Introduction and aim. Matrix metalloproteinase (MMP)-2 and MMP-9 are reported to participate in several pregnancy-related diseases, including intrahepatic cholestasis of pregnancy (ICP), which is a severe liver disorder in pregnant women. Meanwhile, ample evidences have demonstrated that celastrol inhibits the activity and expression of MMPs. The present study aims to examine the effect of celastrol to alleviate symptoms of ICP in rat model.
Material and methods. By inducing ICP with 17 - ethinylestradiol in pregnant female rats, we assessed the impact of celastrol administration on symptoms of ICP, such as the rate of bile flow, the level of total bile acids (TBA), and the activities of MMP-2 and -9. Furthermore, the correlations between the levels of MMPs with the examined ICP symptoms were investigated.
Results. In rats with ICP, both MMP-2 and -9 exhibited significantly elevated activities, which were inhibited by the administration of celastrol. Furthermore, ICP symptoms such as bile flow rate and total TBA were restored by celastrol. Lastly, there were strong correlations between levels of the two MMPs and TBA.
Conclusion. Our findings described for the first time the effects of celastrol to attenuate ICP symptoms through an inhibition of both MMP-2 and -9, providing evidence for a potential role of celastrol as a new drug for the treatment of ICP.
Intrahepatic cholestasis of pregnancy (ICP), otherwise clinically referred to as obstetric hepatosis, obstetric cholestasis or jaundice during pregnancy, is a serious liver abnormality only observed in pregnant women.1 The primary characteristic of ICP is pruritus that begins at the second or third trimester of pregnancy, but the symptom disappears following delivery.2 About one in ten patients in documented cases exhibited moderate jaundice no later than 4 weeks after beginning of itching.3,4 Additional atypical symptoms that have been reported include abdominal pain, subclinical steatorrhea possibly due to vitamin K deficiency5,6 and encephalopathy.7 It has also been indicated that geographical location and ethnic origin greatly influence the prevalence of ICP.1,8,9 The incidence rates in Chile, Scandinavia and Bolivia are among the highest, up to 15%.2,10 Environmental elements and diets in those areas have been identified as major risk factors that may cause the high ICP prevalence, which in fact has shown a steady decline over the past years.10,11 At the same time, ICP incidence in Asia has gradually climbed up in recent years.12,13 Unfortunately, despite the high risk of preterm labor and unexpected intrauterine fetal death,8 in clinics ICP is still mostly considered a minor disorder, and the treatment for ICP mainly aims to improve fetal outcome as well as ameliorate maternal symptoms.
Currently, the precise etiology for ICP remains unclear. As a result, accurate diagnosis of ICP and the development of pharmacological treatments have been greatly hindered. A number of studies investigating ICP reported that ICP patients exhibited increased flux of total bile acids (TBA) to the fetus from the expecting mother.14-16 High level of maternal TBA greatly affects the production of placental hormones, placental transport and chorionic vessel constriction. Continued elevation of serum TBA is believed to be the most sensitive symptom of ICP in the laboratory. However, it is further noted by several reports that the basal serum level of TBA appears to be variable depending on the population studied.17,18 This finding argues that serum level of TBA alone is not sufficient to serve as a reliable clinical biomarker for diagnosis.19 During tissue remodeling, matrix metalloproteinases (MMPs), enzymes responsible for the degradation of the extracellular matrix, are produced by and secreted from the placental cells. MMPs, in particular MMP-2 and -9, are often implicated in various diseases during pregnancy, including fetal inflammatory response, peripartum cardio-myopathy,20 preterm labor,21 pre-eclampsia,22,23 as well as ICP.24
Celastrol is a natural triterpenoid extracted from the Chinese herb Tripterygium wilfordii Hook.f., which is known to exert various biological effects.25 A growing number of reports have highlighted its potent property in inhibiting MMPs. For instance, it was able to inhibit the migration and invasion of both breast cancer cells and rheumatoid fibroblast-like synoviocytes, through suppressing MMP-9 expression.26-29 Celastrol was also reported to function as an inhibitor of heat shock protein 90P, and suppress the expressions of several MMPs including MMP-9 in primary human osteoarthritic chondrocytes.30 In addition, nanomicelles loaded with celastrol could attenuate cytokine secretion in macrophages and inhibit macrophage-induced corneal neovascularization, likely by inhibiting MMP-9 expression.31
Given the widely reported functions of celastrol in inhibiting MMPs, we aimed in the present study to investigate whether celastrol exerts similar inhibitory effects on MMP-2 and -9 in a rat model of 17 a-ethinylestradiol (EE)-induced ICP. Furthermore, we assessed the potential protective role of celastrol against symptoms of ICP in rats.
Materials and MethodsAnimalsExperiments in the present study were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The animal protocols were approved by the Ethical Committee of Cangzhou Central Hospital. Special attention and efforts were paid to ensure minimum suffering of the animals.
