Viral infections have been described to increase the risk of decompensation in patients with cirrhosis. We aimed to determine the effect of SARS-CoV-2 infection on outcome of hospitalized patients with cirrhosis and to compare the performance of different prognostic models for predicting mortality.
PatientsWe performed a prospective cohort study including 2211 hospitalized patients with confirmed SARS-CoV-2 infection from April 15, 2020 through October 1, 2020 in 38 Hospitals from 11 Latin American countries. We registered clinical and laboratory parameters of patients with and without cirrhosis. All patients were followed until discharge or death. We evaluated the prognostic performance of different scoring systems to predict mortality in patients with cirrhosis using ROC curves.
ResultsOverall, 4.6% (CI 3.7–5.6) subjects had cirrhosis (n = 96). Baseline Child-Turcotte-Pugh (CTP) class was assessed: CTP-A (23%), CTP-B (45%) and CTP-C (32%); median MELD-Na score was 19 (IQR 14−25). Mortality was 47% in patients with cirrhosis and 16% in patients without cirrhosis (P < .0001). Cirrhosis was independently associated with death [OR 3.1 (CI 1.9−4.8); P < .0001], adjusted by age, gender, and body mass index >30. The areas under the ROC curves for performance evaluation in predicting 28-days mortality for Chronic Liver Failure Consortium (CLIF-C), North American Consortium for the Study of End-Stage Liver Disease (NACSELD), CTP score and MELD-Na were 0.85, 0.75, 0.69, 0.67; respectively (P < .0001).
ConclusionsSARS-CoV-2 infection is associated with elevated mortality in patients with cirrhosis. CLIF-C had better performance in predicting mortality than NACSELD, CTP and MELD-Na in patients with cirrhosis and SARS-CoV-2 infection. Clinicaltrials.gov:NCT04358380.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus disease 2019 (COVID-19), causes substantial pulmonary disease [1]. Patients with increased age and preexisting comorbid conditions, such as diabetes and obesity, are at increased risk of death [1]. Chronic liver disease and cirrhosis are common conditions presenting a systemic immunocompromised status [2]. Thus, bacterial infections are a common cause of liver-related complications in patients with cirrhosis [3]. Viral infections have been less described in this population. In a recent study from India, 82% of patients with cirrhosis and A/H1N1/09 infection died of pneumonia and acute respiratory distress syndrome (ARDS), with bacterial or fungal infection in some cases [4]. Circulating cytokines and chemokines have been proposed to further contribute to hepatocyte and endothelial damage and the consequent hepatic decompensation [5].
SARS-CoV-2 infection in patients with cirrhosis has been associated with detrimental outcomes. We know from different retrospective studies that mortality in this population is high, ranging from 16% to 42% [6–10]. A large international study identified greater age and alcohol-related liver disease as risk factors for death in patients with cirrhosis hospitalized for COVID-19 [9]. As expected, high rates of acute hepatic decompensation and acute-on-chronic liver failure (ACLF) were also observed in patients with cirrhosis and SARS-CoV-2 infection [7–9]. However, the prognostic performance of different scoring systems to predict mortality in patients with cirrhosis have not been completely evaluated.
COVID-19 pandemic has affected later in Latin America, giving us the unique opportunity to build a multinational prospective registry. Abnormal liver tests on admission were independently associated with increased mortality in patients with no history of liver disease [11]. Thus, we now sought to evaluate the impact of COVID-19 on the clinical outcome of hospitalized patients with cirrhosis and compare different prognostic scores ability to predict patient survival.
2Patients2.1Study design, setting and participating centersThis prospective cohort study was performed from April 15, 2020 through September 15, 2020 in 38 Hospitals from Argentina, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guatemala, Mexico, Paraguay, Peru and Uruguay. The study was supported and coordinated by the Latin American Association for the Study of the Liver, Viral Hepatitis Group of Interest and registered in an open public registry (NCT04358380; www.clinicaltrials.gov). Each Ethics Committee from all the participating centers approved the study protocol, following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [12]. The study followed ethical standards (institutional and national) and those mandated by the Helsinki Declaration of 1975, as revised in 2008. Authors had access to the study data, reviewed, and approved the final version of this manuscript.
2.2Cohort characteristics and study variablesEligibility criteria for enrolment included patients ≥17 years old, hospitalized with SARS-CoV-2 infection confirmed by the real-time polymerase method (RT-PCR) as per the local site-specific protocol. In asymptomatic cases, a nasopharyngeal swab was obtained according to each country surveillance algorithm (i.e., contact with positive subjects). We also included patients admitted for a different condition and tested positive for COVID-19 during their hospitalization. Patients with high-clinical and epidemiological suspicion of SARS-CoV-2 without RT-PCR testing, those with solid organ transplantation, or pregnant were excluded. All eligible patients were enrolled at each clinical site. Cirrhosis and its etiology was determined by the site investigator or treating physician based on liver biopsy, liver elastography or a combination of clinical signs of portal hypertension (i.e. presence of gastroesophageal varices on endoscopy), biochemical parameters (i.e. presence of a platelet count less than 100.000/mm3) and/or radiologic findings consistent with cirrhosis (i.e. splenomegaly (spleen larger than 120 mm on radiographic imaging) [13]. Study data were prospectively registered into a web-based electronic system. All patients were followed until discharge or death. Baseline exposure variables were collected for all enrolled subjects, including detailed demographic data, laboratory parameters and comorbid conditions. We also recorded the hospital course, and treatment regimens.
2.3Liver disease evaluationSeverity of liver disease was evaluated according to Child-Turcotte-Pugh (CTP) score, model for end-stage liver disease (MELD), and MELD-Na. Patients with cirrhosis who developed ascites, variceal bleeding or hepatic encephalopathy were classified as having acute decompensation. Acute-on-chronic liver failure (ACLF) was prospectively defined according to the European Association for the Study of the Liver Chronic Liver Failure (CLIF) consortium definition and by the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) [3,14]. The Asian Pacific Association for the Study of the Liver ACLF criteria was not comprised in our analysis. This score includes patients without cirrhosis and excludes those with previous hepatic decompensation [15]. Thus, for each patient with cirrhosis who developed ACLF, NACSELD and CLIF-C scores were considered.
To assess the impact of SARS-CoV-2 infection on clinical outcomes of hospitalized patients with cirrhosis, we compared clinical data, laboratory features, and survival of this cohort with that of a control group that included patients hospitalized with COVID-19 infection and no history of liver disease.
2.4COVID-19 severityThe severity of COVID-19 was classified based on clinical examination results, symptoms, chest radiography and medical support. Severe COVID-19 cases were defined as those who developed ARDS, required intensive care unit (ICU) monitoring, and/or ventilatory support as reported elsewhere [6].
