Liver function tests (LFT) abnormalities are reported in up to 50% of COVID-19 patients, and metabolic comorbidities are associated with poorer outcomes. The aim of the study was to determine the prevalence of liver steatosis and fibrosis in patients with COVID-19 and their association with clinical outcomes.
Material and methodsRetrospective study in hospitalized COVID-19 patients was conducted. The risk for liver steatosis was estimated by HSI > 36, and risk for advanced liver fibrosis with APRI > 1.0, NAFLD FS > 0.675 and/or FIB-4 > 3.25. Clinical outcomes were admission to Intensive Care Unit (ICU) and mortality.
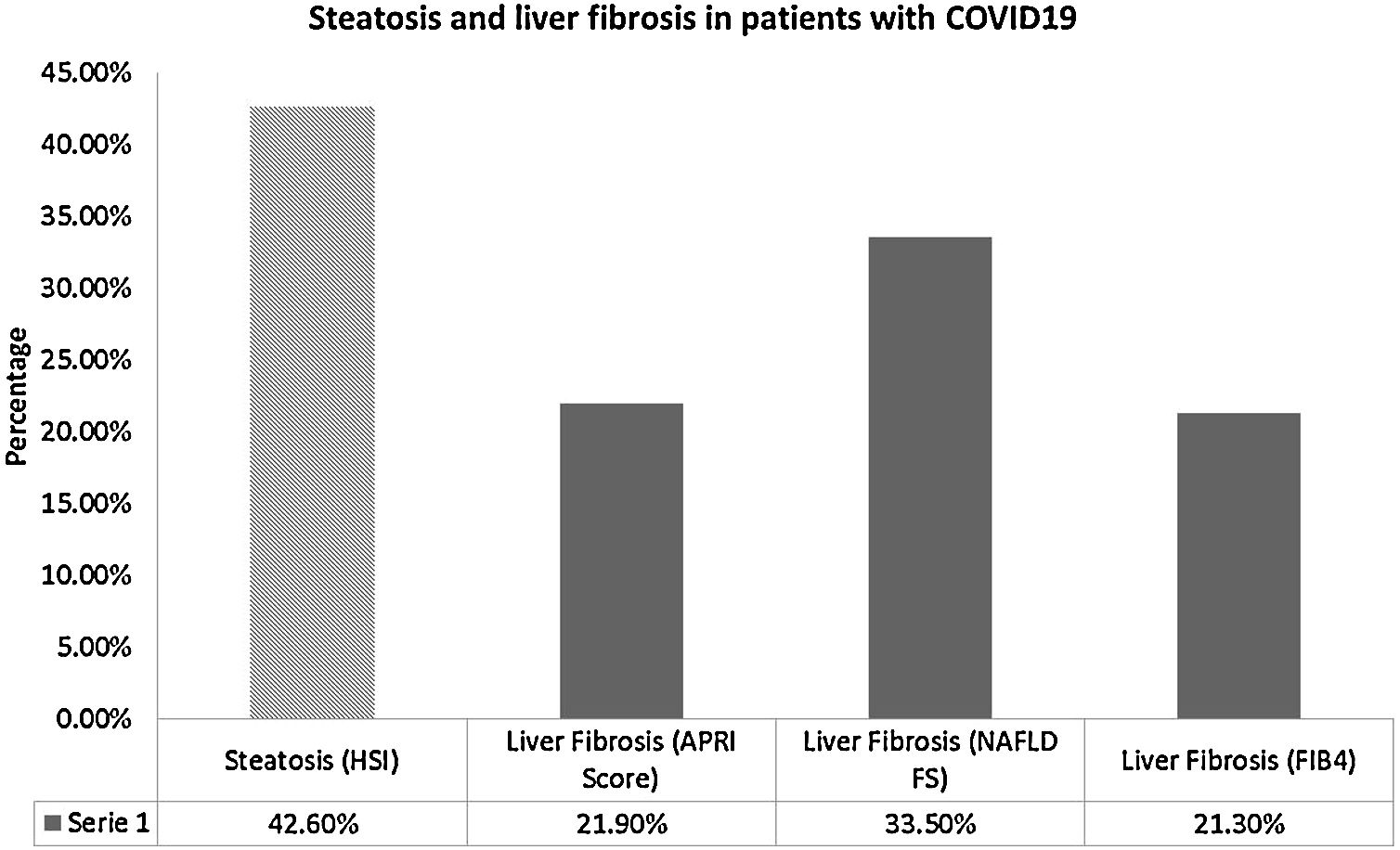
ResultsOf 155 patients, 71.6% were male (n = 111), and 28.4% (n = 44) were obese. Abnormal LFT were present in 96.8% (n = 150), prevalence of steatosis was 42.6% (n = 66) and of significative liver fibrosis was 44.5% (n = 69). Liver fibrosis by FIB-4 was associated with risk of ICU admission (OR 1.74 [95%CI 1.74–2.68; p = 0.023]) and mortality (OR 6.45 [95%CI 2.01−20.83, p = 0.002]); no independent associations were found.
