Asthma diagnosis in preschoolers is mostly based on clinical evidence, but a bronchodilator response could be used to help confirm the diagnosis. The objective of this study is to evaluate the utility of bronchodilator response for asthma diagnosis in preschoolers by using spirometry standardised for this specific age group.
MethodsA standardised spirometry was performed before and after 200mcg of salbutamol in 64 asthmatics and 32 healthy control preschoolers in a case-control design study.
ResultsThe mean age of the population was 4.1 years (3–5.9 years) and 60% were females. Almost 95% of asthmatics and controls could perform an acceptable spirometry, but more asthmatics than controls reached forced expiratory volume in one second (FEV1) (57% vs. 23%, p=0.033), independent of age. Basal flows and FEV1 were significantly lower in asthmatics than in controls, but no difference was found between groups in forced vital capacity (FVC) and FEV in 0.5s (FEV0.5). Using receiver operating characteristic (ROC) curves, the variable with higher power to discriminate asthmatics from healthy controls was a bronchodilator response (% of change from basal above the coefficient of repeatability) of 25% in forced expiratory flow between 25% and 75% (FEF25–75) with 41% sensitivity, 80% specificity. The higher positive likelihood ratio for asthma equalled three for a bronchodilator response of 11% in FEV0.5 (sensitivity 30%, specificity 90%).
ConclusionsIn this sample of Chilean preschoolers, spirometry had a very high performance and bronchodilator response was very specific but had low sensitivity to confirm asthma diagnosis.
Although the diagnosis of asthma in children is mainly clinical, confirming it by post-bronchodilator reversibility reassures the diagnosis and is useful for evaluating response to therapy.1–3 Measuring lung function in children generally entails two main alternatives: techniques requiring little collaboration (e.g. measuring airway resistance by impulse oscillometry (IOS), plethysmography or resistance by the interrupter technique (Rint)); or by spirometry. Performing the first techniques usually requires specialised pulmonary function laboratories with costly equipment, sometimes yielding results that are difficult to interpret.4 In contrast, spirometry, the most commonly used test in schoolchildren and adults across the globe, is generally available in lung function laboratories, and is easy to interpret.
Chile has a public health system named FONASA, funded mostly by the goverment, and which is used by 80% of the population. The remaining 20% belong to a private health institution named ISAPRE, providing financial services and health insurance, whose costs are mostly assumed by the patient. Since 2005 in Chile legislation has been established with explicit guarantees in health (GES) and constitutes a set of benefits guaranteed by law for persons affiliated to FONASA and ISAPRE. Patients who suffer from any disease included in the GES (e.g. asthma) are entitled to early diagnosis, treatment and follow-up by medical specialists, with zero-cost or a low amount by the patient co-payment. Therefore, if the physician suspected asthma, he/she may request a spirometry. On the other hand, spirometers and physiotherapists and technologists trained to carry out spirometry are widely available at primary care centres across the country, as well as in hospitals and private practice. The organisation of the Chilean health system allows spirometry to be widely available and accessible around all the country.
In the last 15 years, many studies have been carried out to standardise spirometry in preschoolers.4–10 An important feature of preschool spirometry is that forced expiratory volume in 0.5s (FEV0.5) is more appropriate than FEV in one second (FEV1) to interpret their results because FEV1 is difficult to obtain and its meaning differs from older children and adults.4,7 Some studies determined the value of measured bronchodilator response to short beta-2 agonists (e.g. salbutamol) for asthma diagnosis in all ages, using different techniques with diverse results.11–16 However, the studies that included preschooler spirometry reported only FEV112–15 and in two of them, spirometry was performed using standardised criteria for adults.12,15
The objective of the present study was to evaluate the utility of measured bronchodilator response to short beta-2 agonists for asthma diagnosis in Chilean preschoolers using spirometry standardised for this specific age group.
