INTRODUCTION
The search for a new subcutaneous immunotherapy administration schedule of the cluster or grouped type entails the consideration of various factors. In first place, it is necessary to have allergen extracts in which major allergens are correctly identified and quantified in micrograms/milliliter and minor allergens are identified. Of the standardization methods that are currently available, this is the one that best guarantees batch-to-batch equivalence between allergen extracts. In second place, the schedule must significantly reduce the number of doses and visits needed to reach the maintenance dose, compared to conventional schedules. This should reduce the cost of administering such treatments for both patients and the institutions where treatment is given, in addition to improving compliance with treatment. In third place, the schedule must be sufficiently flexible to allow dose adjustments, as needed, for factors such as the appearance of adverse reactions or an increase in allergen pressure.
Different multicenter studies have been carried out that take all these factors into consideration1,2, resulting in the development of a cluster schedule of extracts quantified in Mass Units of mites and pollens (olive and/or grasses), as well as the development of a conventional regimen with an important reduction in the number of doses required compared to the 13 doses traditionally recommended3. The tolerance demonstrated from the beginning was good. Nevertheless, the number of systemic reactions to pollens in a specific cluster suggested that the regimen should be modified to improve results. Once the above studies finalized, a second question emerged: Can a short-term clinical benefit be obtained with a cluster schedule administered in the months just before the pollen season?
In order to find a schedule that would reduce the percentage of adverse reactions and test a first approach that could produce a short-term benefit to patients, the present observational, open, prospective, multicenter, controlled study was designed.
MATERIAL AND METHODS
Patients
A total of 191 patients (ages 7 to 50 years) were included in 8 clinical groups. The inclusion criteria were: clinical history of seasonal allergic rhinitis or rhinoconjunctivitis with/without asthma symptoms due to sensitization to Olea europaea and/or a mixture of grasses (Dactylis, Festuca, Lolium, Phleum, and Poa) of at least 1 year duration, with positive skin tests and/or specific IgE in serum (≥ class 2, CAP Pharmacia, Uppsala, Sweden). Patients who had received previous immunotherapy, were sensitized to other clinically relevant allergens, could not receive the treatment under the supervision of an allergy specialist, or had a contraindication for the administration of immunotherapy according to WHO criteria were excluded from the study4. Of 191 patients, 34 acted as controls (these patients that met the same inclusion criteria, but were seen so close to pollen season that it was impossible to initiate immunotherapy). Therefore, they received only the symptomatic medication required for adequate control of their allergy symptoms.
Immunotherapy
Immunotherapy was administered subcutaneously using an extract of Olea europaea and/or a mixture of 5 grasses (Pangramin® Depot-UM, ALK-ABELLÓ, S.A., Madrid, Spain). In both cases, aluminum hydroxide gel-adsorbed allergen extracts were used. Extracts were biologically standardized with the major allergens (Ole e1 and Group 5 respectively) quantified in Mass Units, according to the methodology described by the manufacturer5.
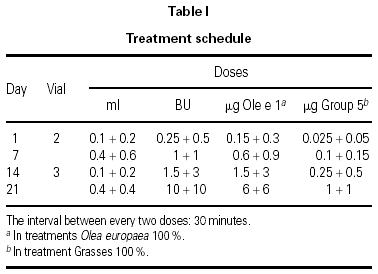
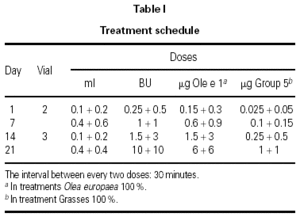
The dosing schedule used is shown in table I.
All doses were administered under the supervision of an allergist at each participating center. The clinical situation of each patient before each dose was evaluated following the instructions described in the Position Paper of the EAACI6.
No patient received premedication with antihistamines before any dose.
Safety monitoring
Adverse reactions were recorded according to the classification cited in the Position Paper of the EAACI6.