2.5Primary outcome and statistical analysisCategorical data were compared using Fisher’s exact test (2-tailed) or Chi-Square (X2) test as appropriate. Continuous variables were reported with a mean (± standard deviation, SD) or median (Interquartile ranges 25–75%, IQR) and compared with Student’s T or Mann-Whitney U tests according to their respective distributions. Multivariable logistic regression was used to evaluate the association between cirrhosis and the odds of death (OR) with corresponding 95% confidence intervals (CI). We first fit univariate models to evaluate crude effects on mortality of prior medical history, clinical and laboratory findings on admission, then outcomes and treatment prescribed during hospitalization. We constructed the final multivariable models including exposure variables with a P-value <0.1 in univariate analysis, using a step-by-step procedure, in order to develop a parsimonious model. The final model’s performance was evaluated including calibration (Hosmer-Lemeshow goodness-of-fit test) and discrimination power through the area under the receiving operator curve (AUROC).
We also used AUROC to determine the score accuracy (c-statistic) of baseline CTP score, MELD-Na, CLIF-C organ failure score and NACSELD, as predictors of 28 days in-hospital mortality in patients with cirrhosis. Data were analyzed with STATA 13.0 (StataCorp, Texas, USA).
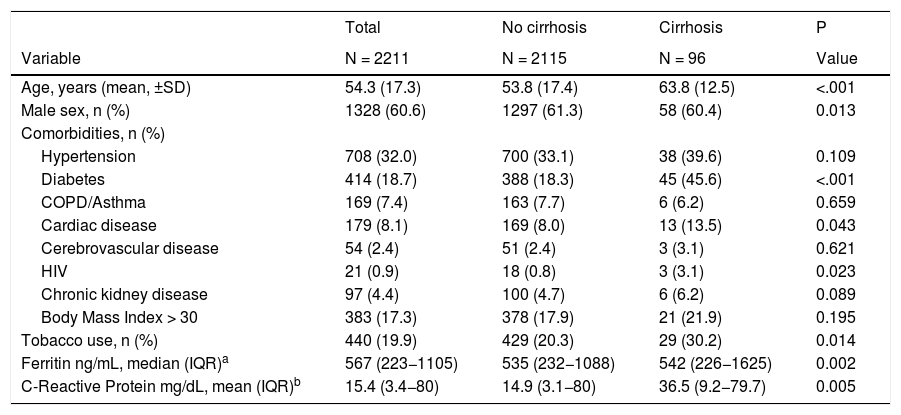
3ResultsA cohort of 2286 patients hospitalized with SARS-CoV-2 infection were enrolled in the Latin American Association for the Study of the Liver registry. After excluding pregnant patients (n = 26) and those who underwent solid organ transplantation (n = 45), 2211 remained for the analysis (Supplementary Fig. 1). Overall, 4.6% (CI 3.7–5.6) subjects had cirrhosis (n = 96). From the entire cohort, 8.6% (CI 7.2–9.6) were admitted for other causes and acquired SARS-CoV-2 infection during hospitalization (n = 186), including 7 patients with cirrhosis (7.3%). Baseline characteristics of the study population are displayed in Table 1. Radiological signs on admission showed pneumonia in 73.9% of the cohort (n = 1636). Common signs and symptoms reported by patients are presented in Supplementary Table 1. Ninety-seven (4.4%) patients were asymptomatic at presentation.
Baseline characteristics of patients hospitalized for COVID-19.
| Total | No cirrhosis | Cirrhosis | P | |
|---|---|---|---|---|
| Variable | N = 2211 | N = 2115 | N = 96 | Value |
| Age, years (mean, ±SD) | 54.3 (17.3) | 53.8 (17.4) | 63.8 (12.5) | <.001 |
| Male sex, n (%) | 1328 (60.6) | 1297 (61.3) | 58 (60.4) | 0.013 |
| Comorbidities, n (%) | ||||
| Hypertension | 708 (32.0) | 700 (33.1) | 38 (39.6) | 0.109 |
| Diabetes | 414 (18.7) | 388 (18.3) | 45 (45.6) | <.001 |
| COPD/Asthma | 169 (7.4) | 163 (7.7) | 6 (6.2) | 0.659 |
| Cardiac disease | 179 (8.1) | 169 (8.0) | 13 (13.5) | 0.043 |
| Cerebrovascular disease | 54 (2.4) | 51 (2.4) | 3 (3.1) | 0.621 |
| HIV | 21 (0.9) | 18 (0.8) | 3 (3.1) | 0.023 |
| Chronic kidney disease | 97 (4.4) | 100 (4.7) | 6 (6.2) | 0.089 |
| Body Mass Index > 30 | 383 (17.3) | 378 (17.9) | 21 (21.9) | 0.195 |
| Tobacco use, n (%) | 440 (19.9) | 429 (20.3) | 29 (30.2) | 0.014 |
| Ferritin ng/mL, median (IQR)a | 567 (223−1105) | 535 (232−1088) | 542 (226−1625) | 0.002 |
| C-Reactive Protein mg/dL, mean (IQR)b | 15.4 (3.4−80) | 14.9 (3.1−80) | 36.5 (9.2−79.7) | 0.005 |
Abbreviation: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
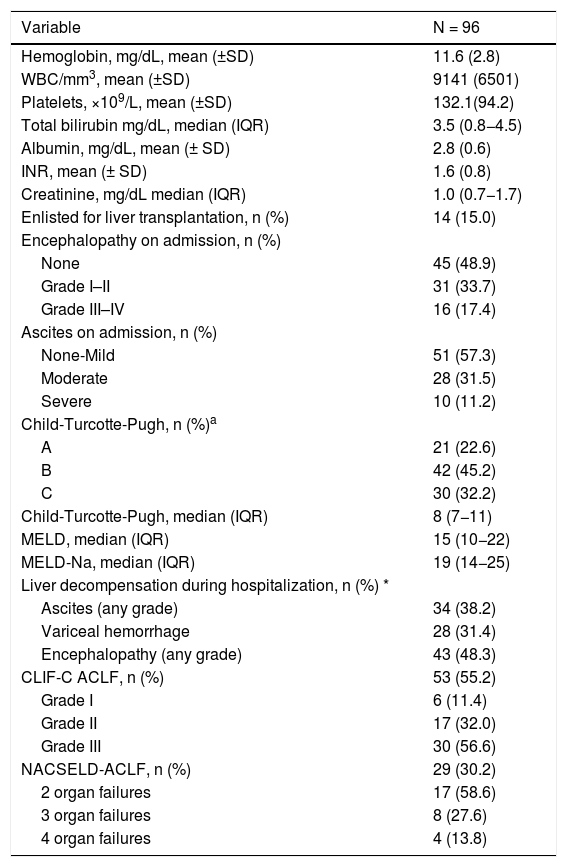
When compared to admitted patients without cirrhosis, patients with cirrhosis were older (53.8 ± 17.4 vs. 63.8 ± 12.5 years old; P < .0001), a higher proportion had diabetes (18.3% vs. 45.6%; P < .0001) and reported history of tobacco use (20.3% vs. 30.2%; P = 0.01) (Table 1). The most common etiologies of cirrhosis were non-alcoholic steatohepatitis in 44 patients, alcohol-induced in 22 cases, chronic hepatitis C in 6 individuals and cholestatic diseases in 5 subjects (Table 2). Baseline CTP class on admission was assessed in 93 patients: CTP-A (23%), CTP-B (45%) and CTP-C (32%); median MELD was 15 (IQR 10−22) and median MELD-Na score was 19 (IQR 14−25). On admission, 15% patients were enlisted for liver transplantation (n = 14) and some grade of encephalopathy or ascites was present in 51.1% and 42.7% of the patients; respectively (Table 2).