Materials and methodsA case-control study was performed in asthmatic preschoolers matched by age, gender and height to healthy controls. Asthmatic children were selected by two paediatric pulmonologists for the study (ML, IC) according to Global Initiative for Asthma (GINA) guidelines.1 Asthmatic children had, at least once a month, one wheezing episode, atopic eczema or parents with asthma, eczema or allergic rhinitis diagnosis. In all of them clinical improvement was demonstrated with short-acting bronchodilators and inhaled corticosteroids (ICS). Preschoolers with moderate to severe persistent asthma presenting at the asthma clinic at the Hospital Padre Hurtado, Santiago, Chile were consecutively included in the study. All asthmatics were on ICS, mean doses of 400mcg/d of budesonide or equivalent for at least three months prior to entering the study. Using a previously validated questionnaire to rule out asthma and other chronic respiratory conditions, healthy preschoolers (controls) were selected from eight random preschool day care institutions from the Santiago Metropolitan Region. Three hundred questionnaires were sent to parents or guardians of those preschoolers. After one week, 216 (72%) questionnaires were returned, of which 143/216 (66%) had complete information and signed informed consent forms for participation in the study. This protocol was approved by the Hospital's Ethics Committee.
Demographic data including information about the neonatal period, tobacco exposure, type of heating used in the home, and history of personal and parental atopy (rhinitis and dermatitis) were collected from asthmatics and controls. Exclusion criteria of the study were prematurity, low weight for gestational age, undernourishment, cardiac or other chronic pulmonary conditions, and parental or guardian failure to sign the informed consent form. Also, children with acute respiratory infection in the preceding seven days were excluded.
All participants (asthmatics and controls) performed pre- and post-salbutamol spirometry (Jaeger MasterScreen® IOS, Würzburg, Germany). Two puffs (200mcg total dosis) of salbutamol (Aerolin®, GSK, Aranda de Duero, Spain) were administrated with three minutes of separation in between puffs and using a mouthpiece chamber (Volumatic®, Glaxo Wellcome GmbH & Co. Bad Oldesloe, Germany). The use of short acting and long acting beta-2 agonists were stopped in asthmatics at least six and 12h, respectively, before performing spirometry. The spirometer was calibrated daily using a 2L syringe.
Weight and height measurements were taken before spirometry, which was performed according to American Thoracic Society/European Respiratory Society guidelines for preschoolers.4 None of the participants had performed a spirometry before. Children belonging to the control group attended the lung function laboratory in groups of five and accompanied by an assistant. They came together to the laboratory, and after a demonstration and verbal explanation by the operator, began to test the child who voluntarily offered. This allowed all the children to try to do spirometry with great enthusiasm, imitating and competing with their peers. Asthmatic preschoolers performed spirometry accompanied only by a parent. Each child did all possible expiratory force curves over a 15-min period, without a nasal clip and standing up. Sometimes, according to operator criteria, animation programmes included in the software (e.g. candles, balloons) were used. All of the spirometry was conducted by one research coordinator (RM) for the study.
We considered acceptable spirometric curves as those with evident PEF, without abrupt end of flow>10% of PEF, with expiratory time>0.5s and without evidence of cough or glottis closure.4,10 For each test, time-volume and flow-volume curves were recorded and the real expiratory time (ET) of the curve was tabulated with the best forced vital capacity (FVC). Additionally, to calculate the reproducibility, variation coefficient, and coefficient of repeatability, the three best basal FEV0.5, FEV1, FEF25–75, FEF25, FEF50 and FEF75 values for each patient were registered.
Statistical analysisThe minimum calculated sample size was 56 asthmatics and 28 controls, taking into account an average difference between groups of bronchodilator response of 15% in FEV0.5, a standard deviation of 20% and 15% for cases and controls, respectively. There was a ratio of samples of 2 to 1, alpha error 5%, 90% power, and a confidence interval of 95% (EPIDAT software, v.3.1). For continuous variables with normal distribution, an average comparison and analysis of variance (ANOVA) between two groups were conducted. A Wilcoxon test was used in other cases. For categorical variables, contingency tables were used (χ2 with Fisher exact test). Comparisons of lung function tests for sensitivity, specificity, and predictive value were done with the optimal cut-off point as determined from the receiver operating characteristic curve (ROC) expressed as the sensitivity versus 1-specificity for each possible cut-off point. Statistically significant differences were considered for a p value<0.05. All statistical analyses were carried out with SPSS software, v. 16.0. To ensure that the bronchodilator response was a clinically significant change and was not due to technique variability, we considered it above the coefficient of repeatability.4
ResultsSixty-four preschoolers with moderate to severe persistent asthma (cases) were consecutively selected from the Hospital Padre Hurtado. From preschool day care institution centres, 43 out of 143 (33%) children who agreed to participate in the study were classified as healthy (controls); 38/43 came to our pulmonary function laboratory and six were excluded (four presented an acute respiratory tract infection and two did not want to perform a spirometry). Therefore, 64 asthmatics and 32 healthy preschoolers were available for analysis.
