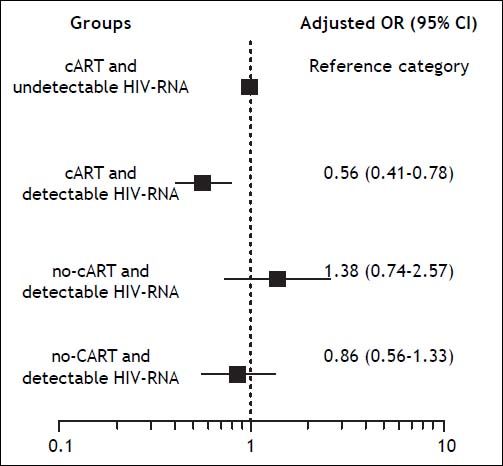
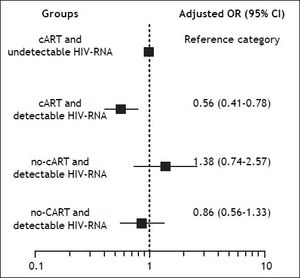
Background and rationale for the study. We assessed the association of CD4+ T-cell counts and HIV-RNA on sustained viral response (SVR) after therapy with pegylated interferon and ribavirin (PR) in HIV/HCV coinfected patients. We examined two large cohorts of coinfected patients treated with PR in Spain between 2000 and 2008. SVR was defined as undetectable HCV-RNA at 24 weeks after the end of PR. Results. We studied 1682 patients, of whom 38% achieved SVR. Baseline factors independently associated with reduced odds of SVR included genotype 1 or 4, HCV-RNA > 500,000 IU/mL, advanced liver fibrosis, CDC clinical category C, and detectable HIV-RNA. By multivariate logistic regression analysis, we found that, in comparison with patients with combination antiretroviral therapy (cART) and undetectable HIV-RNA, the odds ratio [95% confidence interval (CI)] of SVR was 0.56 (0.41-0.78) for cART and detectable HIV-RNA, 0.86 (0.56-2.57) for no-cART and detectable HIV-RNA, and 1.38 (0.74-2.57) for no-cART and undetectable HIV-RNA. Conclusions. Detectable HIV-RNA, but not CD4+ T-cell count, was associated with reduced odds of SVR. However, this finding was only confirmed for cART and detectable HIV-RNA, raising the question as whether this represents a true association of HIV-RNA on response to PR or a spurious association due to poor adherence to treatment.
The most important baseline predictors of sustained virologic response (SVR) after dual therapy with pegylated interferon and ribavirin in patients coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) include virus-related factors, such as HCV genotype and serum HCV-RNA load, and host-related factors, such as liver fibrosis stage and IL28B genotype.1,2 Little is known, however, about the influence of HIV-related factors such as CD4+ T-cell count and HIV-RNA load on the response to pegylated interferon plus ribavirin, largely because patients with low CD4+ T-cell counts and/or with insufficient control of HIV infection have been under-represented in most clinical trials.3–5 Our aim was to assess the association of baseline CD4+ T-cell count and HIV-RNA load on the response to pegylated interferon plus ribavirin in two large observational cohorts of HIV/HCV-coinfected patients.
Material and MethodsThis is a retrospective observational study conducted with patients from two cohorts of HIV/HCV-coinfected individuals initiating their first anti-HCV treatment with pegylated interferon plus ribavirin in Spain between 2000 and 2008. Data for these cohorts have been reported elsewhere.6 Of the 1,701 patients included, we selected the 1682 patients for whom both CD4+ T-cell counts and HIV-RNA load determinations were available when pegylated interferon plus ribavirin was initiated (baseline). Combination antiretroviral therapy (cART) was defined as the use of three or more antiretroviral drugs, one of which had to be a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, one of the nucleoside reverse transcriptase inhibitors abacavir or tenofovir, an integrase inhibitor, or an entry inhibitor. Liver fibrosis was staged according to the METAVIR criteria.7 SVR was defined as an undetectable HCV-RNA load 24 weeks after discontinuation of pegylated interferon plus ribavirin. Multivariate logistic regression models were constructed to identify baseline factors that were associated with the achievement of SVR. To further assess the association of HIV-RNA load at baseline on SVR, we categorized patients into four subgroups according to whether or not they were on cART and whether or not they had detectable HIV-RNA at baseline. We also used logistic regression analysis to calculate the adjusted odds ratio (OR) and 95% confidence interval (CI) for SVR in the different subgroups, taking patients on cART with undetectable HIV-RNA load as the reference group. All analyses were performed on an intention-to-treat basis.
