CDT: carbohydrate-deficient transferrin.
CHC: chronic hepatitis C.
DAAs: direct-antiviral agents.
ETR: end of treatment response.
EVR: early virological response.
HCC: hepatocellular carcinoma.
HCV: hepatitis C virus.
IDUs: injection drug users.
ITT: intention to treat.
IV: intravenous.
OR: Odds Ratio.
PCR: polymerase chain reaction.
Peg-IFN: pegylated interferon.
PP: per protocol.
PR: PegIFN/Ribavirin.
RBV: ribavirin.
RT-PCR: real time-polymerase chain reaction.
RVR: rapid virological response.
SOC: standard of care.
SVR: sustained virological response.
TVR: telaprevir.
WHO: World Health Organization.