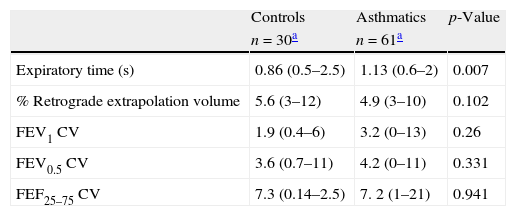
There were no significant differences in most of the demographic characteristics between groups (Table 1). There were more children from the control group using kerosene to heat their homes than in the asthmatic group (48% vs. 13%, p=0.0013 respectively). As was expected, significantly more asthmatics had parents with atopy (rhinitis and dermatitis) than controls; also asthmatics had significantly more prevalence of rhinitis and dermatitis than controls (Table 1).
Demographic characteristics of healthy controls and asthmatics preschoolers. Data shown as mean (DS or range) for continuous and n (%) for categorical variables.
| Controls | Asthmatics | p-Value | |
| n=32 | n=64 | ||
| Age (years), mean (range) | 4.1 (3–5.6) | 4 (3–5.9) | ns |
| Female (%) | 60 | 55.7 | ns |
| Height (cm), mean (±SD) | 101.2 (7.5) | 102.5 (6.8) | ns |
| Weight (kg), mean (range) | 17 (12.5–24.4) | 18.9 (14–26) | ns |
| Tobacco exposure | 12 (38%) | 26 (40%) | ns |
| Use of kerosene for heating | 15 (48%) | 8 (13%) | 0.004 |
| Rhinitis or dermatitis | 10 (33%) | 41 (65%) | 0.005 |
| Parents with rhinitis or dermatitis | 15 (48%) | 51 (80%) | 0.002 |
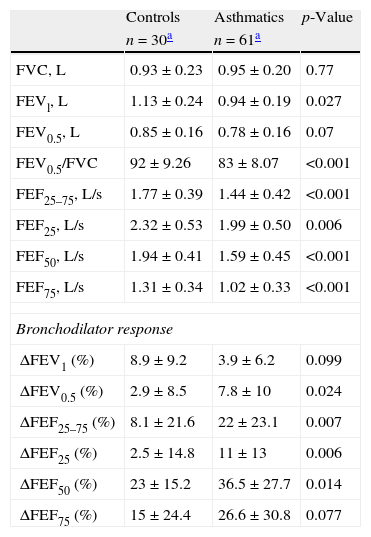
Among participants, 95% of asthmatics and 94% of controls performed at least two acceptable spirometric curves. However, more asthmatics were able to reach an ET of at least one second than controls (56% vs. 23%, p=0.0039 respectively). The mean of retrograde extrapolation volume, coefficient of variation and reproducibility reached for FVC, FEV0.5 and FEV1 values were similar between groups (Table 2). All the flows and FEV1 were significantly lower among asthmatics than controls, and there were no differences in FVC, and FEV0.5 values between groups (Table 3). The bronchodilator response (% of change from basal) was significantly higher in asthmatics than in controls in FEV0.5, FEF 25–75, FEF25, and FEF50 (Table 3).
Characteristics of the spirometry variables in controls and asthmatics preschoolers. CV: coefficient of variation.
Spirometric values and bronchodilator response (calculated as percentage of change from basal value).
