The prevalence of atopic diseases in children with type 1 diabetes mellitus (DM1) has been reported as lower. The aim of this study was to evaluate the prevalence of allergic diseases and allergic sensitisation in Brazilian children and adolescents with DM1.
Patients and methods96 patients with DM1 (aged 4–18 years, 45 boys) followed for at least one year were evaluated for allergic disease through a detailed allergological anamnesis and skin prick tests (SPT) to inhalant allergens (Dermatophagoides pteronyssinus, D. farinae, Blomia tropicalis, Blattella germanica, Periplaneta americana, dog epithelium, cat epithelium, mix fungi), foods (cow's milk, egg-white, yolk, soy, wheat, corn), and positive (histamine 1mg/ml) and negative (saline) controls. Wheals with a mean diameter of induration equal to or greater than 3mm identified a positive SPT.
ResultsThe prevalence values of rhinitis, asthma and atopic eczema (isolated or associated) were 68.0%, 59.1% and 44.4%, respectively. 20.6% of the patients had no allergic disease. 46.8% of the patients had been diagnosed with DM1 for at least four years and there was no relationship between the period of DM1 and the presence of allergic disease, nor of the gender. 48.0% patients were sensitised with predominance of D. pteronyssinus, B. topicalis and D. farinae. The frequency of positive SPT was significantly higher among patients with history of allergic disease (OR=6.98, 95%CI: 2.60–18.74, p<0.001).
ConclusionThe prevalence of allergic diseases and sensitisation in patients with DM1 was higher than usually expected and deserves further investigation to identify possible causes for these findings and to evaluate their importance and influence on the metabolic control.
Data about the duality of the immune response mediated by CD4+T helper (Th) lymphocytes enabled a better understanding of the participation of these cells in the pathogenesis of various allergic diseases and autoimmunity. Both types of Th lymphocytes identified (Th1 and Th2) depend on the profile of the cytokines they produce. Th1 cells are involved in cell-mediated immunity and phagocyte-dependent inflammation producing interferon-gamma (IFN-γ), interleukin (IL) -2 and tumour necrosis factor beta (TNF-β). Additionally, Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 and participate in antibody response (including IgE), eosinophil accumulation and inhibit various functions of phagocytic cells (inflammation independent of phagocytes). Both genetic and environmental factors act jointly to determine the opposite response towards Th1 or Th2.1
Thus far, activation of Th1 cells gives the host protection against infections by intracellular bacteria as well as protection in the pathogenesis of autoimmunity diseases such as Crohn's disease, sarcoidosis, acute renal transplant rejection, type 1 diabetes mellitus (DM1). In contrast, Th2 immune response protects against infection by extracellular agents, but in genetically susceptible individuals determines the allergen-specific sensitisation and development of atopic diseases. Similarly, it has also a role in the pathogenesis of progressive systemic sclerosis, cryptogenic fibrosing alveolitis, and accelerated development of HIV infection.1
Regarding the frequency of autoimmune and allergic diseases, some studies have evaluated a possible decrease in the prevalence of asthma and/or allergic diseases among patients with DM1. Although some reports refer to an inverse relationship between both, there are conflicting results, possibly due to the inadequacy of studies’ design, including the small number of patients evaluated. Nonetheless, the classification of the immune response based on pure Th1 or pure Th2 is very simplistic and may not explain subsequent reports of association between DM1 and asthma and/or allergic diseases.2
Despite the controversial aspects and several limitations of previous studies there is some evidence that DM1 could possibly be a protective factor against the development of allergic disease in children. Recently, a meta-analysis assessed 25 studies evaluating the association between atopic disease (asthma, allergic rhinitis and eczema) in children with DM1 and documented a significant inverse association between asthma and DM1. However, no associations between DM1 and eczema and allergic rhinitis were confirmed.3
The aim of this study was to evaluate the frequency of atopic diseases and sensitisation to inhalant and food allergens in patients with DM1.
Materials and methodsStudy populationThis was a descriptive cross-sectional study carried out with 96 patients with DM1 (47 males), aged between 3 and 18 years, followed at the Division of Pediatric Endocrinology, Department of Pediatrics, Escola Paulista de Medicina-Federal University of São Paulo (EPM-UNIFESP), Brazil. The diagnosis of DM1 was performed considering clinical and laboratorial parameters.4 All patients were under daily replacement of insulin (NPH, twice a day and insulin regular multiple doses) and appropriate nutritional programme. Only patients followed for at least one year were included in the study. Few patients were under other medications, as follows: enalapril (2), laevothyroxine (2), fluoxetine (2), methotrexate (1), phenobarbital (1), laevothyroxine (1), azathioprine and methotrexate (1), phenobarbital (1), fenoxetina (1) and alendronate (1).