Rat model of 17 -ethinylestradiol (EE)-induced ICPAdult female Sprague-Dawley rats, obtained from the animal facility of Cangzhou Central Hospital, were housed under 40%-60% humidity with a 12:12 light/dark cycle at 22 ± 2¯C. Before experiments, rats were acclimatized for two weeks on regular laboratory chow. Female rats (~ 8 weeks) were mated with males of similar age. After 12 h, females were inspected for solid ivory pessaries, followed by examinations under the microscope to identify the presence of sperms in the vagina. Once pregnancy is determined, female rats (n = 48) were randomly assigned into three groups including sham, ICP and ICP + celastrol group (n = 16 each). At 10 days into pregnancy, rats in the sham group were given 1% methylcellulose (as vehicle) through oral gavage daily for 5 consecutive days. To induce ICP, rats in the ICP group received a daily oral administration of EE (E4876, purity ±98%; Sigma, St. Louis, MO, USA) dissolved in 1% methylcellulose at the dose of 5 mg per kilogram of body weight (bw) for 5 consecutive days, as previously described.32 Rats in the ICP + celastrol group were also treated with EE to establish ICP, followed by an oral administration of celastrol (Sigma, St. Louis, MO, USA) at the dose of 5 mg per kg bw daily for 5 consecutive days, as previously described.33
Bile flow rate analysisAfter various treatments, bile samples were collected while animals were under deep anesthetization. To minimize circadian fluctuations, all procedures were conducted between 9-11 am. An incision near the middle abdomen was made, then the common bile duct was cannulated with a PE-10 polyethylene tubing. Rats were kept warm at 37 ¯C using heating equipment, in order to avoid variations in bile flow caused by hypothermia. Rate of bile flow was calculated by gravimetrical methods as fiL per min per kg bw, and subsequently standardized using the gravity of bile at 1.0 g/mL.
Blood sample analysisBlood samples were drawn via heart puncture before animals were sacrificed, then immediately centrifuged to obtain the serum, which was subsequently stored at -80 ¯C for analysis of the biochemical parameters. Measurements of enzymatic activities, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and y-glutamyltranspeptidase (GGT), were performed according to established protocols. After using Sep-Pak to extract the solid-phase, TBA was quantified as previously described with an enzymatic-fluorimetric method.34 Finally, serum levels of MMP-2 and -9 were determined by enzyme-linked immunosorbent assay (ELISA) kits specific for rat MMP-2 and -9 (USCN Life Science, Wuhan, China).
Quantitative real time polymerase chain reaction (RT-PCR)Animals were euthanized with cervical dislocation, then liver tissues were collected and quickly transferred into liquid nitrogen. Extraction of total ribonucleic acid (RNA) from the liver homogenate was carried out using the RNeasy MiniPrep Kit (Qiagen, Valencia, CA, USA), followed by reverse-transcription using Superscript II First-Strand Synthesis kit (Life Technologies, Pleasanton, CA, USA) according to the manufacturer's instructions. Relative levels of gene expression were quantified as 2-Ct with GAPDH as the internal control. Primer sequences used are as follows: MMP-2: forward 5'-CCC ATA CTT TAC TCG GAC CA-3', reverse 5'-TGA CCT TGA CCA GAA CAC CA-3'; MMP-9: forward 5'-CCA CCG AGC TAT CCA CTC AT-3', reverse 5'-GTC CGG TTT CAG CAT GTT TT-3'; GAPDH: forward 5'-CAG TGC CAG CCT CGT CTC AT-3', reverse 5'-AGG GGC CAT CCA CAG TCT TC-3'.
Western blotPurification of the proteins from the liver tissues was performed with the radioimmunoprecipitation assay lysis buffer, then added with 15 fiL of 2X sample buffer (BioRad, Hercules, CA, USA) that had been boiled for 10 min before electrophoresis. Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis, then transferred to nitrocellulose membrane (BioRad, Hercules, CA, USA), followed by incubation with antibodies specific for MMP-2, MMP-9 and GAPDH (Ab-cam, Cambridge, MA, USA). Blots were finally visualized using the ECL kit (Millipore, Billerica, MA, USA).
StatisticsValues are presented as mean ± standard deviation (SD). Differences between groups were determined by two-tailed Student’s t test. Pearson's correlation coefficient test was conducted to determine the correlation between parameters for ICP symptoms and levels of MMPs. A p value less than 0.05 is considered statistically significant.