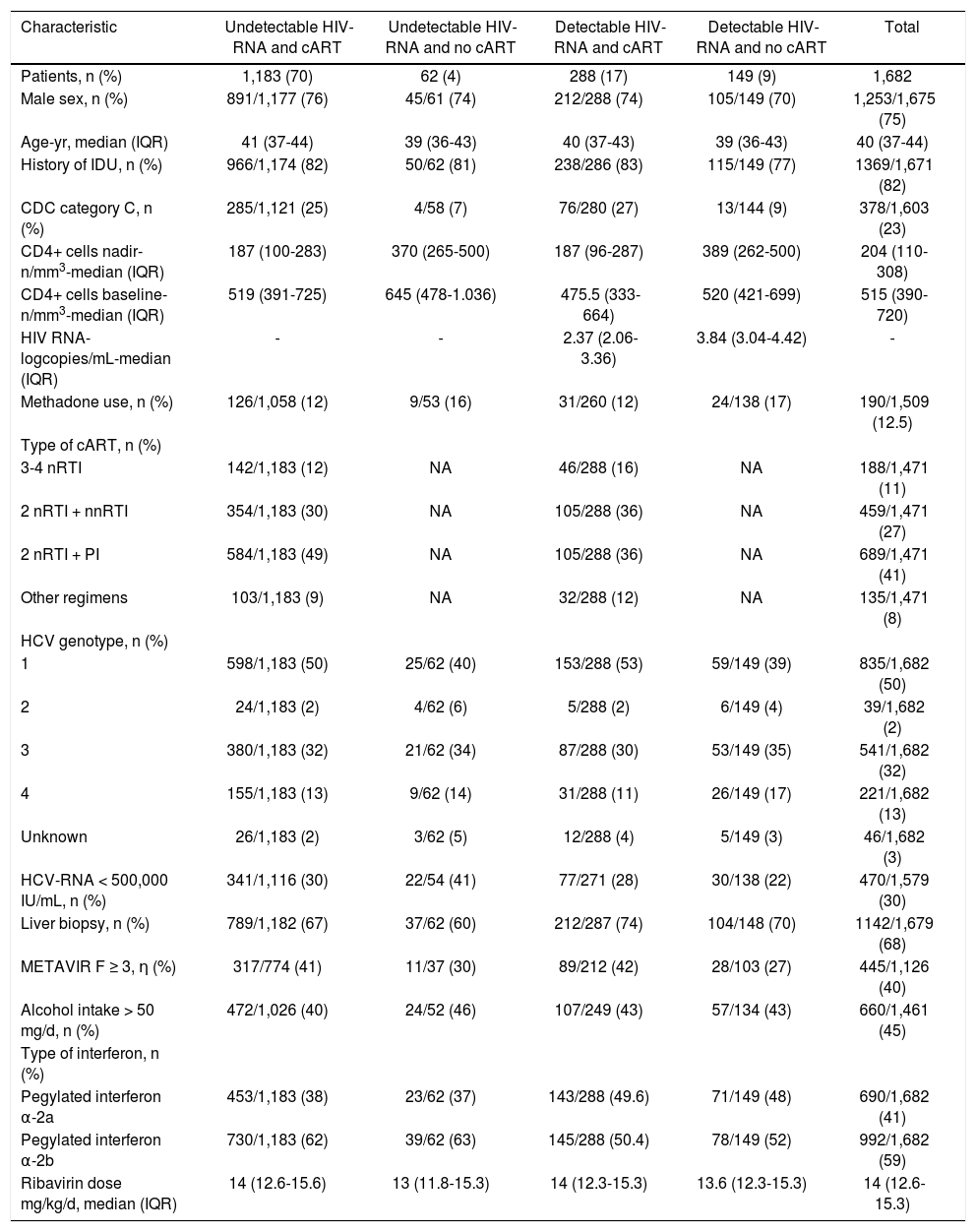
Results and DiscussionThe baseline characteristics of the patients, overall and according to whether or not they were on cART and whether or not they had detectable HIV-RNA at baseline, are shown in the table 1.