Baseline characteristics of patients with cirrhosis and COVID-19 infection.
| Variable | N = 96 |
|---|---|
| Hemoglobin, mg/dL, mean (±SD) | 11.6 (2.8) |
| WBC/mm3, mean (±SD) | 9141 (6501) |
| Platelets, ×109/L, mean (±SD) | 132.1(94.2) |
| Total bilirubin mg/dL, median (IQR) | 3.5 (0.8−4.5) |
| Albumin, mg/dL, mean (± SD) | 2.8 (0.6) |
| INR, mean (± SD) | 1.6 (0.8) |
| Creatinine, mg/dL median (IQR) | 1.0 (0.7−1.7) |
| Enlisted for liver transplantation, n (%) | 14 (15.0) |
| Encephalopathy on admission, n (%) | |
| None | 45 (48.9) |
| Grade I–II | 31 (33.7) |
| Grade III–IV | 16 (17.4) |
| Ascites on admission, n (%) | |
| None-Mild | 51 (57.3) |
| Moderate | 28 (31.5) |
| Severe | 10 (11.2) |
| Child-Turcotte-Pugh, n (%)a | |
| A | 21 (22.6) |
| B | 42 (45.2) |
| C | 30 (32.2) |
| Child-Turcotte-Pugh, median (IQR) | 8 (7−11) |
| MELD, median (IQR) | 15 (10−22) |
| MELD-Na, median (IQR) | 19 (14−25) |
| Liver decompensation during hospitalization, n (%) * | |
| Ascites (any grade) | 34 (38.2) |
| Variceal hemorrhage | 28 (31.4) |
| Encephalopathy (any grade) | 43 (48.3) |
| CLIF-C ACLF, n (%) | 53 (55.2) |
| Grade I | 6 (11.4) |
| Grade II | 17 (32.0) |
| Grade III | 30 (56.6) |
| NACSELD-ACLF, n (%) | 29 (30.2) |
| 2 organ failures | 17 (58.6) |
| 3 organ failures | 8 (27.6) |
| 4 organ failures | 4 (13.8) |
Abbreviations: CLIF-C, Chronic Liver Failure Consortium; INR, international normatized ratio; MELD, model for end-stage liver disease;NACSELD, North American Consortium for the Study of End-Stage Liver Disease; WBC, white blood cells.
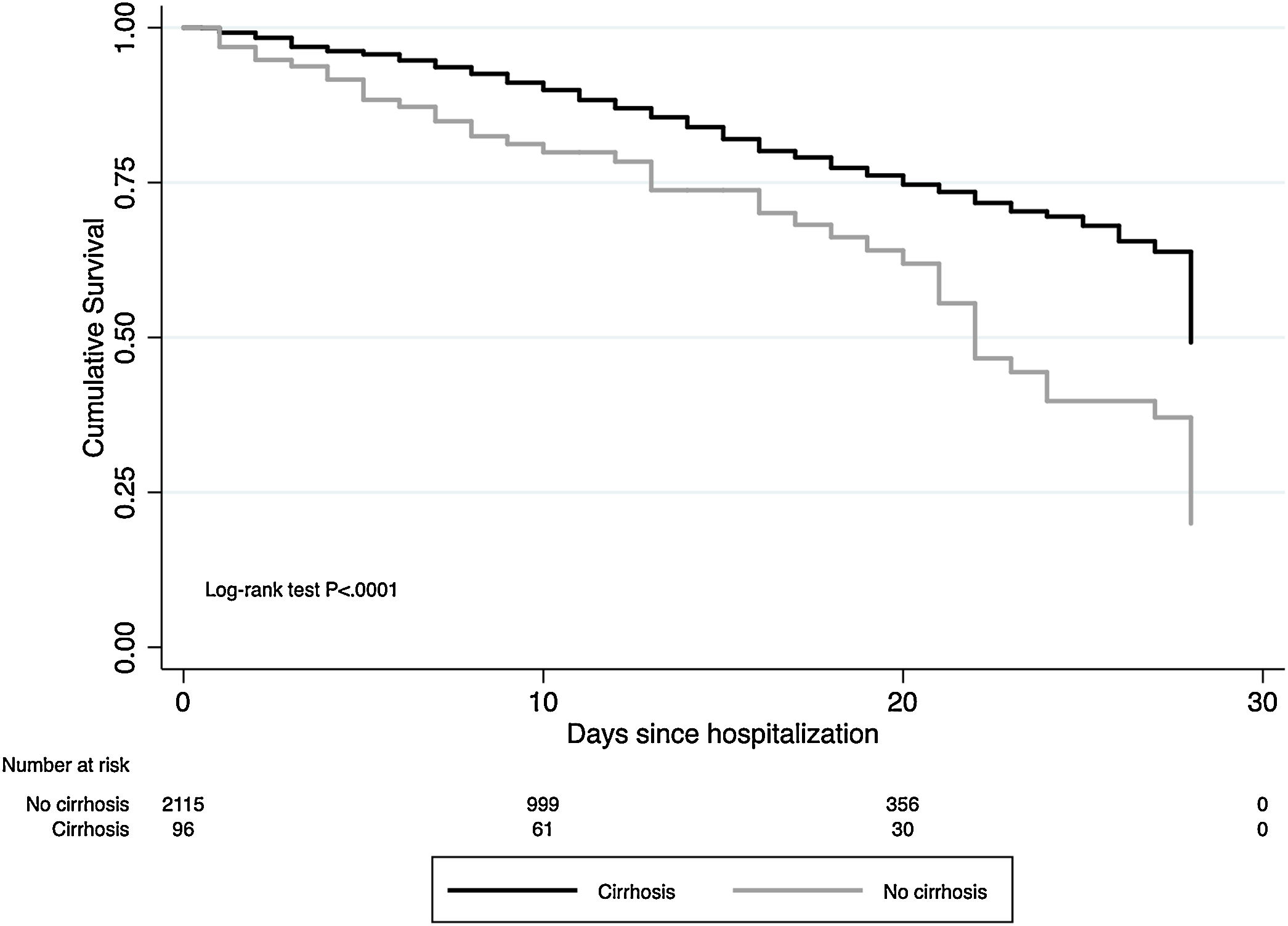
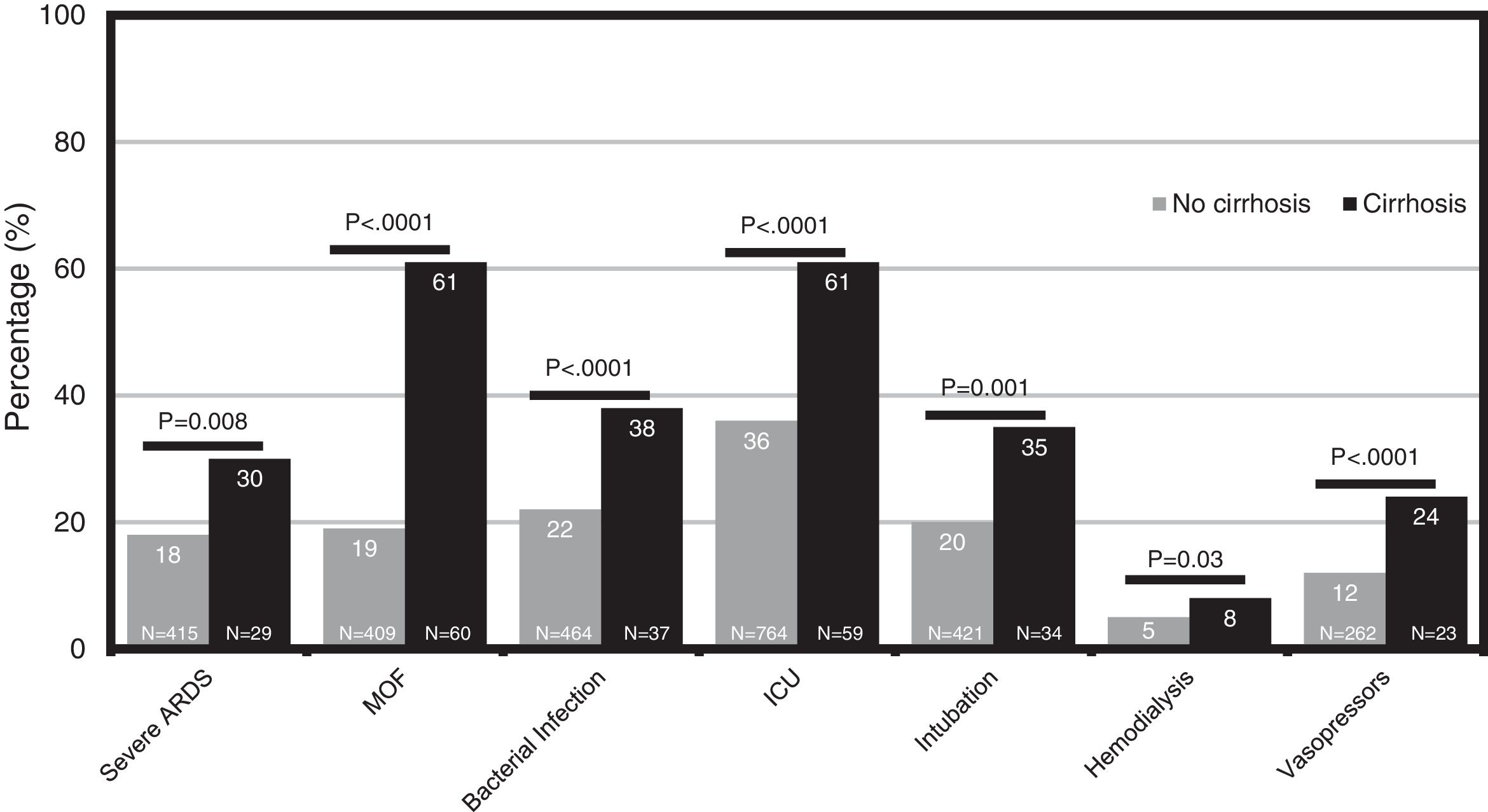
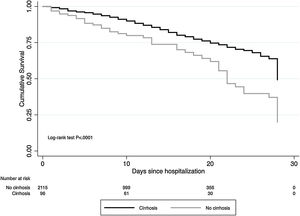