| Controls | Asthmatics | p-Value | |
| n=30a | n=61a | ||
| FVC, L | 0.93±0.23 | 0.95±0.20 | 0.77 |
| FEVl, L | 1.13±0.24 | 0.94±0.19 | 0.027 |
| FEV0.5, L | 0.85±0.16 | 0.78±0.16 | 0.07 |
| FEV0.5/FVC | 92±9.26 | 83±8.07 | <0.001 |
| FEF25–75, L/s | 1.77±0.39 | 1.44±0.42 | <0.001 |
| FEF25, L/s | 2.32±0.53 | 1.99±0.50 | 0.006 |
| FEF50, L/s | 1.94±0.41 | 1.59±0.45 | <0.001 |
| FEF75, L/s | 1.31±0.34 | 1.02±0.33 | <0.001 |
| Bronchodilator response | |||
| ΔFEV1 (%) | 8.9±9.2 | 3.9±6.2 | 0.099 |
| ΔFEV0.5 (%) | 2.9±8.5 | 7.8±10 | 0.024 |
| ΔFEF25–75 (%) | 8.1±21.6 | 22±23.1 | 0.007 |
| ΔFEF25 (%) | 2.5±14.8 | 11±13 | 0.006 |
| ΔFEF50 (%) | 23±15.2 | 36.5±27.7 | 0.014 |
| ΔFEF75 (%) | 15±24.4 | 26.6±30.8 | 0.077 |
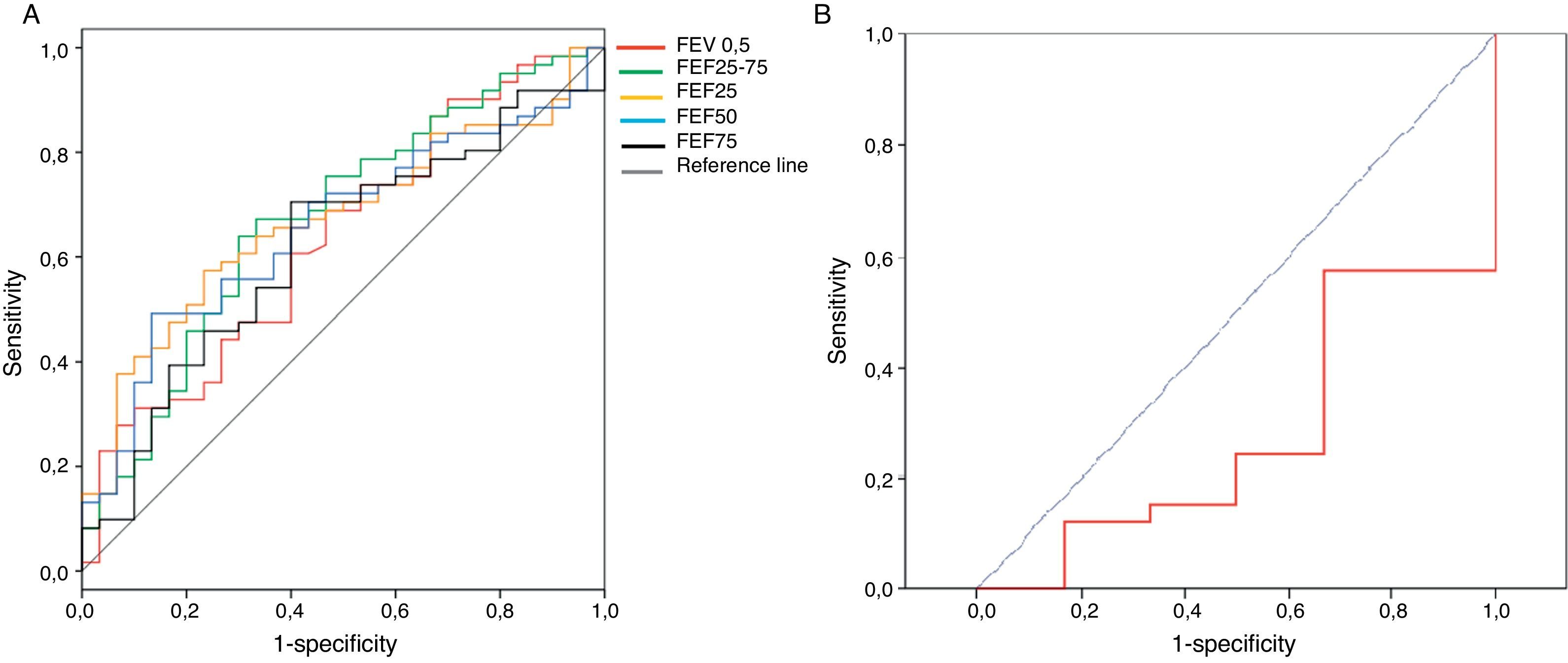
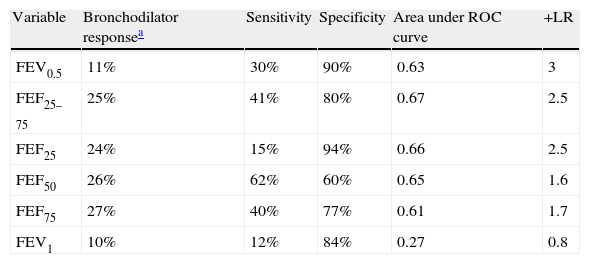
The sensitivity, specificity and positive likelihood ratio of bronchodilator response for all spirometric values are shown in Table 4. As the spirometric values had a normal distribution, ROC curves for bronchodilator response were performed for different cut-offs (Fig. 1). The highest value for distinguishing asthmatics from controls using ROC curves was the bronchodilator response of 25% in FEF25–75 with 40% of sensitivity, 80% of specificity and positive likelihood ratio of 2.5 (Table 4). The best positive likelihood ratio was three for the bronchodilator response of 11% in FEV0.5 (with 30% of sensitivity and 90% of specificity) (Table 4).