ConclusionsThe prevalence of steatosis and significant liver fibrosis was high in COVID-19 patients but was not associated with clinical outcomes.
The novel Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) virus emerged in Wuhan, China, in December 2019; it is a highly transmittable pathogen that causes the coronavirus disease-19 (COVID-19). The two previous betacoronavirus epidemics of global concern where the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) in 2003 and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) in 2012. These had mortality rates ranging between 10% for SARS-CoV and 37% for MERS-CoV. Up to 10,000 cumulative cases were reported [1].
COVID-19 patients typically report respiratory or nonspecific symptoms, as well as gastrointestinal (GI) symptoms including diarrhea, nausea, vomit, anorexia, and abdominal pain, with a reported prevalence between 11.3% and 50.7% [2]. The prevalence of abnormal liver function tests (LFT) in COVID-19 patients has been reported as high as 76.3%, and an association with higher odds of progressing to severe disease was found with hepatocellular (OR 2.73 [CI95% 1.19–6.3]) or mixed type of liver injury (OR 4.44 [CI95% 1.93–10.23]) [3].
The Centers for Disease Control and Prevention (CDC) revised and updated a summary of medical conditions associated with increased risk of severe illness derived from COVID-19, which included among others, obesity (body mass index (BMI) >30 kg/m2) and Type 2 Diabetes Mellitus (T2DM). In Mexico, 72.5% of the adult population is overweight and 9.4% have T2DM [4]. Additionally, the prevalence of hepatic steatosis in Mexico ranges from 14.4% to 62.9% [5], and the prevalence of liver fibrosis has been reported in 8.1% (non-invasive assessment) [6]. Currently, Mexico City is one of the most affected regions in the world, with rising numbers of cases and deaths caused by COVID-19, and we have very few data regarding GI symptoms and LFT abnormalities and their prognostic value in Mexican patients.
2Patients and methods2.1Study design and data collectionWe conducted a retrospective, cross sectional, descriptive study using electronic medical records of adult patients (>18 years old) with a positive RT-PCR SARS-CoV-2 test (GeneFinder™ COVID-19 PLUS RealAmp Kit, OSANG Healthcare Co., Ltd. Korea) in nasopharyngeal swab, admitted to Medica Sur Clinic & Foundation between March 14th through June 5th, 2020. Patients with COVID-19 diagnosis but without LFT determination in the first 48 h after admission were excluded. Clinical and biochemical data were included in the analysis. The study was approved by the Medica Sur Ethics Committee (2020-EXT-487).
2.2Steatosis and liver fibrosis evaluationThe presence of steatosis was determined by Hepatic Steatosis Index (HSI) by the formula 8*Alanine Aminotransferase (ALT) / Aspartate Aminotransferase (AST) + BMI (+2 if T2DM, +2 if female), which detects Metabolic Associated Fatty Liver Diseases (MAFLD) with a value above 36, and a specificity of 92.4% [7], whereas liver fibrosis was assessed by three different predictive scores for advanced fibrosis: AST to Platelet Ratio Index (APRI), NAFLD Fibrosis Score and Fibrosis-4 index (FIB4). The APRI was calculated by the formula (AST [IU/L]) / (AST Upper Limit of Normal [IU/L]) / (Platelets [×109/L]) in which a >1 score has a sensitivity of 61% for advanced fibrosis [8]. The NAFLD Fibrosis Score (NAFLDFS) was calculated by the formula −1.675 + (0.037*age) + (0.094*BMI [kg/m2]) + (1.13*impaired fasting glucose/diabetes [yes = 1, no = 0]) + (0.99*AST/ALT) – (0.013*platelets [×109/L]) – (0.66*albumin [g/dl]) in which a >0.675 score has a positive predictive value of 90% for fibrosis F3–F4 [9]. Finally, the FIB-4 Index was calculated by the formula (Age*AST [IU/L]) / (Platelets [×109/L] * sqrt(ALT [IU/L])) in which a >3.25 score has a positive predictive value of 65% and a specificity of 97% for Ishak’s fibrosis F4–F6 [10].