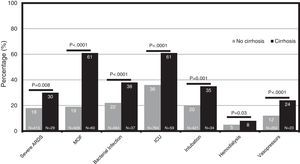
The cumulative mortality rate in the overall cohort was 17.7% (CI 16.1–19.3) after a median time since admission of 12 (IQR 6–20) days (n = 391). Mortality was significantly higher in patients with cirrhosis compared to those without cirrhosis (46.9% vs. 16.4%, P < .0001; respectively) (Fig. 1). From the 46 patients with cirrhosis who died, the cause of death was secondary to COVID-19 lung disease in 36 (78.2%) and liver-related in 10 (21.8%) cases. Of those patients with cirrhosis who died, 4 acquired SARS-CoV-2 infection during hospitalization. Mortality rates in patients with cirrhosis increased according to CTP class; CTP-A (23.8%), CTP-B (40.5%) and CTP-C (66.7%). Patients with cirrhosis required more ICU-level care (P < .0001), developed more frequently severe COVID-19 during hospitalization (60.4% vs. 46.8%, P < .0001), and required more days of hospitalization (13.5 [IQR 7–22] vs. 9 [IQR 5–15], P = 0.0005). Thirty-four patients with cirrhosis received invasive ventilation, 24 (70.6%) for ARDS and 10 (29.4%) for encephalopathy progression. Clinical outcomes are described in Fig. 2.
During hospitalization, antibiotics were more commonly administered to patients with cirrhosis than those without cirrhosis (84.4% vs. 62.7%, P < .0001; respectively). Antiviral therapy with lopinavir/ritonavir was only used in the group of patients without cirrhosis (7.6% vs. 0%; P = 0.001). Overall, the proportion of patients receiving COVID-targeted therapy was significantly higher in those with cirrhosis compared to those without cirrhosis (98.9% vs. 76.6%, P < .0001; respectively). Treatment received by hospitalized patients with COVID-19 are reported in Supplementary Table 2.