Characteristics of 1,682 HIV/HCV-coinfected patients treated with pegylated interferon plus ribavirin.
| Characteristic | Undetectable HIV-RNA and cART | Undetectable HIV-RNA and no cART | Detectable HIV-RNA and cART | Detectable HIV-RNA and no cART | Total |
|---|---|---|---|---|---|
| Patients, n (%) | 1,183 (70) | 62 (4) | 288 (17) | 149 (9) | 1,682 |
| Male sex, n (%) | 891/1,177 (76) | 45/61 (74) | 212/288 (74) | 105/149 (70) | 1,253/1,675 (75) |
| Age-yr, median (IQR) | 41 (37-44) | 39 (36-43) | 40 (37-43) | 39 (36-43) | 40 (37-44) |
| History of IDU, n (%) | 966/1,174 (82) | 50/62 (81) | 238/286 (83) | 115/149 (77) | 1369/1,671 (82) |
| CDC category C, n (%) | 285/1,121 (25) | 4/58 (7) | 76/280 (27) | 13/144 (9) | 378/1,603 (23) |
| CD4+ cells nadir-n/mm3-median (IQR) | 187 (100-283) | 370 (265-500) | 187 (96-287) | 389 (262-500) | 204 (110-308) |
| CD4+ cells baseline-n/mm3-median (IQR) | 519 (391-725) | 645 (478-1.036) | 475.5 (333-664) | 520 (421-699) | 515 (390-720) |
| HIV RNA-logcopies/mL-median (IQR) | - | - | 2.37 (2.06-3.36) | 3.84 (3.04-4.42) | - |
| Methadone use, n (%) | 126/1,058 (12) | 9/53 (16) | 31/260 (12) | 24/138 (17) | 190/1,509 (12.5) |
| Type of cART, n (%) | |||||
| 3-4 nRTI | 142/1,183 (12) | NA | 46/288 (16) | NA | 188/1,471 (11) |
| 2 nRTI + nnRTI | 354/1,183 (30) | NA | 105/288 (36) | NA | 459/1,471 (27) |
| 2 nRTI + PI | 584/1,183 (49) | NA | 105/288 (36) | NA | 689/1,471 (41) |
| Other regimens | 103/1,183 (9) | NA | 32/288 (12) | NA | 135/1,471 (8) |
| HCV genotype, n (%) | |||||
| 1 | 598/1,183 (50) | 25/62 (40) | 153/288 (53) | 59/149 (39) | 835/1,682 (50) |
| 2 | 24/1,183 (2) | 4/62 (6) | 5/288 (2) | 6/149 (4) | 39/1,682 (2) |
| 3 | 380/1,183 (32) | 21/62 (34) | 87/288 (30) | 53/149 (35) | 541/1,682 (32) |
| 4 | 155/1,183 (13) | 9/62 (14) | 31/288 (11) | 26/149 (17) | 221/1,682 (13) |
| Unknown | 26/1,183 (2) | 3/62 (5) | 12/288 (4) | 5/149 (3) | 46/1,682 (3) |
| HCV-RNA < 500,000 IU/mL, n (%) | 341/1,116 (30) | 22/54 (41) | 77/271 (28) | 30/138 (22) | 470/1,579 (30) |
| Liver biopsy, n (%) | 789/1,182 (67) | 37/62 (60) | 212/287 (74) | 104/148 (70) | 1142/1,679 (68) |
| METAVIR F ≥ 3, η (%) | 317/774 (41) | 11/37 (30) | 89/212 (42) | 28/103 (27) | 445/1,126 (40) |
| Alcohol intake > 50 mg/d, n (%) | 472/1,026 (40) | 24/52 (46) | 107/249 (43) | 57/134 (43) | 660/1,461 (45) |