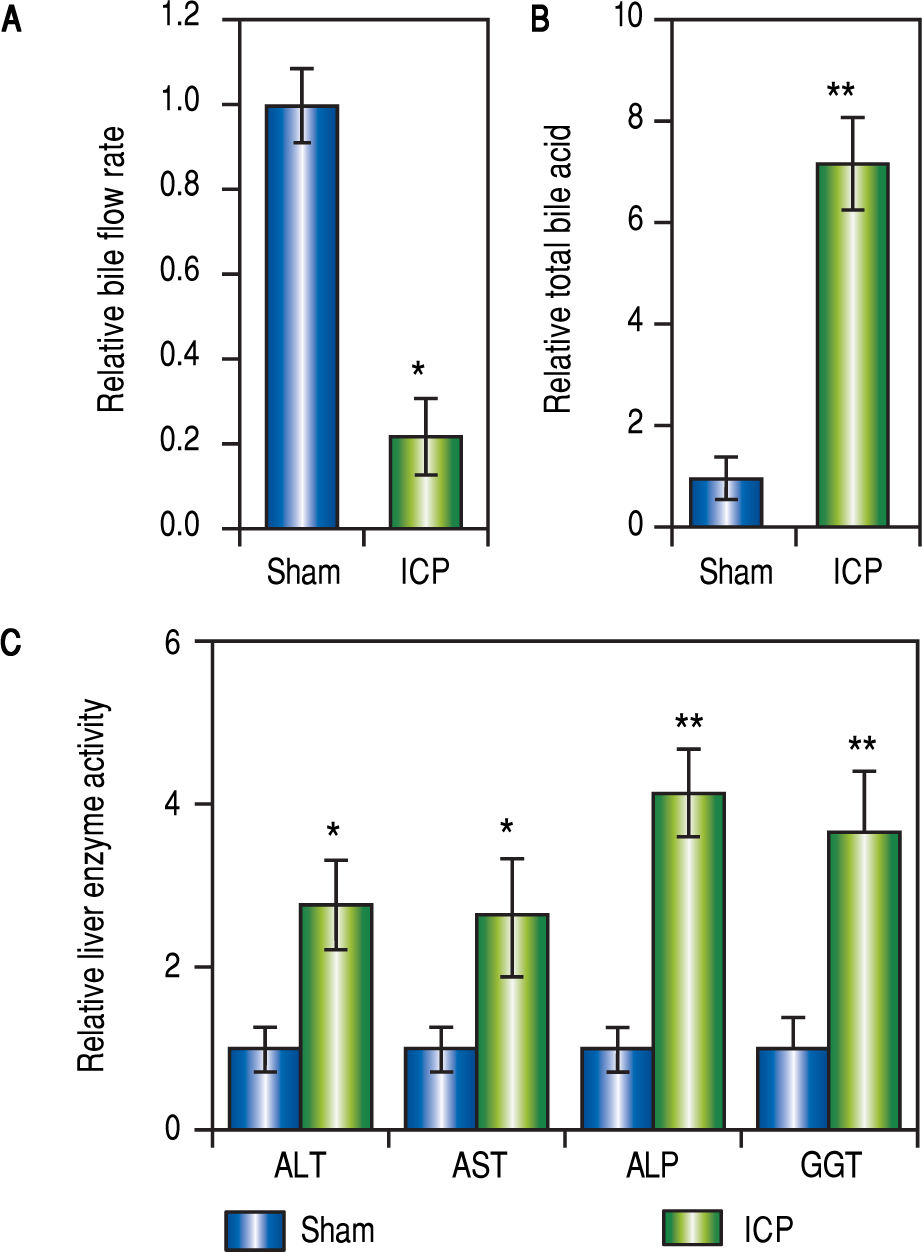
ResultsEstablishing ICP rat model by 17a-ethinylestradiol (EE) inductionIt has been previously reported that EE could induce cholestasis in rats,35,36 which provided a suitable rat model of ICP for the current study. Female rats that have been pregnant for 10 days were given EE at a dose of 5 mg per kg bw daily through oral gavage for 5 consecutive days (ICP group, n = 16). Meanwhile, rats in the sham group (n = 16) with comparable pregnancy stage received 1% methylcellulose (as vehicle) for 5 consecutive days through oral gavage.
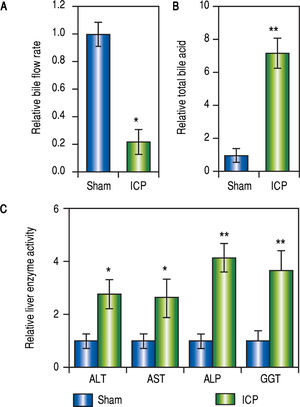
Following the treatment with EE or vehicle, the rates of bile flow in each group were measured to reveal the first signs of ICP. For rats of the ICP group, the bile flow rate was significantly lower than that of the sham rats (Figure 1A). Furthermore, since the elevation of serum TBA concentration is a most sensitive diagnostic trait of ICP clinically, it was important to compare the levels of serum TBA from both treatment groups. Indeed, treatment with EE caused a marked increase of serum TBA levels in the ICP group in comparison to the sham group (Figure 1B).
Establishing ICP rat model by 17 a-ethinylestradiol (EE) induction. (A and B) Relative levels of bile flow rate (A) and total bile acids (B) in sham and ICP groups of rats (n = 16 each), (C) Enzymatic activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and y-glutamyltranspeptidase (GGT) in sham and ICP groups of rats (n = 16 each). Values were mean ± SD, ** p < 0.01, * p < 005, between sham and ICP,
Besides serum TBA, elevated activities of ALT, GGT, AST and ALP were characteristics of ICP as well. Therefore, we also examined these parameters to confirm that ICP rats exhibit similar clinical symptoms. As expected, the serum activities of AST, GGT, ALT and ALP were all significantly and consistently upregulated in ICP rats (Figure 1C). These results demonstrated that EE elicited ICP in rats with symptoms closely resembling those described in human ICP patients.