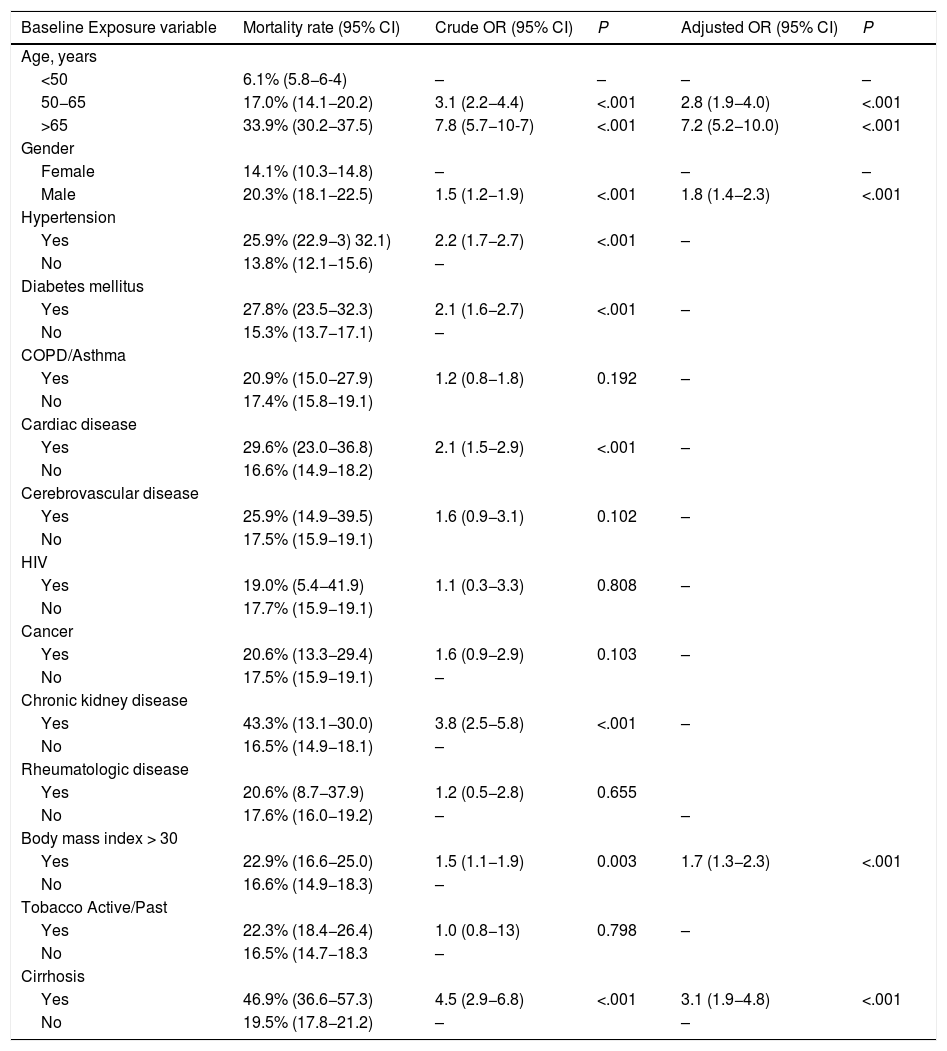
3.3Association of cirrhosis with deathWe evaluated the effect of cirrhosis on the risk of mortality adjusted for other variables previously described to be associated with death. Baseline factors significantly associated with death by univariable analysis were age >65 years (OR 7.8, CI 5.7−10.7; P < .0001), male gender (OR 1.5, CI 1.2−1.9; P = 0.0003), hypertension (OR 2.2, CI 1.7−2.7; P < .0001), diabetes (OR 2.1, CI 1.6−2.7; P < .0001), cardiac disease (OR 2.1; CI 1.5−2.9; P < .0001), chronic kidney disease (OR 3.8; CI 2.5−5.8; P < .0001), BMI > 30 (OR 1.5, CI 1.1−1.9; P < .0001) and cirrhosis (OR 4.5, CI 2.9−6.8; P < .0001) (Table 3). Regarding the etiology of liver disease, we found no association with mortality (data not shown). Multivariable analysis of factors associated with death demonstrated persisting association between age >65 years (OR 7.2, 5.2−10.0; P < .0001), male gender (OR 1.8, CI 1.4−2.3; P < .0001), BMI > 30 (OR 1.7, CI 1.3−2.3, P < .0001 and cirrhosis (OR 3.1, CI 1.9−4.8; P < .0001) (Table 3). The model showed adequate calibration (P = 0.1) with an AUROC of 0.75 (CI 0.73−0.77).
Logistic regression analysis for the primary outcome (death) evaluating prior medical history. Odds Ratios (OR).
| Baseline Exposure variable | Mortality rate (95% CI) | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| Age, years | |||||
| <50 | 6.1% (5.8−6-4) | – | – | – | – |
| 50−65 | 17.0% (14.1−20.2) | 3.1 (2.2−4.4) | <.001 | 2.8 (1.9−4.0) | <.001 |
| >65 | 33.9% (30.2−37.5) | 7.8 (5.7−10-7) | <.001 | 7.2 (5.2−10.0) | <.001 |
| Gender | |||||
| Female | 14.1% (10.3−14.8) | – | – | – | |
| Male | 20.3% (18.1−22.5) | 1.5 (1.2−1.9) | <.001 | 1.8 (1.4−2.3) | <.001 |
| Hypertension | |||||
| Yes | 25.9% (22.9−3) 32.1) | 2.2 (1.7−2.7) | <.001 | – | |
| No | 13.8% (12.1−15.6) | – | |||
| Diabetes mellitus | |||||
| Yes | 27.8% (23.5−32.3) | 2.1 (1.6−2.7) | <.001 | – | |
| No | 15.3% (13.7−17.1) | – | |||
| COPD/Asthma | |||||
| Yes | 20.9% (15.0−27.9) | 1.2 (0.8−1.8) | 0.192 | – | |
| No | 17.4% (15.8−19.1) | ||||
| Cardiac disease | |||||
| Yes | 29.6% (23.0−36.8) | 2.1 (1.5−2.9) | <.001 | – | |
| No | 16.6% (14.9−18.2) | ||||
| Cerebrovascular disease | |||||
| Yes | 25.9% (14.9−39.5) | 1.6 (0.9−3.1) | 0.102 | – | |
| No | 17.5% (15.9−19.1) | ||||
| HIV | |||||
| Yes | 19.0% (5.4−41.9) | 1.1 (0.3−3.3) | 0.808 | – | |
| No | 17.7% (15.9−19.1) | ||||
| Cancer | |||||
| Yes | 20.6% (13.3−29.4) | 1.6 (0.9−2.9) | 0.103 | – | |
| No | 17.5% (15.9−19.1) | – | |||
| Chronic kidney disease | |||||
| Yes | 43.3% (13.1−30.0) | 3.8 (2.5−5.8) | <.001 | – | |
| No | 16.5% (14.9−18.1) | – | |||
| Rheumatologic disease | |||||
| Yes | 20.6% (8.7−37.9) | 1.2 (0.5−2.8) | 0.655 | ||
| No | 17.6% (16.0−19.2) | – | – | ||
| Body mass index > 30 | |||||
| Yes | 22.9% (16.6−25.0) | 1.5 (1.1−1.9) | 0.003 | 1.7 (1.3−2.3) | <.001 |
| No | 16.6% (14.9−18.3) | – | |||
| Tobacco Active/Past | |||||
| Yes | 22.3% (18.4−26.4) | 1.0 (0.8−13) | 0.798 | – | |
| No | 16.5% (14.7−18.3 | – | |||
| Cirrhosis | |||||
| Yes | 46.9% (36.6−57.3) | 4.5 (2.9−6.8) | <.001 | 3.1 (1.9−4.8) | <.001 |
| No | 19.5% (17.8−21.2) | – | – |
Note: Calibration P = 0.1 (Hosmer-Lemeshow) and discrimination for this model was ROC 0.75 (CI 0.73−0.77).