| Type of interferon, n (%) | |||||
| Pegylated interferon α-2a | 453/1,183 (38) | 23/62 (37) | 143/288 (49.6) | 71/149 (48) | 690/1,682 (41) |
| Pegylated interferon α-2b | 730/1,183 (62) | 39/62 (63) | 145/288 (50.4) | 78/149 (52) | 992/1,682 (59) |
| Ribavirin dose mg/kg/d, median (IQR) | 14 (12.6-15.6) | 13 (11.8-15.3) | 14 (12.3-15.3) | 13.6 (12.3-15.3) | 14 (12.6-15.3) |
cART: œmbination antiretroviral therapy. CDC: Centers for Disease Control and Prevention. nRTI: nucleoside reverse transcriptase inhibitor. nnRTI: non-nucleoside reverse transcriptase inhibitor. PI: protease inhibitor. HCV: hepatitis C virus. IQR: interquartile range. NA: not applicable.
The baseline CD4+ T-cell count was < 100 cells/ mm3 in 17 (1%) patients and < 200 cells/mm3 in 54 (3%) patients. Baseline HIV-RNA load (copies/mL) was determined using commercially available tests with different lower limits of detection: < 400 (n = 17), < 200 (n = 168), < 80 (n = 34), and < 50 (n = 1463). A total of 1,245 (74%) patients had undetectable HIV-RNA at baseline. Liver biopsy was performed in 1,142 (68%) patients, 445 (39%) of whom had METAVIR fibrosis stages 3 or 4. All patients were scheduled to receive pegylated interferon plus weight-based ribavirin for 48 weeks.
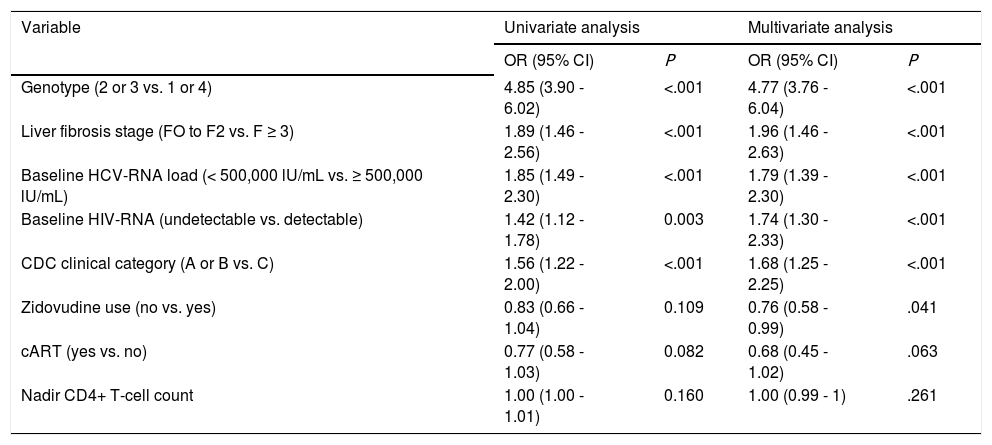
SVR was achieved by 641 patients overall (38%), by 359 of those infected by genotype 2 or 3 (61%), and by 265 of those infected by genotype 1 or 4 (24%). In the univariate analysis, the baseline variables associated with SVR were genotype, liver fibrosis stage, HCV-RNA load, HIV-RNA load, and CDC clinical category; baseline CD4+ T-cell count was not associated with SVR. The final multivariate logistic regression model included variables associated with SVR by univariate analysis as well as covariates of clinical significance such as nadir CD4+ T-cell count, and use of cART. Zidovudine use was also included in the model because it has been shown to be the only antiretroviral drug that adversely affected SVR to pegylated interferon plus ribavirin in HIV/HCV-coinfected patients.6 According to this model, the presence of undetectable HIV-RNA at baseline —among other covariates— was independently associated with increased odds of SVR (Table 2).