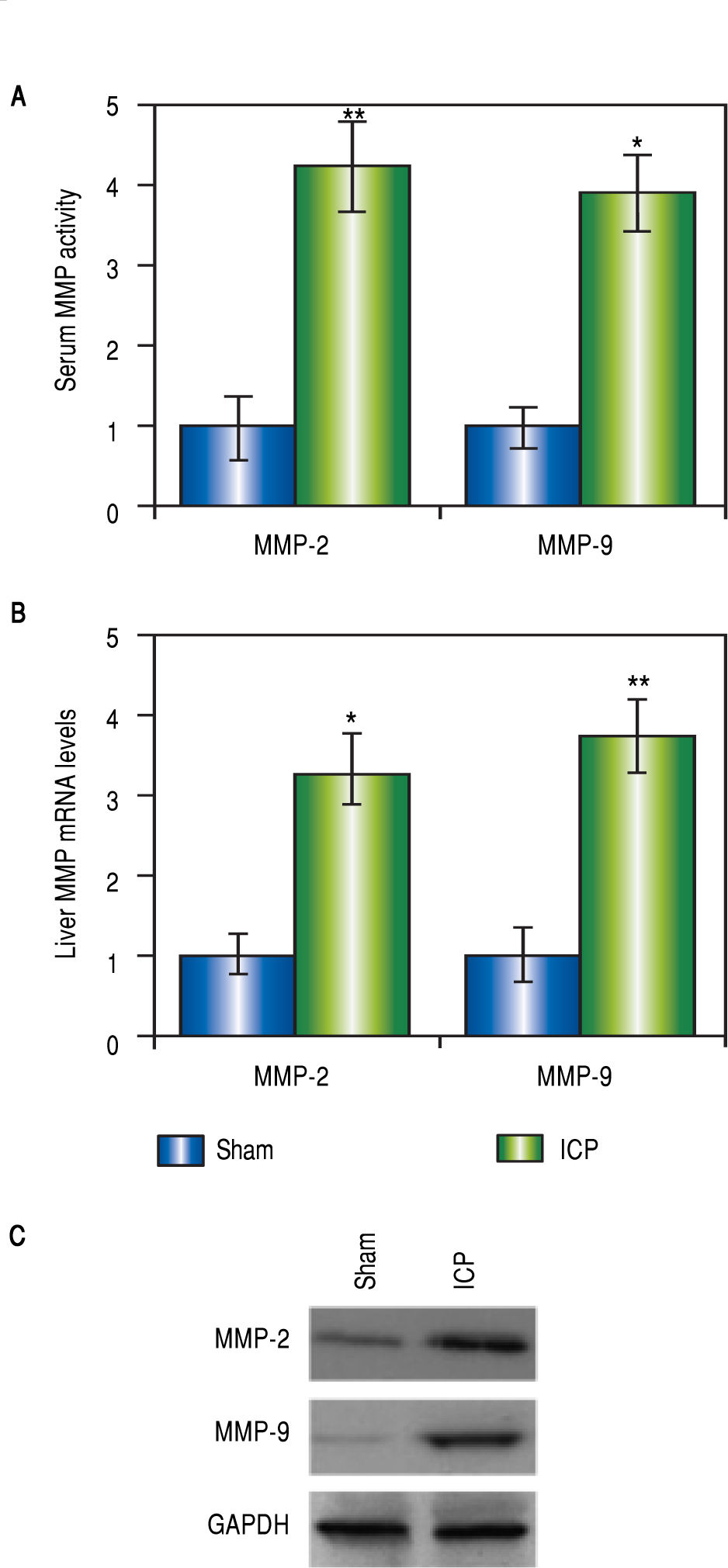
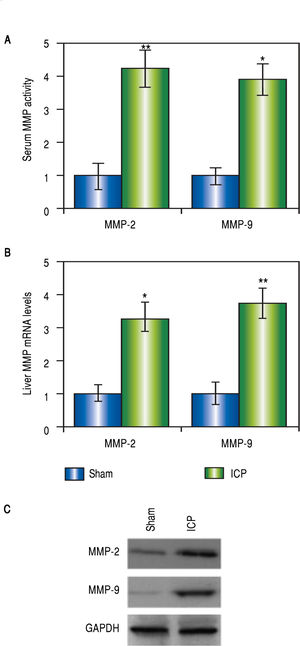
Both MMP-2 and -9 were elevated in rats with ICPMMP-2 and -9 are both implicated in pregnancy-related disorders, however, there is no evidence for a direct connection between MMPs and ICP yet. In our current study, we began with assessing these two MMP levels in the ICP rats. Not surprisingly, there were dramatic elevations of the serum levels of both MMPs in the ICP group but not the sham group (Figure 2A). To confirm the results, liver tissues of both sham and ICP rats were harvested and prepared for Western blot analysis and RT-PCR to determine the expressions of MMPs at both protein and messenger RNA (mRNA) levels. Agreeing with our findings from the serum levels, we observed significantly higher mRNA levels of both MMP-2 and -9 in the livers of ICP rats than the sham rats (Figure 2B). A similar trend was found in the protein levels of both MMPs (Figure 2C). These results provided direct evidences that both MMP-2 and -9 were involved in ICP.