Sensitivity, specificity and positive likelihood ratio (+LR) of bronchodilator response (calculate as % of change from basal) using ROC (receiver operating curve).
| Variable | Bronchodilator responsea | Sensitivity | Specificity | Area under ROC curve | +LR |
| FEV0.5 | 11% | 30% | 90% | 0.63 | 3 |
| FEF25–75 | 25% | 41% | 80% | 0.67 | 2.5 |
| FEF25 | 24% | 15% | 94% | 0.66 | 2.5 |
| FEF50 | 26% | 62% | 60% | 0.65 | 1.6 |
| FEF75 | 27% | 40% | 77% | 0.61 | 1.7 |
| FEV1 | 10% | 12% | 84% | 0.27 | 0.8 |
The present study showed that the best bronchodilator response (% change from basal above the coefficient of repeatability) to distinguish between asthmatics from controls at preschool age is the bronchodilator response of 25% in FEF25–75 with 41% of sensitivity, 80% of specificity. However, the higher positive likelihood ratio was three for a bronchodilator response of 11% in FEV0.5. That means that if a preschooler with recurrent wheezing had a 10%, 20%, or 40% chance of having an asthma diagnosis (pre-test probability) after performing this bronchodilator test, if it is positive, the probability of having an asthma diagnosis increased to 22%, 40% or 60%, respectively (post-test probability).
As we know, most asthma starts at preschool age17; and the use of the asthma predictive index [API] with a positive likelihood ratio of seven in preschoolers with recurrent wheezing gives a higher probability for asthma diagnosis18 than the use of FEV0.5 bronchodilator response reported in the present study. Nevertheless, other studies have demonstrated that bronchodilator response in preschoolers may predict pulmonary function in adolescents and adults3; and also, it may be related to some ICS response.19,20
In our study, almost 95% of preschoolers were able to perform at least two acceptable and reproducible spirometric curves, which is slightly greater than that in other reports (70–90%).5–10,12,15 While healthy children conducted spirometry in small groups and asthmatics were only accompanied by one of their parents, there were no differences in performance and quality of the manoeuvres between groups. Even with the lack of collaboration that preschools sometimes present, the mean of coefficients of variation for FEV1, FEV0.5, and FEF25–75 reported here were similar to others studies in preschoolers4 and also in adults.6
Our value of flows, FEV1 and FEV0.5/FVC ratio were significantly different between asthmatics and healthy preschoolers, but not the FVC and FEV0.5 values. That can be explained by the higher sensitivity that flows have in comparison to volumes for detecting airway obstruction. This finding of lower lung function among asthmatic preschoolers than controls, even using ICS, was described in other studies using different lung function techniques.11,21,22 The airway obstruction not reversible to inhaled corticosteroids in our subjects could be explained by lack of response or by poor adherence (we did not evaluate it) or by another non-inflammatory component, e.g. remodelling.22
In our study, the bronchodilator response was significantly higher among asthmatics than healthy preschoolers. That difference was not observed for FEV1 values, possibly because few subjects breathe out for longer than one second. Only 57% of our asthmatics and 23% of healthy preschoolers reached an ET of at least one second, independent of age. That difference can be explained by the displacement of the equal pressure point to the periphery among asthmatics, performing a higher ET. It is known that at preschool age, only a modest number of subjects can reach FEV1 because their volume is totally expelled in one second.4,7 This is the reason why a spirometry with expiratory time less than 1 second is acceptable in preschool children.4 Even though FEV1 could be reached in preschoolers, their interpretation differs from school children and adults because their value is close to FVC and the FEV1/FVC ratio is higher than 90%, therefore it could be misleading in the presence of obstructive illnesses.7,10 For that reason, the recommendation for spirometry interpretation in preschoolers is to use FEV0.5, FEV0.75, and FEV0.5/FVC ratio.4,7,8,10 In the present study, FEV0.75 was not evaluated because it was not available in the software used. Other studies performed in preschoolers did not find significant differences in spirometry but did find significant differences in IOS bronchodilator response between asthmatics and healthy children13,15; however; those studies used FEV1 instead of FEV0.5 or FEV0.75.
