Since the discovery of immunoglobulin E (IgE) in the late 1960s, there has been an ongoing debate about the role of allergic sensitisation or ‘atopy’ (raised specific IgE levels and skin prick test (SPT) positivity to environmental allergens) in childhood eczema (also called ‘atopic dermatitis’ or ‘atopic eczema’). From early on, it became apparent that many individuals with childhood eczema had very high serum total IgE levels. In addition, eczematous skin is rich in IgE-bearing antigen-presenting dendritic cells, and topical application of allergens, such as house dust mite, can induce skin inflammation in sensitised individuals, as demonstrated in the atopy patch test. But does allergic sensitisation really play a key role in eczema aetiology? In other words, is sensitisation to environmental allergens a primary event in the development of childhood eczema or is atopy an epiphenomenon of the disease process?
Assuming a uniform causal role for allergic sensitisation, one would expect a strong association between atopy and childhood eczema with little variation between populations. There should be a dose-response-relationship between the degree of allergic sensitisation (number of positive SPTs or raised specific IgE levels) and eczema probability, and, most importantly, allergic sensitisation should occur before clinical eczema develops.
The strength of the association between atopy and childhood eczemaA recent systematic review of the literature has shown that the prevalence of allergic sensitisation among eczema sufferers is lower than originally thought, ranging between 7 % and 75 %1. Whereas up to three quarters of hospital patients are sensitised, in community settings sensitisation rates can be below 10 %. One explanation for this discrepancy is disease severity, with more severe cases being seen in hospital departments2.
It may well be that severely inflamed eczematous skin allows easier contact between IgE-bearing antigen-presenting cells and environmental allergens across an impaired skin barrier. Indeed, the majority of epidemiological studies that have examined the link between eczema severity and allergic sensitisation have confirmed a significant positive association. For instance, the Early Prevention of Asthma in Atopic Children (EPAAC) Study conducted among over 2,000 children age 13 to 24months with established moderate to severe childhood eczema from 10 European countries, Australia and South Africa demonstrated that the frequency of positive specific IgE responses to a standard panel of food and inhalant allergens increased with greater eczema severity3.
Another factor that might explain part of the varying degrees of association between eczema phenotype and sensitisation is geography. The EPAAC Study has shown great variation in sensitisation rates to foods and aeroallergens among children with eczema between countries, with particularly high prevalences of atopic eczema found in Australia (83 %), the UK (79 %), and Italy (76 %), whereas infants with eczema from Belgium had significantly lower sensitisation rates (52 %). Some of these differences may be due to varying degrees of environmental allergen exposure, but other lifestyle related influences are likely to be involved, as a number of population-based cross-sectional studies from across the globe have suggested a significantly stronger association between allergic sensitisation and eczema in developed compared to developing nations1.
As part of the second phase of the International Study of Asthma and Allergies in Childhood (ISAAC), around 30,000 schoolchildren aged 8 to 12years old from 20 affluent and non-affluent countries were physically examined for flexural eczema, using the same standardised diagnostic criteria and physical examination4. Children were also skin prick tested to a panel of six common environmental allergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, Alternaría tenuis, mixed tree and grass pollen). Investigators were encouraged to add allergens of local relevance. Large variations in the proportion of allergic sensitisation among children with flexural eczema were observed across study centres. Not a single child with eczema was sensitised in Ghana and Palestine, while 74 % of eczema sufferers in Hong Kong had at least one positive SPT. The odds of allergic sensitisation in children with flexural eczema vs healthy controls varied correspondingly between 0.74 (95 % CI 0.31-1.81) in Pichincha (Ecuador) and 4.53 (95 % CI 1.72-11.93) in Madrid (Spain). It is interesting to note that the ISAAC Study found a significant association between a country's socio-economic status (based on World Bank criteria) and the strength of the association between atopy and flexural eczema, with significantly stronger associations seen in affluent compared to non-affluent settings (combined ageand sex-adjusted odds ratio (OR)affluent = 2.69 [95 % CI 2.31-3.13] vs ORnon.affluent = 1.17 [95 % CI 0.81-1.70]). However, there was a linear relationship between the number of positive SPT responses and the probability of having flexural eczema in the majority of affluent study centres. While the ISAAC Study results overall point towards an association between allergic sensitisation and childhood eczema due to shared aetiologies linked to a ‘western lifestyle’ and affluence rather than direct causality, causality cannot be excluded where a dose-response-relationship between the number of positive SPTs and flexural eczema probability was observed.