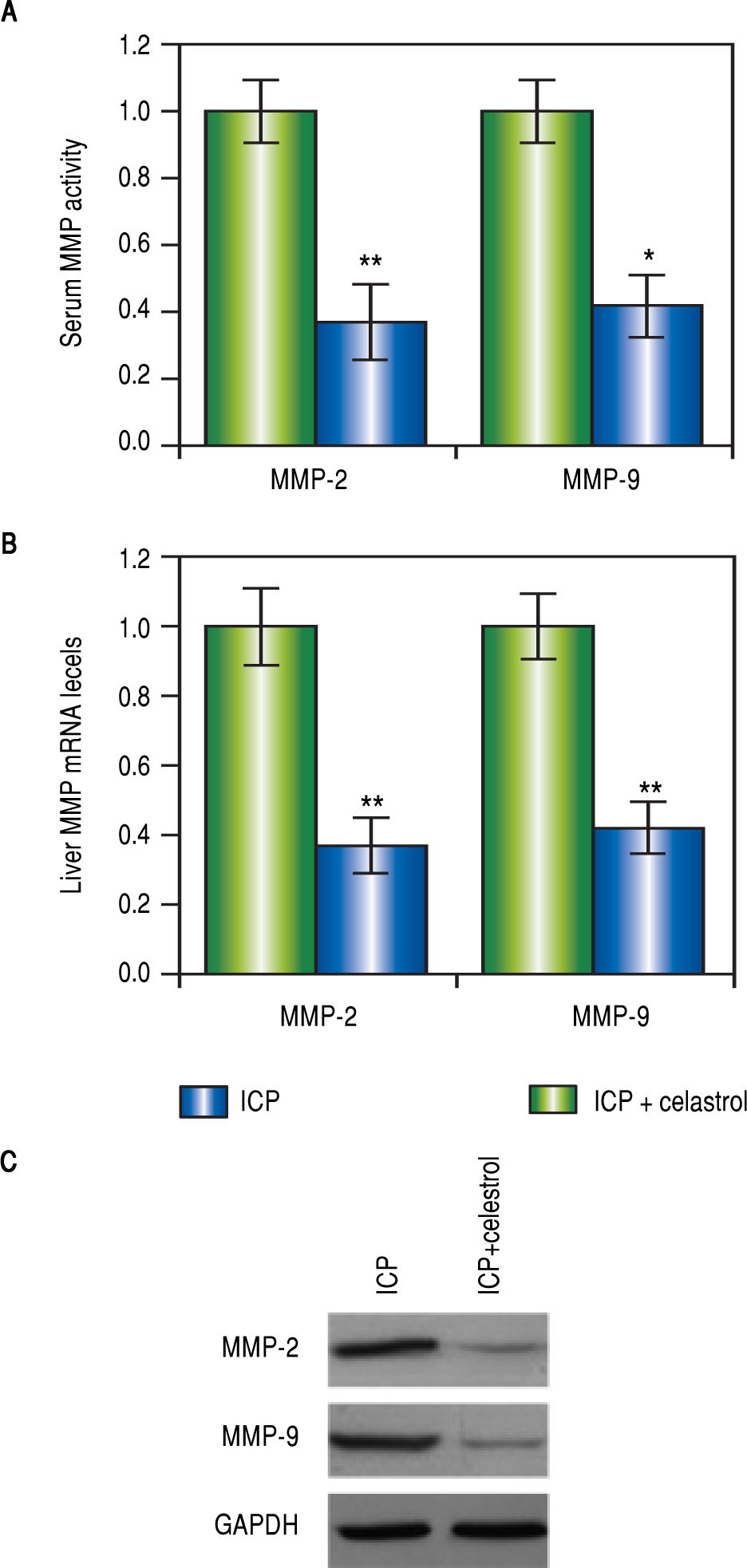
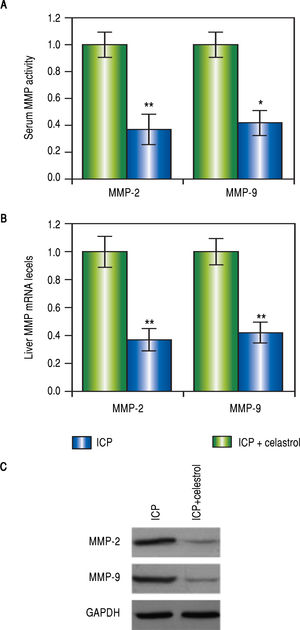
Celastrol reduced levels of MMP-2 and -9 in rats with ICPVarious studies have demonstrated that celastrol functions to repress MMP-9 activity, we therefore speculated that celastrol might exert similar functions in the EE-induced ICP rat model. After the treatment with EE, rats in the ICP + celastrol group (n = 16) received additional administration of celastrol at 5 mg per kg bw, and rats in the ICP group (n = 16) served as control. At the end of the celastrol treatment, the levels of MMP-2 and -9 in the serum and liver tissues from both groups were measured. As expected, activities of both MMPs in the serum were greatly lowered after celastrol treatment, as compared to the ICP group (Figure 3A). Also, both mRNA and protein levels of MMP-2 and -9 in the liver were significantly lower in the ICP + celastrol rats than the ICP group (Figure 3BFigure 3C). These findings suggested that celastrol treatment in ICP rats reduced serum activities and liver expressions of both MMPs.
