Cow's milk allergy (CMA) is the most frequent food allergy in childhood.1 Treatment consists of cow's milk proteins eviction, with the use of an extensively hydrolysed milk formula (eHF) being the first option in most cases, due to its good tolerance, availability and cost.1 Few cases of IgE-mediated allergy to eHF have been reported2–6 but oral tolerance induction (OIT) has not been previously described in children with allergy to eHF.
We report the case of a boy, referred to our Immunoallergy department in 2010, at the age of five years, whose parents reported a severe IgE-mediated CMA diagnosed at six months of age following an episode of anaphylaxis, with generalised urticaria, angio-oedema, rhinoconjunctivitis and wheeze after yoghourt ingestion, and vomiting after eating a milk-containing puree. The child began cow's milk avoidance and was started on an eHF (Aptamil®Pepti Junior, Milupa). However, several episodes of reproducible urticaria occurred immediately after eHF introduction, for which it was stopped and a soy milk formula was prescribed. At that time the child had specific IgE (ImmunoCAP®, Phadia – Thermo Fisher Scientific, Uppsala, Sweden) positive for whole cow's milk (55.2kU/L), casein (54.8kU/L), β-lactoglobulin (BLG) (16.6kU/L) and α-lactalbumin (ALA) (42.9kU/L), and negative for soy. Due to food protein-induced enterocolitis syndrome with soy milk, manifesting as recurrent diarrhoea beginning three weeks after soy milk introduction, the child was started on an aminoacid formula (Neocate®, Nutricia), which he maintained at the time of the consultation with good tolerance and adequate growth. The parents reported several episodes of contact urticaria on occasional mucocutaneous contact with cow's milk, and no episodes of accidental cow's milk ingestion.
The child also presented with symptoms of asthma since early childhood, controlled with inhaled fluticasone through a spacer device, mild persistent rhinitis treated with nasal mometasone furoate, mild atopic dermatitis and a family history of atopy (father and brother with asthma and rhinitis).
Skin prick tests (SPT) (Laboratorios Leti, Madrid, Spain) at the age of five years were positive for whole cow's milk (mean wheal diameter=9mm), casein (10mm), BLG (9mm), ALA (8mm) and eHF (Aptamil®Pepti Junior, Milupa) (8mm). As a control, due to the fact that the SPT with eFH is not standardised, we performed SPT with this eHF in 10 CMA children, which was negative in all of them. SPT were negative for aeroallergens and soy extract. Specific IgE (ImmunoCAP®, Phadia – Thermo Fisher Scientific) was positive for whole cow's milk (36.0kU/L), casein (33.4kU/L), BLG (12.3kU/L) and ALA (22.1kU/L).
At the age of six years, an open oral provocation test with cow's milk was performed, which was positive 17min after ingestion of 1mL of cow's milk, with generalised urticaria, rhinoconjunctivitis and palpebral oedema. The child presented no respiratory, gastrointestinal or cardiovascular symptoms and was treated with oral antihistamine and corticosteroid with complete resolution in 3h.
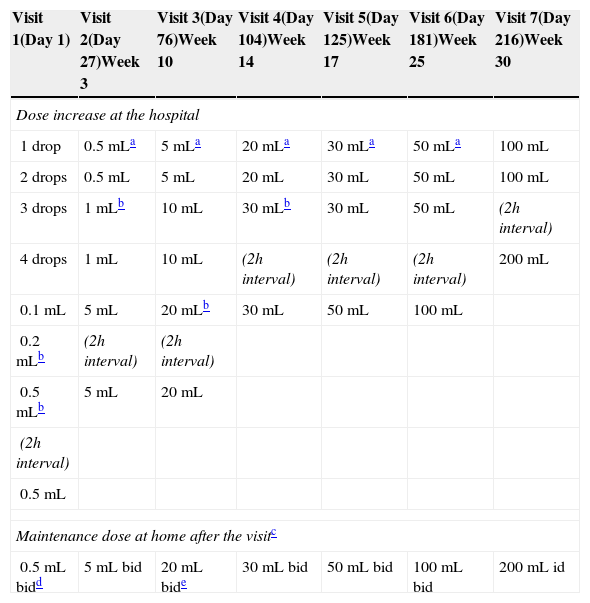
Due to the persistence of cow's milk allergy at the age of six years, an oral tolerance induction (OTI) procedure was proposed and consented by the parents, which was completed in 30 weeks (Table 1). Since then, the child maintains a daily intake of 200mL once a day, with progressive introduction of other milk containing foods with tolerance.
Cow's milk oral tolerance induction protocol.
| Visit 1(Day 1) | Visit 2(Day 27)Week 3 | Visit 3(Day 76)Week 10 | Visit 4(Day 104)Week 14 | Visit 5(Day 125)Week 17 | Visit 6(Day 181)Week 25 | Visit 7(Day 216)Week 30 |
|---|---|---|---|---|---|---|
| Dose increase at the hospital | ||||||
| 1 drop | 0.5mLa | 5mLa | 20mLa | 30mLa | 50mLa | 100mL |
| 2 drops | 0.5mL | 5mL | 20mL | 30mL | 50mL | 100mL |
| 3 drops | 1mLb | 10mL | 30mLb | 30mL | 50mL | (2h interval) |
| 4 drops | 1mL | 10mL | (2h interval) | (2h interval) | (2h interval) | 200mL |
| 0.1mL | 5mL | 20mLb | 30mL | 50mL | 100mL | |
| 0.2mLb | (2h interval) | (2h interval) | ||||
| 0.5mLb | 5mL | 20mL | ||||
| (2h interval) | ||||||
| 0.5mL | ||||||
| Maintenance dose at home after the visitc | ||||||
| 0.5mL bidd | 5mL bid | 20mL bide | 30mL bid | 50mL bid | 100mL bid | 200mL id |
OIT initiated with sublingual drops with 20-min interval. Oral doses with 30-min interval between intakes, except when specified otherwise.