2.3Statistical analysisThe distribution of data was assessed by the Kolmogorov-Smirnov test. Continuous data were presented as median and interquartile range; categorical data were presented as percentages and frequencies. The Mann-Whitney U test was used to evaluate differences between patients with and without steatosis, as well as liver fibrosis. The association of categorical data and clinical outcomes was analyzed by Chi square. Univariate and multivariate analyses were performed for associations between steatosis and liver fibrosis with Intensive Care Unit (ICU) admission and mortality. The p-values <0.05 were considered statistically significant. SPSS v.21 was used for statistical analysis.
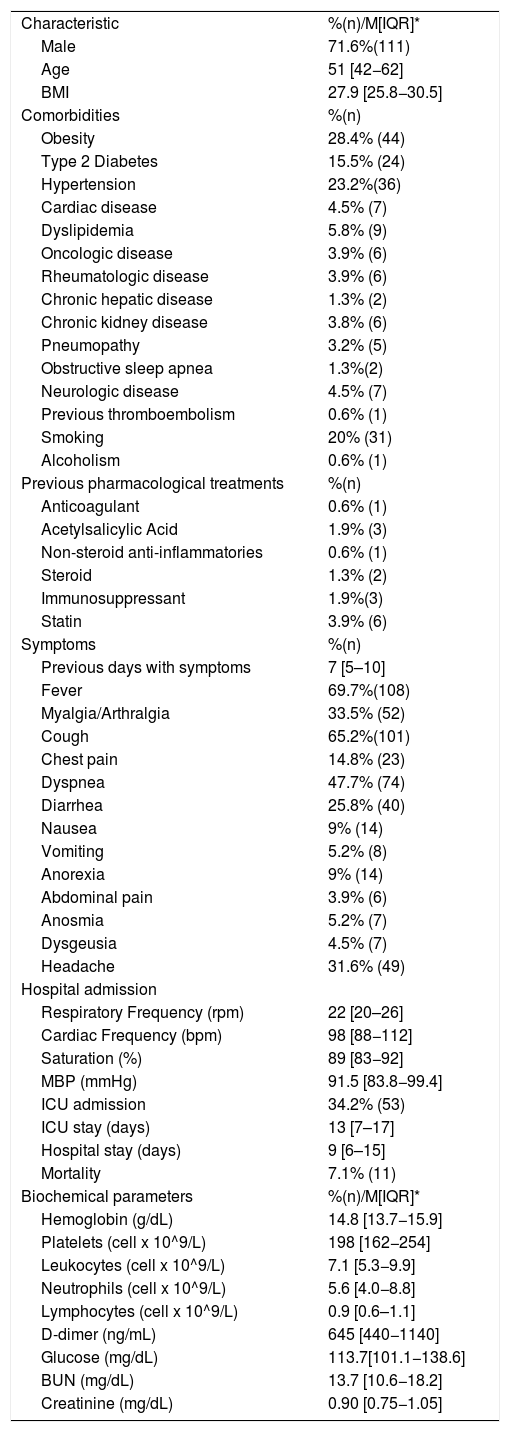
3ResultsA total of 155 patients were included in the final analysis, in which 71.6% (n = 111) were male; median of age and BMI were 51 [42−62] years and 27.9 [25.8−30.5] kg/m2, respectively. Obesity (28.4%, n = 44), hypertension (23.2%, n = 36), and T2DM (15.5%, n = 24) were the most frequent comorbidities. Only 2 patients (1.3%) reported a preexisting liver disease. Among biochemical parameters, the median of hemoglobin was 14.8 [13.7−15.9] g/dL, platelets 198 [162−254] x 109/L, and leukocytes 7.1 [5.3−9.9] x 109/L. Demographic data, clinical characteristics, drug history, and biochemical parameters are shown in Table 1. Patients had a median of 7 [5–10] days with symptoms before admission, which included fever in 69.7% (n = 108), cough in 65.2% (n = 101), and dyspnea in 47.7% (n = 74). The median of hospital stay was 9 [6–15] days and 34.2% (n = 53) of the patients required admission to the ICU, with a median stay of 13 [7–17] days.