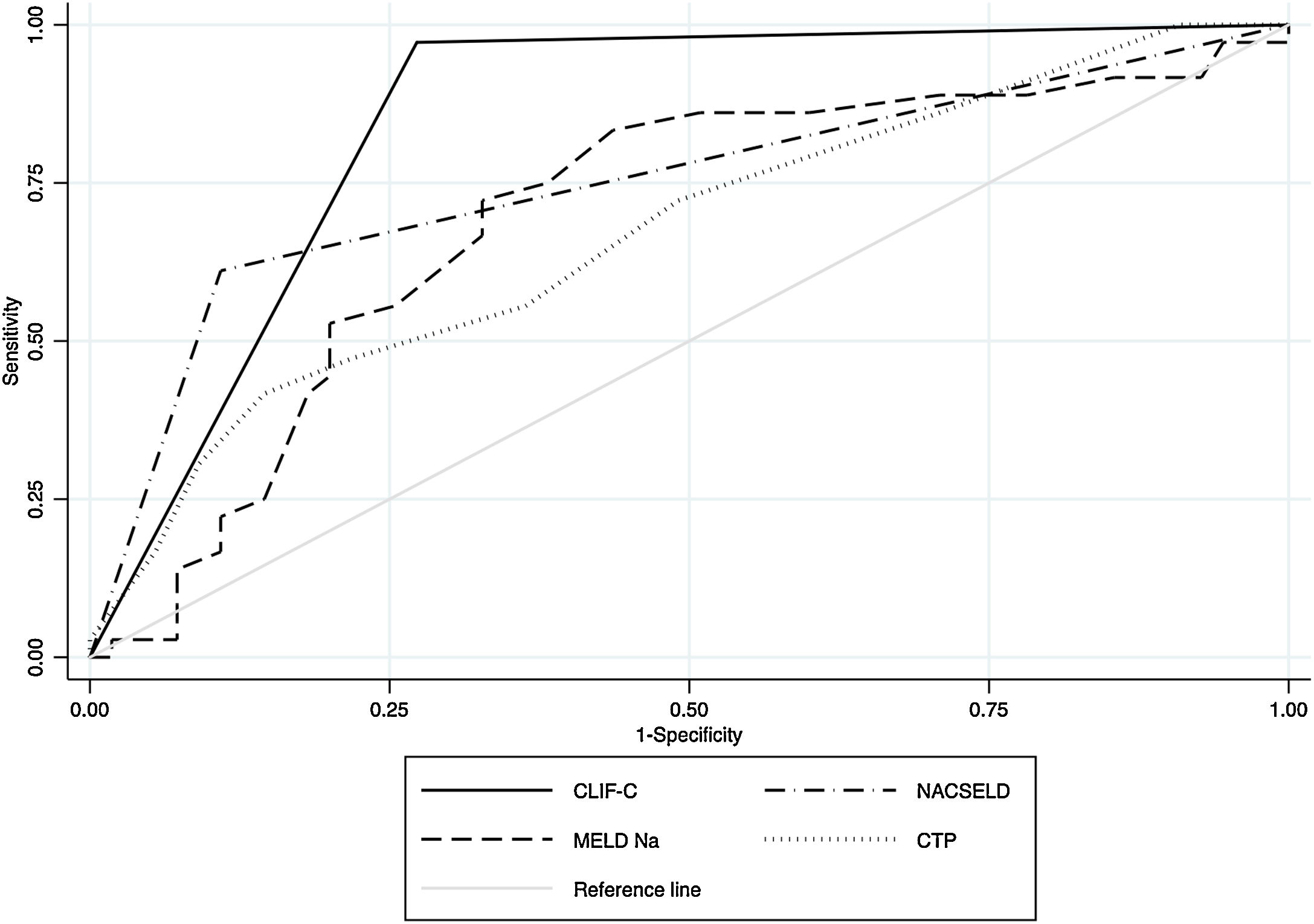
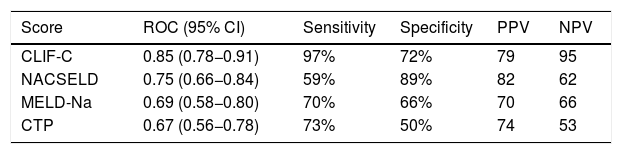
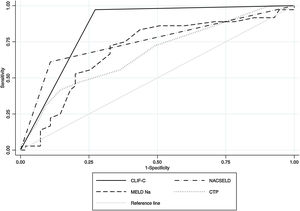
Within the 96 patients with cirrhosis, acute decompensation events or worsening of baseline clinical condition following hospitalization for SARS-CoV-2 infection were reported in 59 (61.4%) cases. Decompensation events included new or worsening encephalopathy 43 (48.3%), ascites 34 (38.2%) and variceal hemorrhage 28 (31.4%). Among the whole cirrhosis cohort, 53 (55.2%) and 29 (30%) patients developed ACLF according to CLIF-C organ failure score and NACSELD, respectively (Supplementary Fig. 1). We used AUROC to assess the ability of scoring systems to predict 28-days mortality in patients with cirrhosis following hospitalization for SARS-CoV-2 infection. The AUROC of CLIF-C organ failure score (AUROC 0.85, CI 0.78−0.91) had better prognostic accuracy than CTP score (AUROC 0.67, CI 0.56−0.78), baseline MELD-Na (AUROC 0.70, CI 0.58−0.80), and NACSELD (AUROC 0.75, CI 0.66−0.84) (Fig. 3). The AUROCs estimated for the CLIF-C organ failure score, CTP score, MELD-Na and NACSELD were compared in pairs and were significantly different for all cases (P = 0.009). Sensitivity, specificity, positive predictive value and negative predictive value of the four different scores regarding 28-days mortality rate are demonstrated in Table 4.