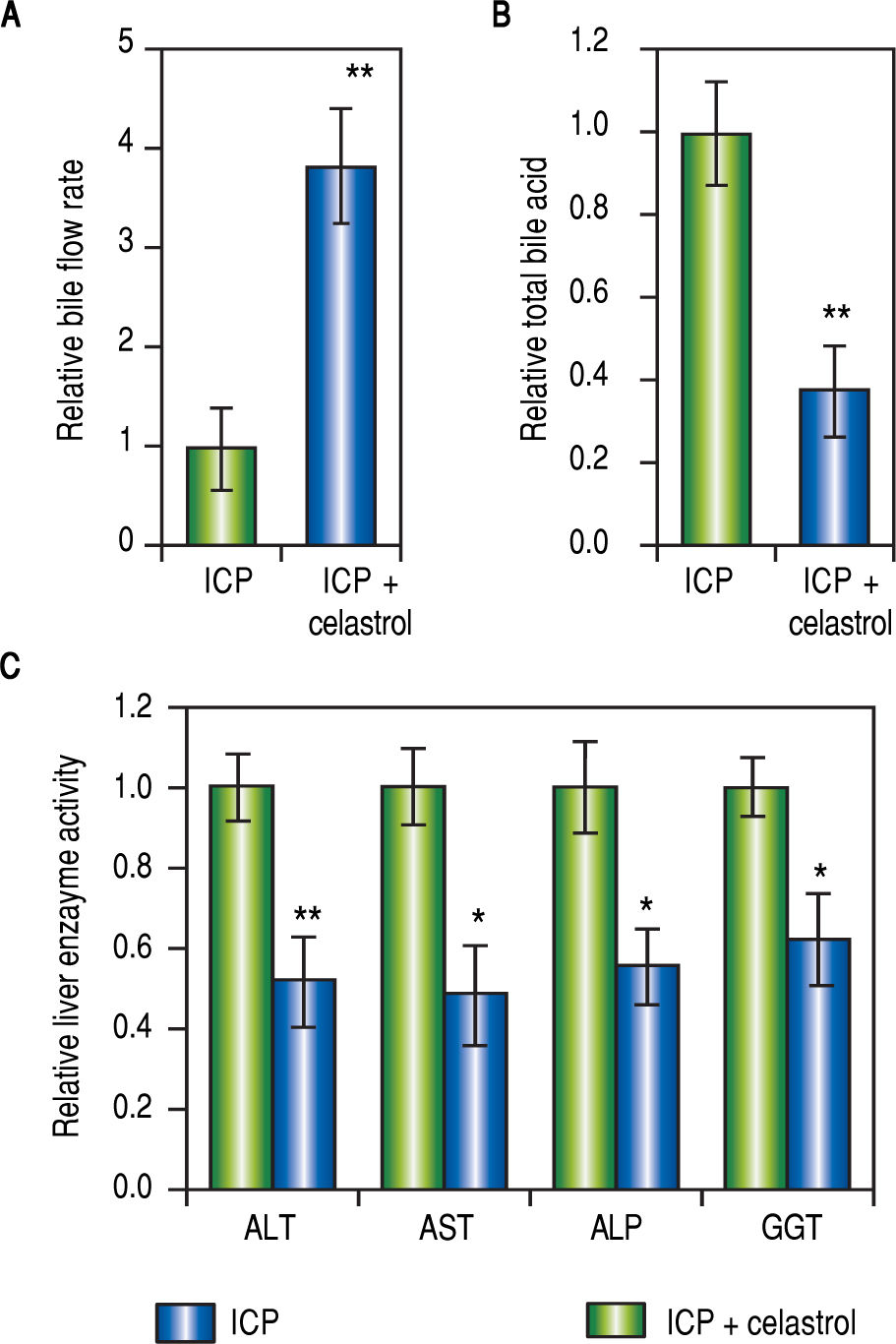
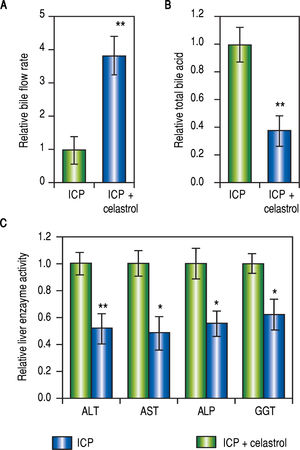
Celastrol ameliorated symptoms in ICP ratsSince both MMPs were up-regulated in ICP rats and inhibited by celastrol treatment (Figure 2 and Figure 3), we next evaluated the effect of celastrol in alleviating ICP symptoms, including serum TBA levels, rate of bile flow and activities of GGT, ALT, ALP and AST. First, celastrol treatment effectively reversed bile flow rates of ICP + celastrol rats compared to the ICP group, as shown in Figure 4A. Second, celastrol treatment dramatically reduced serum levels of TBA in the ICP + celastrol group compared with the ICP rats (Figure 4B). Furthermore, liver enzyme activities of GGT, ALT, ALP and AST were all markedly down-regulated in ICP + celastrol group compared to the ICP group (Figure 4C). Our data clearly presented evidences for the potential role of celastrol as a novel therapeutic tool for the treatment of ICP.