Clinical, demographic and biochemical characteristics of patients. Continuous variables are reported as median (interquartile range), categorical ones as frequency (percentage).
| Characteristic | %(n)/M[IQR]* |
| Male | 71.6%(111) |
| Age | 51 [42−62] |
| BMI | 27.9 [25.8−30.5] |
| Comorbidities | %(n) |
| Obesity | 28.4% (44) |
| Type 2 Diabetes | 15.5% (24) |
| Hypertension | 23.2%(36) |
| Cardiac disease | 4.5% (7) |
| Dyslipidemia | 5.8% (9) |
| Oncologic disease | 3.9% (6) |
| Rheumatologic disease | 3.9% (6) |
| Chronic hepatic disease | 1.3% (2) |
| Chronic kidney disease | 3.8% (6) |
| Pneumopathy | 3.2% (5) |
| Obstructive sleep apnea | 1.3%(2) |
| Neurologic disease | 4.5% (7) |
| Previous thromboembolism | 0.6% (1) |
| Smoking | 20% (31) |
| Alcoholism | 0.6% (1) |
| Previous pharmacological treatments | %(n) |
| Anticoagulant | 0.6% (1) |
| Acetylsalicylic Acid | 1.9% (3) |
| Non-steroid anti-inflammatories | 0.6% (1) |
| Steroid | 1.3% (2) |
| Immunosuppressant | 1.9%(3) |
| Statin | 3.9% (6) |
| Symptoms | %(n) |
| Previous days with symptoms | 7 [5–10] |
| Fever | 69.7%(108) |
| Myalgia/Arthralgia | 33.5% (52) |
| Cough | 65.2%(101) |
| Chest pain | 14.8% (23) |
| Dyspnea | 47.7% (74) |
| Diarrhea | 25.8% (40) |
| Nausea | 9% (14) |
| Vomiting | 5.2% (8) |
| Anorexia | 9% (14) |
| Abdominal pain | 3.9% (6) |
| Anosmia | 5.2% (7) |
| Dysgeusia | 4.5% (7) |
| Headache | 31.6% (49) |
| Hospital admission | |
| Respiratory Frequency (rpm) | 22 [20–26] |
| Cardiac Frequency (bpm) | 98 [88−112] |
| Saturation (%) | 89 [83−92] |
| MBP (mmHg) | 91.5 [83.8−99.4] |
| ICU admission | 34.2% (53) |
| ICU stay (days) | 13 [7–17] |
| Hospital stay (days) | 9 [6–15] |
| Mortality | 7.1% (11) |
| Biochemical parameters | %(n)/M[IQR]* |
| Hemoglobin (g/dL) | 14.8 [13.7−15.9] |
| Platelets (cell x 10^9/L) | 198 [162−254] |
| Leukocytes (cell x 10^9/L) | 7.1 [5.3−9.9] |
| Neutrophils (cell x 10^9/L) | 5.6 [4.0−8.8] |
| Lymphocytes (cell x 10^9/L) | 0.9 [0.6–1.1] |
| D-dimer (ng/mL) | 645 [440−1140] |
| Glucose (mg/dL) | 113.7[101.1−138.6] |
| BUN (mg/dL) | 13.7 [10.6−18.2] |
| Creatinine (mg/dL) | 0.90 [0.75−1.05] |
Laboratory examinations reference range: Hemoglobin 11.–16.3 g/dL; Platelets 150–450 × 10^9/L; Leukocytes 4.5–11 × 10^9/L; Neutrophils 1.8–7 × 10^9/L; Lymphocytes 1.2–4 × 10^9/L; D-dimer 0−499 ng/mL; Glucose 72−99 mg/dL; BUN 8−20 mg/dL; Creatinine 0.44–1.03 mg/dL.