The temporal relationship between atopy and childhood eczemaThe main dilemma is that the majority of studies performed to date are cross-sectional in design. Only longitudinal and intervention studies can infer causality, however, and what is needed are well-designed birth cohorts that closely investigate the temporal relationship between allergic sensitisation and eczema phenotype. A recent Australian cohort study among 500 at risk children followed up from birth until age 5 found that allergic sensitisation at 18months was not associated with eczema at 5years (adjusted OR = 0.78, 95 % CI 0.23-2.64) 5. In the same cohort eczema phenotype at 18months was a predictor of allergic sensitisation at 5years (adjusted OR = 1.67, 95 % CI 1.20-2.33), suggesting that sensitisation is a secondary rather than primary phenomenon in childhood eczema. This is in keeping with findings from a UK birth cohort among almost 600 children, where no clear relationship between levels of house dust mite exposure at 2months of age, and eczema and house dust mite sensitisation risk at 8years could be found6. In fact, higher levels of exposure to house dust mite at 2years of age appeared to confer a degree of protection on later eczema and sensitisation risk, although results missed statistical significance and require replication.
The ‘atopic march’A number of studies have suggested that early allergic sensitisation in childhood eczema predicts later allergic respiratory disease, and some have gone so far as to suggest that children with eczema almost invariably progress to allergic respiratory disease in later childhood: the so-called ‘atopic march’7. This concept was originally based on cross-sectional study evidence and only few prospective cohort studies have been conducted. A recent meta-analysis of 13 longitudinal studies suggested a pooled odds ratio of 2.14 (95 % CI, 1.67-2.75) for the risk of asthma after eczema, compared with children without eczema, with only about a third of eczema sufferers developing asthma at a later stage8. Some studies have shown that only children with eczema plus sensitisation have a higher risk of allergic respiratory disease9, but, as mentioned above, similar observations have even been made in those with allergic sensitisation alone1,5. To complicate matters further, the Melbourne Atopy Cohort Study, a prospective birth cohort among 552 infants with a family history of allergic disease, has recently suggested that allergic sensitisation to foods at 6months of age can predict eczema development up to age 7 (hazard ratio (HR) = 1.63, 95 % CI 1.13-2.35) in those who develop eczema after the first 6months of life, which would be in keeping with a causal relationship between atopy and later childhood eczema. In infants who get the disease before 6months of age the risk of allergic sensitisation within the first two years of life was also significantly increased (HR = 3.47, 95 % CI 1.65-7.32)10.
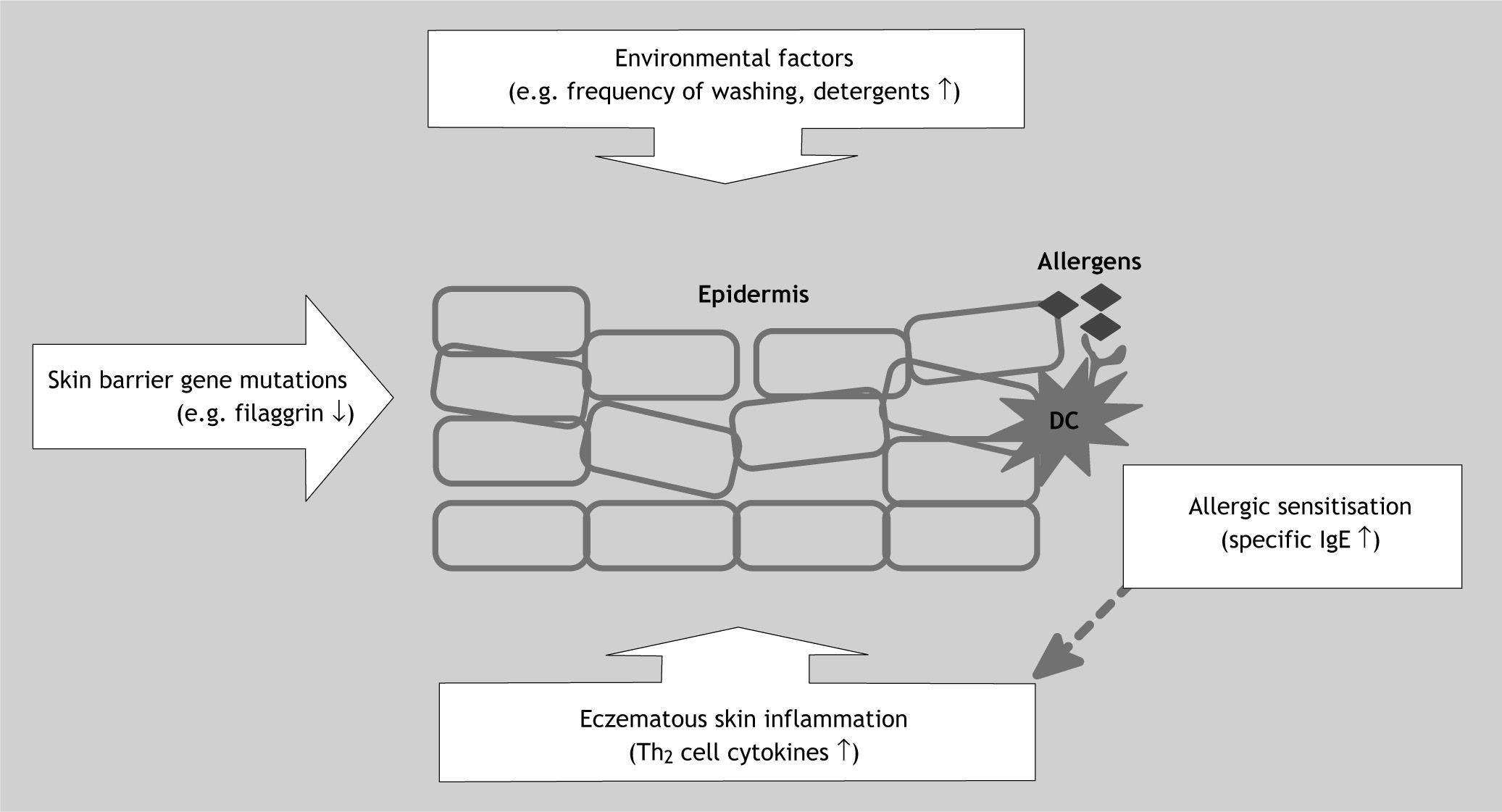
Skin barrier impairment – the missing link?Skin barrier impairment may provide the missing link between allergic sensitisation and childhood eczema and could explain some of the seemingly contradictory study findings discussed above. Mutations in the filaggrin and other skin barrier genes located in the epidermal differentiation complex (EDC) on chromosome 1q21 are strongly associated with childhood eczema, but genetics alone cannot explain the dramatic increase in eczema prevalence over past decades7,11. Environmental factors, such as frequent use of detergents and water hardness, must also play an important role, and through interaction with genetic factors are thought to contribute to skin barrier breakdown (reduction in natural moisturising factor, increase in skin