Celastrol ameliorated symptoms in ICP rats, (A and B) Relative levels of bile flow rate (A) and total bile acids (B) in ICP and ICP + celastrol groups of rats (n = 20 each), (C) Enzymatic activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and y-glutamyltranspeptidase (GGT) in ICP and ICP + celastrol groups of rats (n = 16 each). Values were mean ± SD, **p < 0.01, * p <0.05, between ICP and ICP + celastrol.
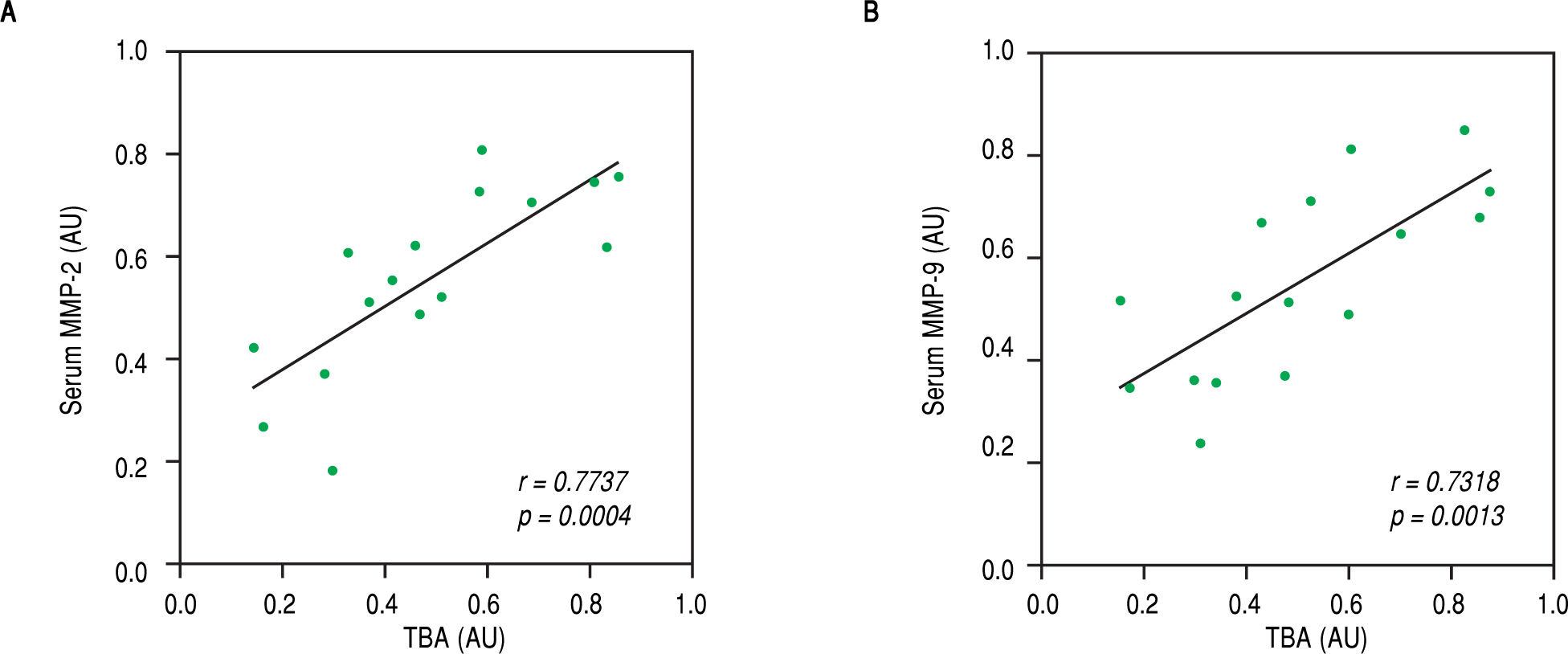
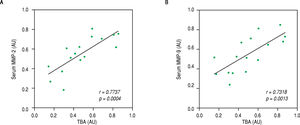
Finally, we speculated that the above observed amelioration in ICP symptoms was attributed to the actions of celastrol in repressing MMP-2 and -9. To evaluate this possibility, we performed Pearson's correlation coefficient test between serum levels of both MMPs with parameters for ICP symptoms in the rats of the ICP + celastrol group. Indeed, strong linear correlations were found between serum TBA levels and levels of both MMP-2 and MMP-9 (Figure 5A and Figure 5B, respectively), which suggested that the inhibitory effect of celastrol on MMPs contributed to the amelioration of ICP symptoms in the ICP rat model.
Levels of TBA were strongly correlated with serum levels of MMP-2 and MMP-9 in ICP rats receiving celastrol treatment. Pearson’s correlation coefficient test was performed between TBA and serum levels of MMP-2 (A) and MMP-9 (B), respectively, in ICP + celastrol group of rats (n = 16). Values were in arbitrary units (AU).