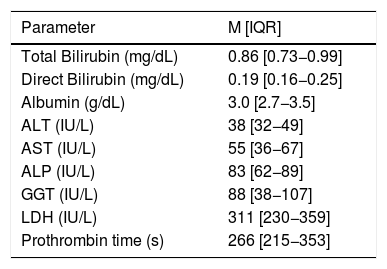
At least one GI symptom was reported in 36.1% (n = 56) of the patients, which included diarrhea in 25.8% (n = 40), nausea in 9% (n = 14), and anorexia in 9% (n = 14). Regarding LFT (Table 2), a total of 96.8% (n = 150) of patients presented at least one abnormality, and lactate dehydrogenase was the most frequent in 89.7% (n = 139) of the cases.
Liver function tests in patients admitted with COVID-19. Continuous Variables are reported as median (interquartile range).
| Parameter | M [IQR] |
|---|---|
| Total Bilirubin (mg/dL) | 0.86 [0.73−0.99] |
| Direct Bilirubin (mg/dL) | 0.19 [0.16−0.25] |
| Albumin (g/dL) | 3.0 [2.7−3.5] |
| ALT (IU/L) | 38 [32−49] |
| AST (IU/L) | 55 [36−67] |
| ALP (IU/L) | 83 [62−89] |
| GGT (IU/L) | 88 [38−107] |
| LDH (IU/L) | 311 [230−359] |
| Prothrombin time (s) | 266 [215−353] |
Laboratory examinations reference range: Total bilirubin 0.4–1.5 mg/dL; Direct bilirubin 0−0.3 mg/dL; Albumin 3.5–4.8 g/dL; ALT 14–54 IU/L; AST 15–41 IU/L; ALP 32–91 IU/L; GGT 7−05 IU/L; LDH 98–192 IU/L.
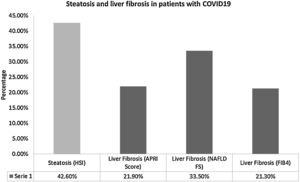
The prevalence of steatosis by HSI score >36 was 42.6% (n = 66) and it was not associated with clinical outcomes. On the other hand, the prevalence of advanced liver fibrosis by any model was 44.5% (n = 69), which is shown in Fig. 1. The prevalence of T2DM was higher among patients with fibrosis (66.7% (n = 16) vs. 33.3% (n = 8), p = 0.025), and mortality was also higher in these patients (81.8% (n = 9) vs. 18.2% (n = 2), p = 0.012); however, no independent associations were found in multivariate analyses.
Differences in basal biochemical levels were found in patients with advanced liver fibrosis. D-dimer (780 [535–1530] vs. 585 [430–907], p = 0.023); glucose (121 [102.9–146] vs. 109 [99.8–133], p = 0.044); AST (67 [58.5–77] vs. 38.5 [29.7–58.5] p ≤ 0.0001); alkaline phosphatase (89 [67.5–89] vs. 75 [58–89] p = 0.001), and gamma glutamyl transpeptidase (107 [70.5–115] vs. 58 [31–101], p ≤ 0.0001) were higher in patients with fibrosis. On the other hand, platelets (172 [136–218.5] vs. 217 [172.7–287.2], p = 0.001) and albumin (3.0 [2.7–3.1] vs. 3.1 [2.7–3.7], p = 0.002) were lower in these patients.