Assessment of clinical efficacy
Clinical efficacy was assessed using dairy cards to record symptoms and medication consumption during the pollen season. Symptoms were scored as follows: 0 (none), 1 (mild), 2 (moderate), and 3 (severe). In the case of medication consumption, the use of short-acting antihistamines (drops, oral, or parenteral formulations) and β2-agonists was recorded: 1 point/dose. Inhaled corticosteroids (nasal/bronchial): 2 points. Oral or parenteral corticoids: 3 points.
Modification of parameters in vitro
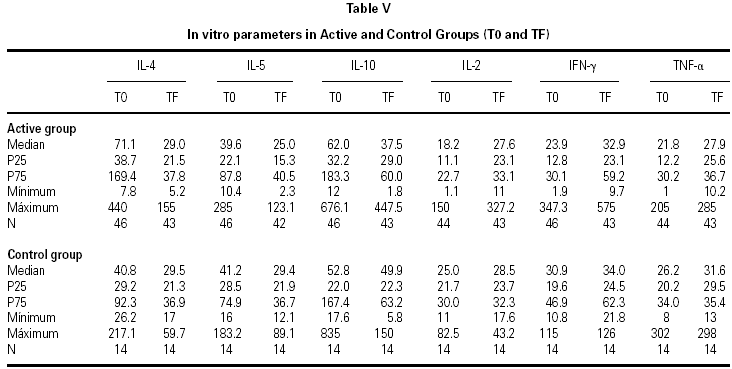
IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ determinations were carried out by means of flow cytometry (Cytometric Bead Array, BD Biosciences)7,8. Determinations were made by the Unit of Investigation of the Hospital Reina Sofía (Cordova). Samples were collected twice in the active and control groups: before initiating treatment (T0) and after finalizing follow-up (1 month after the end of pollen season, TF)
Statistical analysis
All statistical analyses were made with the SAS system, version 8.1. The association between variables was assessed with the Fisher exact test and intervals of confidence were calculated with a reliability of 95 % by means of the exact binomial method.
RESULTS
Sample characteristics
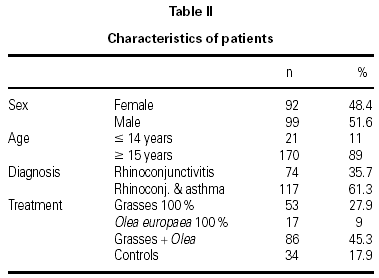
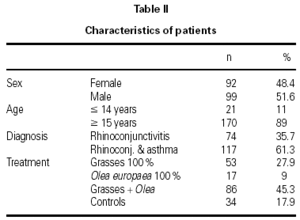
The characteristics of the patients are shown in table II. The mean age of the patients was 26.2 ± 10.2 years and the mean duration of their allergy was 5.8 ± 4.7 years. There were 6 withdrawals for occupational or familial reasons, which were unrelated to the study, and 1 in compliance with the study protocol, due to the presentation of two consecutive systemic reactions. Nonetheless, this patient continued to receive immunotherapy and attained the maximum dose without further incidents.
Treatment characteristics
A total of 1,580 doses were administered, 1,199 in the initiation phase and 381 in the maintenance phase. The number of patients given immunotherapy, type of extract used, and number of controls are shown in table II.
Tolerance
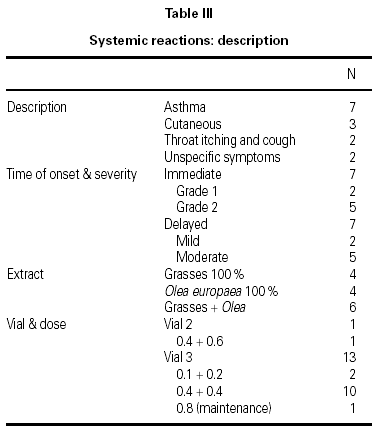
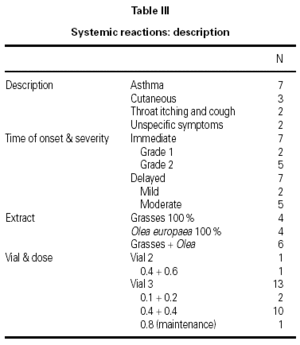
A total of 17 reactions were recorded in 15 patients. Three reactions were local and 14 were systemic. Systemic reactions occurred in 0.88 % of the doses administered and in 12 patients (7.6 % of all patients given immunotherapy). The reactions are described in table III. Of the 12 patients that had systemic reactions, 3 were treated with a 100 % extract of grasses, 3 with a 100 % Olea extract, and 6 with a mixture of both extracts; systemic reactions occurred in 5.7 %, 17.7 %, and 6.9 %, respectively, of the patients treated with each extract. These differences were not statistically significant. By age groups, only 1 patient under the age of 14 years had a systemic reaction; all the other systemic reactions occurred in older patients. Again, the differences were not statistically significant.