Factors associated with a sustained virologie response by multivariate logistic regression analysis.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | Ρ | OR (95% CI) | Ρ | |
| Genotype (2 or 3 vs. 1 or 4) | 4.85 (3.90 - 6.02) | <.001 | 4.77 (3.76 - 6.04) | <.001 |
| Liver fibrosis stage (FO to F2 vs. F ≥ 3) | 1.89 (1.46 - 2.56) | <.001 | 1.96 (1.46 - 2.63) | <.001 |
| Baseline HCV-RNA load (< 500,000 lU/mL vs. ≥ 500,000 lU/mL) | 1.85 (1.49 - 2.30) | <.001 | 1.79 (1.39 - 2.30) | <.001 |
| Baseline HIV-RNA (undetectable vs. detectable) | 1.42 (1.12 - 1.78) | 0.003 | 1.74 (1.30 - 2.33) | <.001 |
| CDC clinical category (A or Β vs. C) | 1.56 (1.22 - 2.00) | <.001 | 1.68 (1.25 - 2.25) | <.001 |
| Zidovudine use (no vs. yes) | 0.83 (0.66 - 1.04) | 0.109 | 0.76 (0.58 - 0.99) | .041 |
| cART (yes vs. no) | 0.77 (0.58 - 1.03) | 0.082 | 0.68 (0.45 - 1.02) | .063 |
| Nadir CD4+ T-cell count | 1.00 (1.00 - 1.01) | 0.160 | 1.00 (0.99 - 1) | .261 |
OR: Odds Ratio. 01: œnfidence interval. ODO: Oenters for Disease Oontrol and Prevention. cART: combination antiretroviral therapy.
We also used a logistic regression model to calculate the adjusted odds ratio and 95% confidence interval for SVR of the different subgroups. The model was built according to cART and HIV-RNA load, and by taking the group of cART and undetectable HIV-RNA load as the reference category. The covariates for adjustment were HCV genotype, liver fibrosis stage, HCV-RNA load, CDC clinical category, nadir CD4+ T-cell count, and zidovudine. We found that, in comparison with patients on cART and undetectable HIV-RNA, the odds of achieving SVR were significantly lower for patients with cART and detectable HIV-RNA load, but not significantly different for those with no cART and detectable HIV-RNA load or for those with no cART and undetectable HIV-RNA load (Figure 1).
Adjusted odds ratio (OR) and 95% confidence intervals (CI) for SVR of the different subgroups according to cART and HIV-RNA load and taking the patients receiving cART with undetectable HIV-RNA load as the reference group. The covariates for adjustment were HCV genotype, HCV-RNA load, liver fibrosis stage, CDC clinical category, zidovudine use, and nadir CD4+ T-cell count.