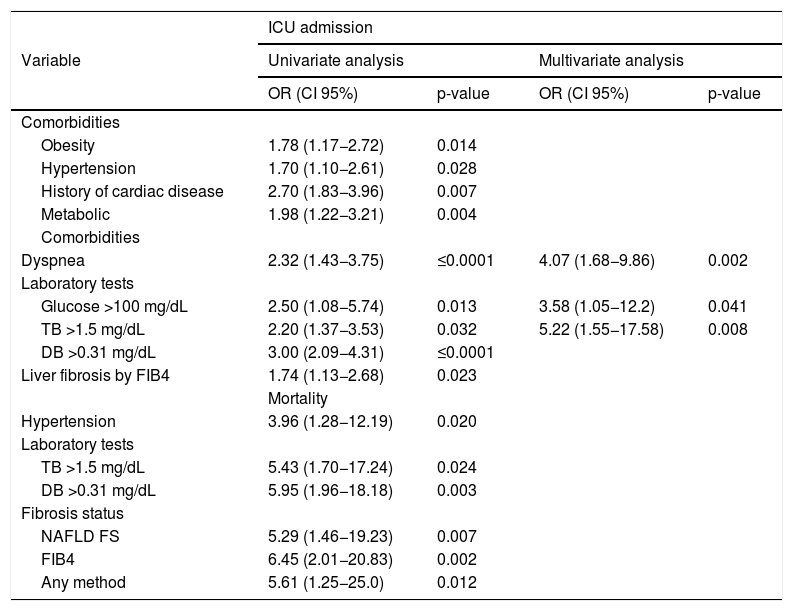
Factors associated with ICU admission were obesity, hypertension, history of cardiac disease, dyspnea, basal glucose higher than 100 mg/dL, basal total bilirubin (>1.5 mg/dL), basal direct bilirubin (>0.31 mg/dL), advanced liver fibrosis by FIB-4, and presence of metabolic comorbidities; in multivariate analyses, the presence of dyspnea, elevated glucose, and direct bilirubin showed an independent association with ICU admission. (Table 3) Significantly higher levels of total bilirubin (0.89 [0.73–1.07] vs. 0.86 [0.71−0.93], p = 0.007), direct bilirubin (0.24 [0.18−0.39] vs. 0.19 [0.14−0.19], p ≤ 0.001), C-reactive protein (209 [117.6–258.7] vs. 118.4 [46.2–180.4], p = 0.001), and procalcitonin (0.25 [0.17−0.58] vs. 0.11 [0.76−0.26], p ≤ 0.001) were found in patients admitted into the ICU.
Univariate and multivariate analysis for factors associated to ICU admission and univariate analysis for mortality risk in patients with COVID-19. Variables are reported as median (interquartile range).
| ICU admission | ||||
|---|---|---|---|---|
| Variable | Univariate analysis | Multivariate analysis | ||
| OR (CI 95%) | p-value | OR (CI 95%) | p-value | |
| Comorbidities | ||||
| Obesity | 1.78 (1.17−2.72) | 0.014 | ||
| Hypertension | 1.70 (1.10−2.61) | 0.028 | ||
| History of cardiac disease | 2.70 (1.83−3.96) | 0.007 | ||
| Metabolic | 1.98 (1.22−3.21) | 0.004 | ||
| Comorbidities | ||||
| Dyspnea | 2.32 (1.43−3.75) | ≤0.0001 | 4.07 (1.68−9.86) | 0.002 |
| Laboratory tests | ||||
| Glucose >100 mg/dL | 2.50 (1.08−5.74) | 0.013 | 3.58 (1.05−12.2) | 0.041 |
| TB >1.5 mg/dL | 2.20 (1.37−3.53) | 0.032 | 5.22 (1.55−17.58) | 0.008 |
| DB >0.31 mg/dL | 3.00 (2.09−4.31) | ≤0.0001 | ||
| Liver fibrosis by FIB4 | 1.74 (1.13−2.68) | 0.023 | ||
| Mortality | ||||
| Hypertension | 3.96 (1.28−12.19) | 0.020 | ||
| Laboratory tests | ||||
| TB >1.5 mg/dL | 5.43 (1.70−17.24) | 0.024 | ||
| DB >0.31 mg/dL | 5.95 (1.96−18.18) | 0.003 | ||
| Fibrosis status | ||||
| NAFLD FS | 5.29 (1.46−19.23) | 0.007 | ||
| FIB4 | 6.45 (2.01−20.83) | 0.002 | ||
| Any method | 5.61 (1.25−25.0) | 0.012 | ||
TB = total bilirubin, DB = direct bilirubin.
Mortality was 7.1% (n = 11) and the associated factors were hypertension, basal total bilirubin, basal direct bilirubin, and liver fibrosis by any method, as well as by NAFLD FS and FIB-4; (Table 3) nonetheless, none of these variables showed an independent association with mortality risk. Non-surviving patients showed higher basal levels of prothrombin time (383 [266–686] vs. 266 [210.2–324.5], p = 0.048), D-dimer (2790 [1140–10570] vs. 642 [448–940], p = 0.007), blood urea nitrogen (23.8 [16.2–30] vs. 13.2 [10.3–17.7], p = 0.007), creatinine (1.18 [1.04–1.62] vs. 0.9 [0.75–1.02], p = 0.042), and direct bilirubin (0.35 [0.19−0.51] vs. 0.19[0.16−0.23], p = 0.029) than surviving patients.