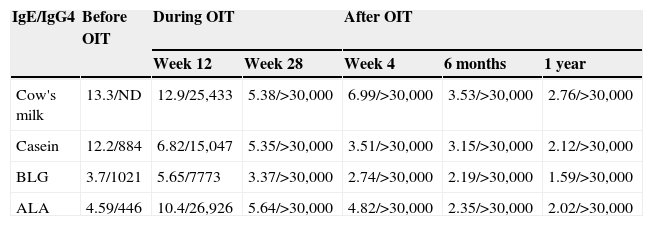
Skin prick tests four weeks after OIT were positive for cow's milk (4mm), casein (4mm), BLG (5mm) and ALA (3mm) and negative for eHF. Skin prick tests one year after OIT were positive for casein (3mm), BLG (4mm) and ALA (3mm) and negative for cow's milk. Data on specific IgE and IgG4 before, during and after OIT are presented in Table 2.
Specific IgE (kU/L) and IgG4 (mg/L) to cow's milk and cow's milk proteins before, during and after oral tolerance induction.
| IgE/IgG4 | Before OIT | During OIT | After OIT | |||
|---|---|---|---|---|---|---|
| Week 12 | Week 28 | Week 4 | 6 months | 1 year | ||
| Cow's milk | 13.3/ND | 12.9/25,433 | 5.38/>30,000 | 6.99/>30,000 | 3.53/>30,000 | 2.76/>30,000 |
| Casein | 12.2/884 | 6.82/15,047 | 5.35/>30,000 | 3.51/>30,000 | 3.15/>30,000 | 2.12/>30,000 |
| BLG | 3.7/1021 | 5.65/7773 | 3.37/>30,000 | 2.74/>30,000 | 2.19/>30,000 | 1.59/>30,000 |
| ALA | 4.59/446 | 10.4/26,926 | 5.64/>30,000 | 4.82/>30,000 | 2.35/>30,000 | 2.02/>30,000 |
ND, not done.
Milk allergens are known to preserve their biological activity even after boiling, pasteurisation, ultra-high temperature processing or evaporation, which is the reason why extensive hydrolysis is necessary to obtain hypoallergenic formulas (1); eHFs can be produced from casein or whey proteins and have been characterised as those in which ≥95% of peptides have a molecular weight <1500Da and less than 0.5% have a molecular weight >6000Da (2), although there is no agreement on these criteria.1
Allergic reactions, IgE and non-IgE mediated, to both casein and whey protein eHFs, have been previously reported (2–6). Our patient presented with anaphylaxis to cow's milk and urticaria to an extensively hydrolysed whey protein formula due to an IgE-mediated mechanism, confirmed by skin prick test positivity.
Although CMA is mainly due to the IgE binding to conformational epitopes, Matsumoto et al.7 have demonstrated the IgE binding to linear epitopes from caseins and also from whey proteins such as ALA and BLG and that the pattern of IgE and IgG4 reactivity to these epitopes could differentiate sensitisation from transient and persistent CMA. Moreover, despite extensively hydrolysed casein formula showing a better safety profile than that of whey hydrolysates,3 it still contains trace amounts of casein and whey,8 which may be responsible for the clinical reactivity in some patients.
No previous studies have compared children's allergy and tolerance to eHF in relation to severity and duration of CMA, or to values of specific IgE or skin prick tests to cow's milk and proteins, but one might hypothesise that allergy to eHF could be a marker of severity, since children react to linear epitopes and/or to trace amounts of cow's milk proteins, which is also consistent with the high specific IgE found in our patient prior to OIT.
García-Ara et al.9 have reported that milk OIT is achieved earlier in children with low specific IgE and that those allergic children with higher cow's milk specific IgE levels (>11.4kU/L) experience more frequent and severe adverse reactions during the procedure. Morais-Almeida et al.10 have reported achievement of a 200mL maintenance dose in a mean of four (from 3 to 6) hospital visits and 12 (from 6 to 20) weeks in children with anaphylaxis to cow's milk, using the same OIT protocol, with 2/10 children presenting anaphylactic reactions and 8/10 children presenting a total of 13 mild to moderate reactions treated with oral antihistamines or corticosteroids during induction phase. This is in contrast with the longer period of time (30 weeks) and number of visits (seven visits) needed to achieve tolerance in the reported patient and to the more frequent adverse reactions, although all the reactions were mild to moderate (five reactions at the hospital and four at home).
To the best of our knowledge, this is the first report of a patient with eHF allergy treated with OIT. We emphasise that despite OIT taking a longer time and with more adverse events than previously reported by the authors,10 tolerance of 200mL of cow's milk plus a free diet was achieved and a decrease of specific IgE/IgG4 ratio as well as in skin prick test size to whole cow's milk and its proteins was observed. Moreover, the previously positive skin prick test to the eHF turned negative after successful OIT.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that the parents of the patient included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the parents of the patient mentioned in the article. The author for correspondence is in possession of this document.