Follow-up of symptoms and medication consumption
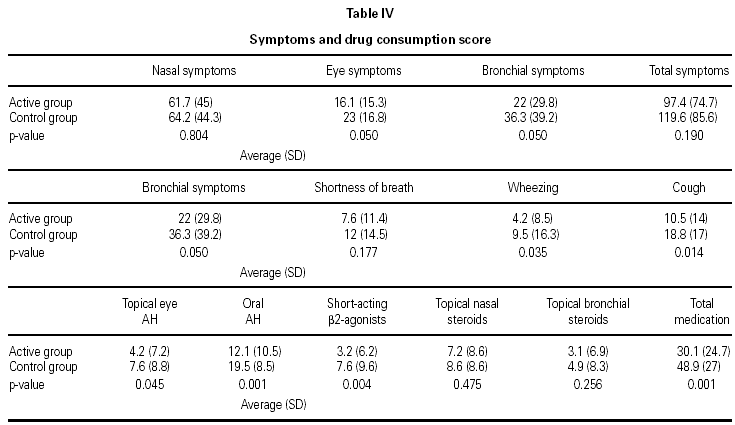
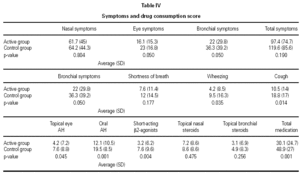
As shown in table IV, there was a significant decrease in the consumption of medications and frequency of symptoms between the active and control groups.
Determinations in vitro
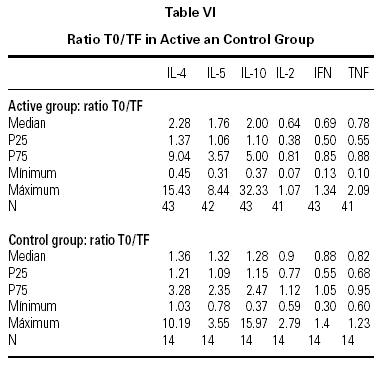
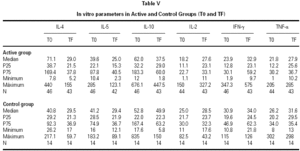
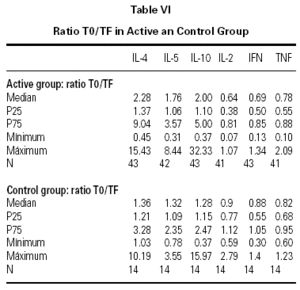
The descriptive data of the variables studied at T0 and TF are summarized in table V. Analysis of the fit to a normal distribution using the Kolmogorov-Smirnov and Shapiro-Wilk tests showed that the variables did not have a Gaussian distribution. Therefore, the evolution of these parameters in the two groups was studied by logarithmic transformation of the data. Results are given in table VI.
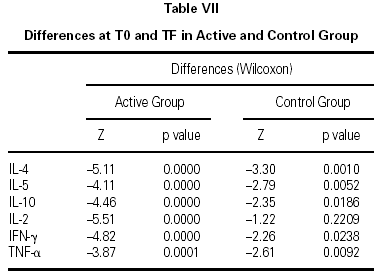
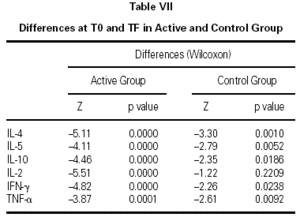
The immunological parameters changed in both groups over the course of the study period (T0-TF). The differences between the active and control groups at TF did not reach statistical significance, the most notable changes occurring in the IL-4 and IL-10 concentrations. All values are given in table VII.