4DiscussionAs expected, due to the epidemiological context of Mexico, in our study, we found a high prevalence of metabolic comorbidities, which included obesity, hypertension, and T2DM, which were associated with clinical outcomes in univariate analyses. Obese patients face an increased risk of severe complications and mortality. They also represent a challenge for therapeutic maneuvers such as imaging diagnosis, intubation, mechanical ventilation, and pronation, among others [11]. A meta-analysis including 3207 patients with COVID-19 described that underlying chronic conditions such as hypertension, diabetes, and cardiovascular and respiratory diseases were higher in critical/non-surviving patients; clinical manifestations such as fever and dyspnea were also associated with the progression of the disease [12]. We found similar results in our study, with dyspnea as the most important associated symptom for ICU admission with an OR 4.07 (CI95% 1.6–9.86).
The pathogenesis of GI symptoms and liver injury in COVID-19 is still a subject of research. SARS-CoV-2’s glycoprotein spikes (S protein) on its outer surface bind to the host cells’ ACE2 (Angiotensin-Converting Enzyme 2) receptor, and a conformational change in the S protein allows viral envelope fusion with the host cells’ membrane [13]. The receptor-binding domain of its S protein share 73.8–74.9% amino acid identity of SARS-CoV that uses the same ACE2 receptor for host cell entry [13,14]. ACE2 micro RNA is present in all tissues and, remarkably, in the surface of lung alveolar epithelial cells and enterocytes of the small intestine [15], which supports theories of GI invasion and shedding of the virus in feces [16].
Although ACE2 is not expressed in Kupffer cells, hepatocytes, and endothelium of liver sinusoids [15], expression of ACE2 is induced by hypoxia in cultured hepatocytes [17] and it has been shown that COVID-19 infection is associated with liver disease, ranging from a mild to severe damage, but it is usually transient [2]. Currently, there is no consensus on the exact mechanism of liver injury, but it may be related to: 1) direct cytopathic damage, 2) systemic inflammatory response, 3) liver hypoxia and ischemia, 4) acute-on-chronic liver injury, or 5) drug-induced liver injury [18]. It has been recently demonstrated the presence of SARS-CoV-2 virions inside hepatocytes [19] and that death of cholangiocytes is induced by SARS-CoV-2 [20]. Even though it has not been demonstrated in humans, hepatic steatosis in mice models increases hepatic ACE2 mRNA [21], which could explain, at least in theory, the relationship between SARS-CoV-2 and liver injury in MAFLD patients. The liver harvests a great pool of macrophages and immune cells, that in addition to a particular susceptibility of hepatocytes to proinflammatory cytokines, the systemic inflammatory response can easily produce liver injury as a collateral damage [19].
The global prevalence of GI symptoms in COVID-19 patients has been reported between 11.3% and 79.1% [22]. In our study, GI symptoms were reported in 36.1% (n = 56), which is similar to another recent Mexican study which reported a prevalence of 20.8% [23]. Additionally, a meta-analysis which included 6686 patients estimated that 15% of them had at least one GI symptom and that 10% only had GI symptoms upon presentation [24]. Another systematic review and meta-analysis which included 12,797 patients reported that mortality in the group of patients with GI symptoms (0.4% [IC95%, 0–1.1%; I2 = 74%]) was similar (p = 0.15) to overall mortality (2.1% [IC95%, 0.2–4.7%; I2 = 94%]) [25].
Abnormal LFT were found in 96.8% (n = 150) of our patients, which is higher that recent studies reporting a prevalence ranging from 16.1% to 76.3% [3,26], including 21.5% of liver injury prevalence during hospitalization [3]. Our study did not find an association between abnormal LFT and mortality, as a multicentric study in China that included 5771 patients which found that abnormal AST was associated with increased mortality risk by any cause with an OR 4.81 (IC95%, 3.38–6.86; p < 0.001), which increased to OR 14.87 (IC95%, 9.64–22.93; p < 0.001) in the group with AST above 120 IU/L [27].