Receiver operating characteristic (ROC) curves and area under the curve (AUC) to determine the score accuracy for CLIF-C, NACSELD, baseline MELD-Na and baseline CTP as predictors of 28-day mortality for patients with SARS-CoV-2 infection and cirrhosis. The AUC were as follows: 0.85 for CLIF-C, 0.75 for NACSELD, 0.69 for MELD-Na and 0.67 for CTP.
Ability of four scoring systems to predict 28-days mortality in patients with cirrhosis following hospitalization for SARS-CoV-2 infection.
| Score | ROC (95% CI) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| CLIF-C | 0.85 (0.78−0.91) | 97% | 72% | 79 | 95 |
| NACSELD | 0.75 (0.66−0.84) | 59% | 89% | 82 | 62 |
| MELD-Na | 0.69 (0.58−0.80) | 70% | 66% | 70 | 66 |
| CTP | 0.67 (0.56−0.78) | 73% | 50% | 74 | 53 |
Abbreviations: CLIF-C, Chronic Liver Failure Consortium; CTP, Child-Turcotte-Pugh; LR, log likelihood ratio; MELD-Na, model for end-stage liver disease; NACSELD, North American Consortium for the Study of End-stage Liver Disease; NPV, negative predictive value; PPV, positive predictive value.
The results from this large Latin American prospective cohort study describe that patients with cirrhosis hospitalized with COVID-19 were more frequently admitted to ICU and had a significantly higher probability of developing severe COVID-19 than those individuals without cirrhosis. Moreover, patients with cirrhosis presented 3-fold increased mortality risk than those with no liver disease. Additionally, a high proportion of patients with cirrhosis developed either acute decompensation or ACLF. When comparing different scores to predict 28-day mortality in a head-to-head manner we found that CLIF-C definition has the better prognostic performance in this population.
Our observed mortality rate was strikingly higher than the rate reported in recently published studies from Europe and North America (30–34%) and similar to a small cohort from India (42%) [7–9]. We can speculate that this might be the consequence of treating patients with advanced liver disease and multi-organ failure in a region with a fragile health care system. Despite this potential limitation, infected patients with cirrhosis have a uniformly worse crude prognosis compared to uninfected patients [3]. Whether SARS-CoV-2 infection outcomes are similar to other acute precipitants, including bacterial infection in patients developing ACLF, remains uncertain. A study from Italy reported significantly higher mortality in patients with cirrhosis and SARS-CoV-2 compared with bacterial infection [7]. Viral infections in patients with cirrhosis have also been associated with high mortality rates. Influenza virus infection has been associated with an 18% mortality rate in patients with cirrhosis [16]. Furthermore, in a study from India, 18 out of 22 patients with H1N1 infection and cirrhosis died of pneumonia and ARDS [4]. These studies underscore the concept that infections are associated with ACLF and excessive systemic inflammation leading to organ dysfunction through direct deleterious effects on microcirculatory homeostasis and mitochondrial function [17]. The accepted strategy for the management of ACLF is treating the precipitating factor whilst providing intensive monitoring and support of failing organs [18]. Specific treatment against SARS-CoV-2 was not standardized in our study. However, we did not find any specific therapy associated with increased survival (data not shown).
Our observed rates of patients with cirrhosis and advanced liver disease (CTP B-C 77%) far exceeded the previously reported by the SECURE-cirrhosis registry (CTP B-C 56%) and by a study from Italy (CTP B-C 62%) [7,9]. We can speculate that this could be the consequence of many factors such as the strict and prolonged quarantine established in many Latin American countries that precluded patients with cirrhosis to be adequately monitored; the scarce availability of beds in the ICU in many cities despite having more time than Europe to condition the health system, and the SARS-CoV-2 infection that led to a rapid clinical deterioration of patients with cirrhosis. Only 33% of hospitalized patients with CTP-C cirrhosis survived, and mortality further increased in those receiving mechanical ventilation. The pandemic represents a challenging scenario for patients with cirrhosis and their treating physicians. We report a strong association between liver disease severity and death after SARS-CoV-2 infection, highlighting the importance of carefully monitoring these patients guided by individual risk, institutional resources and the local burden of COVID-19.
In line with previous studies, we described lung injury as the predominant cause of death in patients with cirrhosis [7,9]. Liver-related complications accounted for only 22% of mortality in patients with cirrhosis from our cohort. Nevertheless, respiratory function had been severely compromised by SARS-CoV-2 since all of them required invasive ventilation. Severe ARDS was significantly more common in patients with cirrhosis compared with those without liver disease. This implicates liver dysfunction as a potential driver of ongoing lung injury. The mechanism for enhanced lung injury in patients with cirrhosis is probably multifactorial. It may include altered pulmonary function through worsening ascites or hepatic hydrothorax, impaired coagulation associated with venous thromboembolic disease and immune dysregulation.
We evaluated the performance of different prognostic scores to determine mortality at 28-days in patients with cirrhosis. In our study, CLIF-C definition of ACLF has the best prognostic performance compared to NACSELD, MELD, and CTP. Moreover, the use of CLIF-C definition led to the diagnosis of a larger number of patients with ACLF. NACSELD presented a poor sensitivity for mortality prediction that can be explained by the restrictive nature of their diagnostic criteria for ACLF. Considering that there is still no consensus on which is the best score to determine 28-day mortality in patients with ACLF, our findings are similar to those reported by a Brazilian prospective study who described that CLIF-C had better performance in predicting mortality when compared to NACSELD criteria [19]. Interestingly, in a North American cohort Bajaj et al. described the Charlson Comorbidity Index to be independently associated with mortality in patients with cirrhosis and COVID-19 [8]. Furthermore, Iavarone et al. have proposed that the combination of CLIF-C and Charlson Comorbidity Index better predict survival in these patients [20]. One of the greatest contributions of these scores is to determine the futility of continued aggressive care in hospitalized patients with cirrhosis [3]. In patients with four organ failures in whom survival is unlikely, escalation of care and use of palliative care should be carefully evaluated. During the COVID-19 pandemic, we have learned that health care expenditures and human resources are valuable and limited supplies.