DISCUSSION
The use of cluster schedules is becoming increasingly important, as indicated by the number of studies in which this type of initiation schedule has been used9. However, most of these studies were carried out at a single center or have small series, which makes it difficult to answer many of the questions inherent to the design of a schedule of this type: Is the schedule adaptable to different types of allergens? Does it have an adequate tolerance profile? Is the risk/benefit ratio favorable? Does it produce a savings in indirect costs to patients and health-care systems? Does raising the initiation doses with respect to those used in conventional schedules clinically benefit patients? Although it was not possible to answer all the questions that have been raised previously, the determination of certain investigators was fundamental for the development of the schedule proposed10,11. These studies provided the clinical base that facilitated the design and choice of the proposed schedule. The assumptions of this study derived from experience obtained previously in two multicenter studies carried out with mite and pollen extracts prepared by the same manufacturer1,2. In both cases, the same cluster schedule was used and a different tolerance profile was found with the two extracts. In the case of pollens, systemic reactions occurred in 1.2 % of doses, whereas in the case of mite extract, they occurred in only 0.3 % of doses. The appearance of delayed systemic reactions after the third cluster (0.1 + 0.2 mL of vial 3) with a pollen extract1 led us to consider several options for improving tolerance, finally choosing to increase the dose in the previous cluster from 0.4 + 0.4 mL of vial 2 to 0.4 + 0.6 mL of the same vial and await the effect.
The results showed a reduction in the percentage of systemic reactions from 1.2 % to 0.9 %, although the difference was not significant. In addition, there were no severe reactions. On the other hand, the increase in dose did not raise the number of local reactions, with only 3 being recorded in the present study. There were no severe systemic reactions (grade 3 or 4 according to the EAACI classification).
In addition, the fact that the schedule of 8 doses given in 4 visits could be maintained resulted in a net reduction in the number of doses and visits with respect to the conventional 13-dose schedule. The reduction was equivalent to 69 % for the number of visits and 38 % for the number of doses required to reach the maintenance dose.
Finally, we had one last question: Would this schedule produce short-term benefits? In view of the fact that, until now, almost all clinical trials of subcutaneous immunotherapy have assessed the clinical effect of this schedule after one or more years of treatment, it was necessary to plan the first clinical approach to evaluating the type of parameters that would be more sensitive to short-term changes. With this aim in mind, our controls were a group of patients who met the same inclusion and exclusion criteria as the patients who received immunotherapy, but had visited the physician at a time when the proximity to the pollen season made treatment with allergen vaccine inadvisable and therefore received only symptomatic medication to control allergy symptoms. This design disclosed significant differences in symptoms and medication consumption in favor of the patients who received immunotherapy. Although a double-blind, placebo-controlled study is needed to confirm these results, our findings indicate a possible short-term effect of allergen vaccines administered in the initiation phase at higher-than-usual initial allergen concentrations and following a schedule with a short initiation period and fewer doses to attain the maintenance dose. Likewise, the profile of 6 cytokines (IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ) was determined in both groups of patients before beginning treatment and after finalizing the follow-up of patients, one month after the end of the pollen season. The aim was to identify short-term immunological changes that might accompany clinical changes. We observed combined changes in the cytokine profile (a decrease in IL-4, IL-5, and IL-10 and an increase in IL-2, TNF-α, and IFN-γ) that appeared in a short interval of time in pollinized patients who received immunotherapy and symptomatic treatment or only symptomatic treatment. The changes in the control group of our study could be due to the action of symptomatic medications12,13, which were consumed in larger amounts by controls than by the active group. The changes in the active group were greater and could have been influenced by immunotherapy, as has been reported by other authors14. The explanation for these findings will have to be sought in larger samples of patients pollinized by natural exposure and in studies with a more prolonged observation of patients receiving immunotherapy versus those not receiving it.
Therefore, in view of the results obtained we can conclude that the changes in the schedule improved the tolerance profile without producing severe adverse reactions. Likewise, it appears that the use of this type of schedule can provide a short-term clinical benefit to patients, although these results must be confirmed in a double-blind, placebo-controlled study.