The bronchodilator response determined as a change percentage with respect to theoretic values23 is recommended because it is independent of basal values, height and age.24 We calculated bronchodilator response as a changing percentage with respect to basal values because we did not have national reference values for our population (mostly Hispanic). The thresholds for a positive bronchodilator response in our study (Table 4) were similar to those obtained in other studies that included younger children.24,25 In a recent publication, designed to assess the reproducibility and repeatability of spirometry in preschool children, thresholds were 14% for FEV 0.75 and 33% for FEF25–75, because coefficients of repeatability were higher than that in our study.26 That could be explained because in the Borrego study they included a placebo inhaler, which may explain the greater variability and consequently an increase in the cut-off points for considering a significant response to bronchodilator.26 Using ROC curves, the best spirometric index to discriminate between asthmatics and healthy preschoolers in our study was FEF25–75 with a cut-off of 25% of bronchodilator response (sensitivity=41% and specificity=80%). In general, the spirometric values had poor sensitivity for asthma diagnosis (Table 4). The potential explanations could be that patients already had ICS therapy for more than three months and most of them reached asthma control, or because the doses of salbutamol used (200mcg) were too low. In another study, 400mcg of salbutamol in asthmatics in whom inhaled corticosteroids (aged 6–20 years) were used showed a significant bronchodilator response in 60% of the cases.21 Dundas et al.27 reported that a 9% change in FEV1 after 400mcg of salbutamol had 50% of sensitivity for asthma diagnosis among school age. Gallant et al.12 found that a 9% bronchodilator response in FEV1 (using 200mcg of albuterol) differentiated asthmatics from healthy children with 43% of sensitivity and 84% of specificity. Although Gallant's results are similar to ours, older children were included (4–17 years), they were not using inhaled corticosteroids, and nearly half of them were mild asthmatics.12
The discriminative power of bronchodilator response for asthma diagnosis among preschoolers was already evaluated using other techniques, such as Rint, IOS, and plethysmography.11,13–16,29 In one study, bronchodilator response using plethysmography had higher power to discriminate asthmatics from controls (sensitivity of 66% and specificity of 89%), compared to the other two techniques.11 McKenzie et al.28 found that bronchodilator response measured by Rint can distinguish wheezing from non-wheezing cough preschoolers (with 76% of sensitivity and 80% of specificity); however, those wheezing preschoolers did not use ICS. A recent study showed that IOS bronchodilator response could identify 77% of asthmatic children (3–18 years old) and excluded 76% of non-asthmatic patients, and FEV1 did not reveal differences between these two groups.13 Nevertheless, the results obtained with other lung function techniques (e.g. Rint, IOS, and plethysmography) cannot be totally compared with spirometry.29
The present study has some limitations that are important to mention. First, the sample is small, although we did reach the sample size required. Second, the dose of salbutamol used was low (200mcg) and maybe with higher doses more bronchodilator response can be observed in asthmatics. Third, it would be reasonable to test the bronchodilator response also in asthmatics without controller drugs when, like in this study, the purpose of spirometry is the confirmation of asthma diagnosed.
Spirometry in Chile is widely available and accessible around all the country, and it has a very high performance in preschoolers when the quality criteria are adapted to this age group. So, spirometry could be a very important tool in the management of preschoolers with clinical asthma diagnosis. We think that in order to find the discriminative power of spirometry bronchodilator response for asthma diagnosis in preschool children, we would need to do a study in asthmatic preschoolers before they start their control therapy, and with 400mcg of salbutamol. But still, bronchodilator response could be more useful for monitoring response to therapy than for diagnosis reassurance.30
In conclusion, Chilean preschoolers from this study had a very high performance spirometry and a bronchodilator response of 11% in FEV0.5 has a 30% sensitivity and 90% specificity for asthma diagnosis. We believe that it could be very important to design a study that will allow to better assess the utility of spirometry in the diagnosis and to follow-up preschool asthmatics.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.