We described a prevalence of 42.6% (n = 66) of steatosis, which was not associated with clinical outcomes. In contrast, a retrospective study [28] of 202 COVID-19 patients in China found a prevalence of MAFLD of 37.6% and higher risk of disease progression. Since MAFLD is currently considered the liver manifestation of metabolic syndrome, [29] these patients often have chronic inflammation. This fact could contribute to the interplay in the cytokine storm described in COVID-19, resulting in the progression of the disease, its complications, and fatal outcomes [30]. This explanation is supported by Zheng et al.’s study [31] in China, in which 30% of the patients presented MAFLD, according to tomographic criteria, and found an increased 6-fold risk of severe COVID-19 and obesity in MAFLD patients, in comparison to non-obese patients (OR 6.32, 95%CI 1.16–34.54, p = 0.033). The addition of metabolic comorbidities may increase the risk of serious complications in this group of patients, but further research is required.
In our study, we found a pooled prevalence of advanced liver fibrosis of 44.5% (n = 69) through any non-invasive liver assessment model, which was associated with hospital outcomes. This result is in concordance to the multicenter study by Ibáñez-Samaniego et al. [32] in Spain, which included 160 patients and estimated a risk for advanced fibrosis in 28.1%. A FIB-4 ≥ 2.67 score increased the risk of ICU admission as an independent risk factor (OR 3.41, 95%CI 1.30–8.92). Interestingly, in the multivariate analysis with exclusion of patients with MAFLD, FIB-4 ≥ 2.67 remained as a risk factor for mechanical ventilation (OR 3.25; 95% CI, 1.24–8.53). In our study, no independent associations were found for liver fibrosis and clinical outcomes.
These associations are interesting but must be interpreted with caution, since most of the non-invasive predictive models include variables that may be affected by the COVID-19 infection as well, resulting in overdiagnosis of liver fibrosis upon presentation. Nonetheless, we consider that screening for liver fibrosis in patients with COVID-19 would contribute to further risk stratification and to design follow-up strategies in surviving patients who were unaware of their liver diseases, especially in regions where obesity and other metabolic comorbidities are highly prevalent.
Our study only included two patients with preexisting chronic liver disease, but a previous report by an International Registry (COVID-Hep.net and COVIDCirrhosis.org) that included 152 patients showed that 23.3% were admitted to the ICU and 17.5% required mechanical ventilation, with a reported mortality of 39.8% [33]. A meta-analysis with 1558 patients from 6 studies did not find a correlation between increased risk of COVID-19 and liver disease [34], but a multicenter study in the United States with 2780 participants (n = 250 with liver disease and COVID-19), reported that preexisting liver disease was associated with overall mortality (RR 3; CI 95%, 1.5–6.0) [35]. As the pandemic evolves, new studies have reported important associations between MAFLD and COVID-19 severity. In a retrospective study of 202 patients in China, MAFLD was associated with COVID-19 progression had (OR 6.4; 95% IC, 1.5–31.2) [36].
Further research, especially in the Americas, is required to elucidate the relationship between liver disease and COVID-19 regarding clinical outcomes.
Limitations of our study include the retrospective design, the assessment of liver fibrosis through non-invasive models, and the conduction of the study in a private hospital; additionally, as this is not a prospective design, decisions in management and timing of laboratory tests are based on each attending physician during hospital course.
5ConclusionsIn COVID-19 patients, the prevalence of liver steatosis and advanced liver fibrosis by non-invasive assessment prediction models was high and was not associated with clinical outcomes; 96.8% of COVID-19 patients had at least one abnormal LFT. The presence of metabolic comorbidities was associated with mortality and ICU admission. The timely diagnosis of COVID-19 patients, including those presenting GI symptoms and abnormal LFT, is of utmost importance in the fight towards reducing mortality.
Conflicts of interestNo potential conflicts of interest were reported by the authors (Lopez-Mendez Ivan, Aquino-Matus Jorge, Murua-Beltrán Gall Sofia, Prieto-Nava Jose David, Juarez-Hernandez Eva, Uribe Misael, Castro-Narro Graciela).
Ethics approval statementThis study was approved by Medica Sur Ethics Committee (2020-EXT-487).
Informed consentNot applicable.
FundingNone.