ICP has posed prominent clinical challenge around the globe given its high risk of preterm labor and unexpected intrauterine fetal death.8 There has been substantial progress in ICP diagnosis, with up-regulated serum concentration of TBA being the most sensitive factor. However, serum TBA level alone is not sufficient to diagnose ICP, for it shows variations depending on the population examined.17,18 Besides the lack of a definite factor for ICP diagnosis, limited knowledge on the pathogenesis of the disease further hinders the development effective treatments, worsened by the lack of appropriate animal models. EE is an agent commonly used to induce cholestasis in rats,35,36 but this model displays limited resemblance to symptoms observed in human patients, therefore making conclusions generated from studies using such models unreliable. We speculate that the reason for this discrepancy is the pregnancy stage at the time of EE administration. In human patients, ICP often manifests near the late second or at the beginning of the third trimester.2 Therefore in our current study, we treated rats at a comparable pregnancy stage, where EE was given at 10 days into pregnancy, corresponding to the beginning of the third trimester of pregnancy in human. The consistency for the time of EE administration elicited symptoms that highly resembled human ICP patients,37 as evidenced by the reduction in bile flow rate, elevated activities of liver enzymes such as GGT, AST, ALP, and ALT, as well as serum levels of TBA.
Our results indicated that MMP-2 and -9, both involved in several pregnancy-related disorders, were greatly up-regulated in the serum as well as liver of rats with ICP. It has been previously shown that reduced maternal MMP-2 levels in the serum is linked to fetal inflammation and preterm labor21, while increased MMP-2 serum levels are commonly observed in peripartum cardiomyopathy.20 Furthermore, changes in synthesis of MMP-2 and - 9 were associated with pre-eclampsia.22,23 It was also reported that during defective placental development, the secretions of MMP-2 and -9 were markedly suppressed, causing intrauterine growth restriction.38 Moreover, the abnormal increase in activities of MMP-2 and -9 on the amnion of fetal membrane was shown to contribute to premature membrane rupture.39During pregnancy, levels of MMP-2 and -9 in the serum are tightly regulated, perturbations of which often leads to various maternal and fetal disorders. In the present study, MMP-2 and -9 were found to be up-regulated, suggesting that both MMPs are indeed involved in ICP, which necessitates further investigations into their functions in the disease.
Besides ICP, cholestasis is reportedly the major syndrome of hepatotoxicity, in particular those induced by ingestion of harmful herbal products.40,41 MMPs are also thought to contribute to hepatotoxicity, supported by reports demonstrating the protective effects against hepato-toxicity exerted through down-regulation of MMP-9.42 On a related note, celastrol has been shown to inhibit MMP-9 activity by several independent reports. For instance, celastrol was shown to inhibit MMP-9 expression in both breast cancer cells and rheumatoid fibroblast-like synoviocytes to suppress their migration and invasion.26-29
Given these accumulating evidences supporting the effect of celastrol to inhibit MMPs, we aimed to examine whether celastrol functioned in a similar fashion in our rat ICP model, to suppress both MMPs and thereby ameliorate ICP symptoms. Indeed, treatment with celastrol significantly down-regulated MMP-2 and -9 in the liver as well as in the serum of the ICP rats. It is worth noting that MMP-2 and -9 were markedly reduced at both mRNA and protein levels by celastrol treatment, suggesting that the suppression of MMPs by celastrol could occur as early as the transcriptional stage. Future investigations are needed to reveal the transcriptional mechanisms underlying the inhibitory effects of celastrol on MMP-2 and MMP-9, as well as to identify potential targets of celastrol other than these two MMPs. Furthermore, our findings strongly support that the protective functions of celastrol against ICP symptoms are likely mediated through its inhibition on MMP-2 and -9, because we found strong correlations between the serum TBA levels and the levels of both MMPs. However, cautions should be taken when applying discoveries in our animal study to clinical settings, since ICP symptoms in patients bare differences with those of animal model.
ConclusionsIn summary, we first established a rat model of ICP induced by EE, with symptoms highly resembling those observed clinically in pregnant women with ICP. Next, using this rat model, we showed that activities and expressions of MMP-2 and -9 were markedly up-regulated in ICP rats. Furthermore, we discovered that the symptoms in the pregnant rats with ICP were significantly ameliorated by celastrol, likely through the inhibition of MMP-2 and -9. Altogether, our findings in the present study have demonstrated for the first time the protective roles of celastrol in alleviating ICP symptoms, providing supports for celastrol as a potential therapeutic tool in treating ICP. Nevertheless, to translate the effects of celastrol from animal models to clinical settings, further investigations are required to confirm the efficacy as well as safety of celastrol.
Abbreviations- •
ICP: Intrahepatic cholestasis of pregnancy.
- •
MMP: Matrix metalloproteinase.
- •
TBA: Total bile acids.
None.
Authors’ ContributionsJunjun Guo, Yong Wang, Na Wang, Yulai Bai: data collection and analysis; Dandan Shi: study design and manuscript writing. All authors read and approved the final submission.
AcknowledgmentsNone.
Conflict of InterestsThe authors declares that there is no conflict of interest regarding the publication of this article.