Our study´s major strength is the inclusion of a large and geographically diverse population, in which data collection and outcome measures have been clearly defined and prospectively collected. Additionally, comparing cases with contemporaneous patients without cirrhosis strengthens the association between liver disease severity and mortality described in patients with cirrhosis. However, we are aware that our study suffers certain limitations. First, few patients have fulfilled ACLF criteria according to NACSELD definition. However, this can be the consequence of a more severe definition of organ failures. Second, some major covariables can be missing in our registry given that we prioritize clinical data and well-known factors associated with poor outcomes. Third, given our study's design, we did not compare COVID-19-associated outcomes in patients with cirrhosis and SARS-CoV-2 infection who did not require hospitalization to those of persons without cirrhosis. Moreover, some patients with undiagnosed cirrhosis could have been incorrectly included in the group of patients with no cirrhosis. Finally, although SARS-CoV-2 infection was diagnosed as per the local site-specific protocol, algorithms followed their local epidemiological situation and available resources.
In summary, in this large multicenter cohort from Latin America, we described that SARS-CoV-2 infection in cirrhosis patients is strongly associated with high rates of acute hepatic decompensation and death. Moreover, CLIF-C definition allowed a greater number of patients to be diagnosed with ACLF and better predicted 28-day mortality compared to NACSELD, CTP, and MELD-Na. Our findings highlight the need to follow the recommended preventive measures in patients with advanced liver disease during COVID-19 pandemic [21].AbbreviationsACLF acute-on-chronic liver failure acute respiratory distress syndrome area under the receiving operator curve Chronic Liver Failure Consortium Coronavirus disease 2019 Child-Turcotte-Pugh intensive care unit model for end-stage liver disease North American Consortium for the Study of End-Stage Liver Disease severe acute respiratory syndrome coronavirus 2
None.
Conflict of interestNone.
AuthorshipContributorship Statement: Dr. Manuel Mendizabal had full access to all of the data in the study and take responsibility for the integrity of data and accuracy of the data analysis.
Concept and design: Drs. Marcelo O. Silva and Manuel Mendizabal.
Acquisition, analysis, or interpretation of data: Drs. Manuel Mendizabal, Ezequiel Ridruejo, Federico Piñero, Margarita Anders, Martín Padilla, Luis G. Toro, Aldo Torre, Pedro Montes, Alvaro Urzúa, Esteban G. Ballerga, María D. Silveyra, Douglas Michelato, Javier Díaz, Mirta Peralta, Josefina Pages, Sandro R. García, Isabel G. Lozano, Yuridia Macias, Daniel Cocozzella, Norberto Chavez-Tapia, Martín Tagle, Alejandra Dominguez, Adriana Varón, Emilia V. Pozo, Fátima Higuera-de la Tijera, Carla Bustios, Damiá Conte, Nataly Escajadillo, Andrés J Gómez, Laura Tenorio, Mauricio Castillo Barradas, Maria Isabel Schinoni, Fernando Bessone, Fernando Contreras, Leyla Nazal, Abel Sanchez, Matías García, Julia Brutti, María Cecilia Cabrera, Godolfino Miranda-Zazueta, German Rojas, Maximo Cattaneo, Graciela Castro-Narro, Fernando Rubinstein and Marcelo O. Silva.
Drafting of the manuscript: Dr. Manuel Mendizabal.
Critical revision of the manuscript for important intellectual content: Drs. Manuel Mendizabal, Ezequiel Ridruejo, Federico Piñero, Margarita Anders, Martín Padilla, Luis G. Toro, Aldo Torre, Pedro Montes, Alvaro Urzúa, Esteban G. Ballerga, María D. Silveyra, Douglas Michelato, Javier Díaz, Mirta Peralta, Josefina Pages, Sandro R. García, Isabel G. Lozano, Yuridia Macias, Daniel Cocozzella, Norberto Chavez-Tapia, Martín Tagle, Alejandra Dominguez, Adriana Varón, Emilia V. Pozo, Fátima Higuera-de la Tijera, Carla Bustios, Damiá Conte, Nataly Escajadillo, Andrés J Gómez, Laura Tenorio, Mauricio Castillo Barradas, Maria Isabel Schinoni, Fernando Bessone, Fernando Contreras, Leyla Nazal, Abel Sanchez, Matías García, Julia Brutti, María Cecilia Cabrera, Godolfino Miranda-Zazueta, German Rojas, Maximo Cattaneo, Graciela Castro-Narro, Fernando Rubinstein and Marcelo O. Silva.
Supervision: Drs. Ridruejo, Rubinstein, Silva.
Final approval of the version to be submitted: Drs. Manuel Mendizabal, Ezequiel Ridruejo, Federico Piñero, Margarita Anders, Martín Padilla, Luis G. Toro, Aldo Torre, Pedro Montes, Alvaro Urzúa, Esteban G. Ballerga, María D. Silveyra, Douglas Michelato, Javier Díaz, Mirta Peralta, Josefina Pages, Sandro R. García, Isabel G. Lozano, Yuridia Macias, Daniel Cocozzella, Norberto Chavez-Tapia, Martín Tagle, Alejandra Dominguez, Adriana Varón, Emilia V. Pozo, Fátima Higuera-de la Tijera, Carla Bustios, Damiá Conte, Nataly Escajadillo, Andrés J Gómez, Laura Tenorio, Mauricio Castillo Barradas, Maria Isabel Schinoni, Fernando Bessone, Fernando Contreras, Leyla Nazal, Abel Sanchez, Matías García, Julia Brutti, María Cecilia Cabrera, Godolfino Miranda-Zazueta, German Rojas, Maximo Cattaneo, Graciela Castro-Narro, Fernando Rubinstein and Marcelo O. Silva.
Statistical analysis: Drs. Manuel Mendizabal, Federico Piñero and Fernando Rubinstein.
We would like to thank ALEH´s executive office for their invaluable help and support on this project, especially to Macarena Muñoz, María Jesús Marcone and Verónica García Huidobro. To Silvina Heisecke from CEMIC-CONICET for the copyediting of this manuscript.
Other authors who collaborated with data acquisition: Jonathan Aguirre-Valadez, Silvana Ocampo, Claudio Toledo, Mauricio Orrego, Victoria Mainardi, Marcos Girala, Beatriz Ameigeiras, Pablo Caballini, Aldana Scarpin, Kelly Stephany Casanova Lau and Jorge José Díaz Rodriguez.