pH and subsequent increase in protease activity), which could represent the first step on the way to eczematous skin inflammation12. Skin barrier impairment without or, even more likely, with inflammation could facilitate contact between environmental allergens, such as house dust mite, and antigen-presenting cells in the epidermis, a process which is likely to drive skin inflammation further (Fig. 1). Support for the hypothesis that disruption of the epidermal skin barrier is an important event in the pathogenesis of childhood eczema comes from a recent German family study. Intriguingly, filaggrin loss-of-function skin barrier gene mutations were more strongly associated with atopic rather than non-atopic eczema. The same was found in three further German and one North American paediatric population13–16. In one study, allergic sensitisation was only associated with filaggrin mutations in the presence of clinical eczema, underlining the role of eczematous inflammation in addition to genetically predetermined skin barrier impairment in the development of allergic sensitisation and respiratory allergy16. In this explanatory model, IgE-driven processes can amplify the inflammation cascade in eczematous skin, partly by leading to further skin barrier breakdown, but allergic sensitisation is not an essential prerequisite of clinical eczema17. This would fit the observation that a significant proportion of children, especially with mild disease, are not sensitised and would also explain why even strict house dust mite avoidance has proven to be a disappointing strategy in eczema prevention and treatment.
Epidemiological studies have shown stronger associations between allergic sensitisation and childhood eczema in hospital vs community populations, partly explained by differences in disease severity. In most cases, clinical eczema precedes allergic sensitisation, but the opposite scenario also occurs. The current hypothesis is that skin barrier breakdown lies at the centre of the association between atopy and eczema. Gene mutations in the epidermal differentiation complex on chromosome 1q21 predispose to skin barrier impairment. Environmental factors, such as frequent washing and detergent use, can further contribute to skin barrier breakdown, eventually resulting in an upregulation of type 2T helper cell cytokines, such as IL-4, IL-5 and IL-13. Contact between environmental allergens and antigen-presenting dendritic cells in the epidermis leads to allergic sensitisation. The latter is probably primarily a secondary phenomenon as a consequence of skin barrier impairment, but raised specific IgE levels can also amplify existing eczematous skin inflammation. DC: dendritic cell; IgE: immunoglobulin E; Th2: type 2T helper cells.
What is now required are large birth cohort studies with clear diagnostic criteria to examine the intricate relationship and sequence of events between skin barrier gene mutations, phenotypic skin barrier impairment, and the immunological changes associated with clinical eczema, its severity, age of onset, chronicity and allergic sensitisation. The potential impact on clinical practice is significant, as the delineation of subtypes of childhood eczema may allow us to develop tailor-made preventative and therapeutic strategies. For example, if children with filaggrin mutations were to develop skin barrier impairment prior to clinical eczema and allergic sensitisation and given the increased allergic respiratory disease risk in children with atopy, topical skin barrier-restoring treatments could help to not only prevent eczema development but also allergic sensitisation and through this later allergic respiratory disease.
Conflict of interestThe author has no conflict of interest to declare.