In this large observational study, we did not observe an association between baseline CD4+ T-cell count and SVR; however, it must be taken into account that the median baseline CD4+ T-cell count in our patients was > 500 cells/mm3 and that only 3% of the patients had a CD4+ T-cell count < 200 cells/mm3. Our findings are consistent with those of three major clinical trials that compared pegylated interferon with non-pegylated interferon (APRICOT,3 RIBAVIC,4 and ACTG A50715), both of which were combined with ribavirin in coinfected patients. However, a retrospective analysis of efficacy and safety data from the APRICOT trial according to baseline CD4+ T-cell count categorized by HCV genotype revealed that for patients infected with HCV genotype 1, the SVR was lower among those with CD4+ T-cell counts < 350/mm3 and was independent of baseline CD4+ T-cell counts for genotypes 2 and 3.8 In our study, we did not find an association between baseline CD4+ T-cell counts and SVR in patients infected with HCV genotype 1 (data not shown). In an observational study of pegylated interferon and ribavirin in 542 coinfected patients, no significant differences were found in efficacy and safety between those with a CD4+ T-cell count ≤ 250 cells/mm3 (N = 39) and those with a CD4+ T-cell count > 250 cells/mm3 (N = 503).9
The association of baseline HIV-RNA load on SVR was analyzed in two of the three large clinical trials mentioned above. Detectable HIV-RNA load was not found to be associated with SVR in the RIB-AVIC trial;4 however, in ACTG A5071, detectable HIV-RNA load at baseline was independently associated with higher odds of SVR.5 The authors of this trial speculated on several factors that could explain this paradoxical finding, such as the enrollment of patients with preserved CD4+ T-cell counts who had not received cART or of patients who had not been receiving cART long enough to have complete suppression of HIV-RNA; they also considered the possibility that this finding may represent a type I error. In our study, detectable HIV-RNA load at baseline was independently associated with a reduced likelihood of SVR; however, subgroup analysis adjusted for important baseline covariates indicated that this finding was only confirmed in patients with detectable HIV-RNA load and cART. It remains to be determined whether this represents a true association of HIV-RNA load on response to pegylated interferon and ribavirin or a spurious association due to poor adherence to treatment.
Our study is subject to a series of limitations; the most important being that it is not entirely prospective. However, follow-up was performed by the same physicians in the same reference hospitals, with standard clinical and laboratory parameters assessed every 6 months. Another important limitation of our study, as mentioned above, is the lack of information about adherence to pegylated interferon and ribavirin therapy, which would have helped to better understand the true meaning of the association found between detectable HIV-RNA load at baseline and SVR. Finally, our study is also limited by the lack of IL28B genotyping data; therefore, we cannot rule out the possibility that differences in this variable could have affected outcome.
In summary, we did not observe an association between baseline CD4+ T-cell count and SVR. We found that detectable HIV-RNA load at baseline was independently associated with a reduced likelihood of SVR. However, subgroup analysis adjusted for important baseline covariates indicated that this association only held in patients with detectable HIV-RNA and cART, thus raising the question of whether it is a true association of HIV-RNA on response to pegylated interferon and ribavirin or a spurious association due to poor adherence to treatment. Response to pegylated interferon and ribavirin was not compromised in patients with a detectable HIV-RNA load who were not receiving cART; therefore, treating HCV infection before HIV infection can be a reasonable option in coinfected patients, particularly those whose CD4+ T-cell counts are high enough to permit a delay in the initiation of cART. These findings are still relevant for clinical practice because interferon plus ribavirin is currently considered an acceptable option for treatment of hepatitis C in settings where none of the new HCV drugs are available.10,11
Abbreviations- •
cART: combination antiretroviral therapy.
- •
CDC: Centers for Diseases Control and Prevention.
- •
CI: confidence interval.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
OR: odds ratio.
- •
SVR: sustained viral response.
Supported in part by grants from the Fundación para la Investigación y la Prevención del SIDA en España (FIPSE) (Refs. 36443/03, 36702/07, and 361020/10), by grants from Fondo de Investigacion de Sanidad en España (FIS) (Spanish Health Funds for Research) (Refs. EC07/90734, PI11/01556, and EC11/241), and by Red de Investigación en SIDA (AIDS Research Network) (RIS) (Ref RD12/0017). Dr. Juan Berenguer is an investigator of the Programa de Intensificación de la Actividad Investigadora en el Sistema Nacional de Salud (I3SNS)
AcknowledgementsThe authors thank Thomas O’Boyle for writing assistance during the preparation of the manuscript.