International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaireDuring clinical evaluation, parents and/or patients were interviewed and filled in the ISAAC's written questionnaire (WQ),5 previously validated to Portuguese (Brazilian culture).6 According to the answers, patients were defined as follows: with asthma if they answer affirmatively to the question “had wheezing in the past 12 months”; with rhinitis if they answer affirmatively to “had nasal symptoms without a flu or cold in the last 12 months”; and with atopic eczema if they answer affirmatively to “had skin rash that appeared and disappeared in the last 12 months or rash in “characteristic regions”.5
Skin prick testsAll the patients underwent skin prick tests (SPT) using the puncture technique (7) with the following food and inhalant allergens: cow's milk, soy, corn, wheat, peanut, egg (yolk and white), fish, Dermatophagoides pteronyssinus, D. farinae, Blomia tropicalis, Blattella germanica, Periplaneta americana, dog epithelium, cat dander, fungi mix, as well as a positive control (histamine 1mg/ml) and a negative control (excipient) (IPI-ASAC® and ALC® Brazil). A wheal with a mean diameter equal to or 3mm bigger than negative control was characterised as a positive SPT.7 Patients with at least one positive SPT were considered as allergic.
This study was approved by the Ethical Research Committee of UNIFESP-EPM. Patients or parents, when appropriate, signed the informed consent to participate in the study.
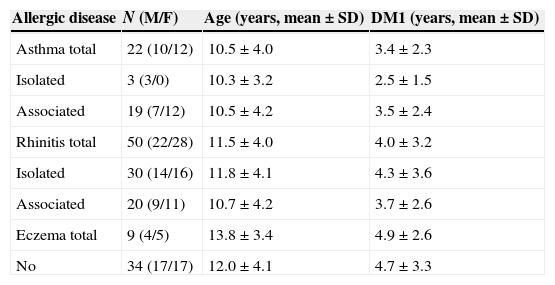
ResultsAmong the 96 patients evaluated 48.9% were male and 46.8% had had DM1 for at least four years with no differences according to gender. With respect to allergic diseases, 64.6% (62/96) reported a minimum of one allergic disease during life, with rhinitis being the most prevalent one, alone or associated with other allergic diseases. 62.2% (28/45) of boys and 66.7% (34/51) of girls reported having an allergic disease associated to DM1; thus showing that gender did not exert any influence upon the prevalence of allergic diseases (OR=0.82, 95%CI: 0.36–1.90, p=0.67). Similarly, the duration of DM1 had no impact on the frequency of allergic disease: 61.7% (29/47) had DM1 for more than four years and 67.3% had DM1 for less than four years (OR=0.78, 95%CI: 0.34–1.81, p=0.67) (Table 1).
Distribution of patients according to allergic disease associated or not to diabetes mellitus type I (DM1).
| Allergic disease | N (M/F) | Age (years, mean±SD) | DM1 (years, mean±SD) |
|---|---|---|---|
| Asthma total | 22 (10/12) | 10.5±4.0 | 3.4±2.3 |
| Isolated | 3 (3/0) | 10.3±3.2 | 2.5±1.5 |
| Associated | 19 (7/12) | 10.5±4.2 | 3.5±2.4 |
| Rhinitis total | 50 (22/28) | 11.5±4.0 | 4.0±3.2 |
| Isolated | 30 (14/16) | 11.8±4.1 | 4.3±3.6 |
| Associated | 20 (9/11) | 10.7±4.2 | 3.7±2.6 |
| Eczema total | 9 (4/5) | 13.8±3.4 | 4.9±2.6 |
| No | 34 (17/17) | 12.0±4.1 | 4.7±3.3 |
ANOVA – no differences in the age and length of DM1.
M – male; F – female; SD – standard deviation.
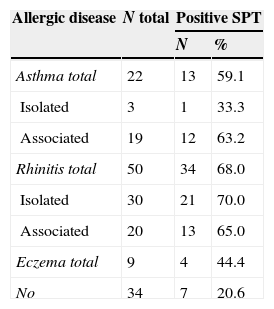
The prevalence values of asthma, rhinitis and atopic eczema were: 22.9%, 52.1% and 9.4%, respectively (Table 2). The evaluation of allergic sensitisation, regardless of the allergic disease presented, revealed that 46.9% (45/96) had positive SPT. There were no differences regarding gender: 46.7% (21/45) of boys and 49.0% (25/51) girls (OR=0.91, 95%CI 0.41–2.03, p=0.9) and duration of DM1. Patients with asthma and/or rhinitis showed a high frequency of sensitisation when compared to those with AD. Positive SPT were observed in 20.6% of DM1 patients considered without allergic disease (OR=6.98; IC95%: 2.60–18.74; p<0.001) (Table 2).
Distribution of diabetes mellitus type I patients according to allergic disease and positive skin prick test (SPT).
| Allergic disease | N total | Positive SPT | |
|---|---|---|---|
| N | % | ||
| Asthma total | 22 | 13 | 59.1 |
| Isolated | 3 | 1 | 33.3 |
| Associated | 19 | 12 | 63.2 |
| Rhinitis total | 50 | 34 | 68.0 |
| Isolated | 30 | 21 | 70.0 |
| Associated | 20 | 13 | 65.0 |
| Eczema total | 9 | 4 | 44.4 |
| No | 34 | 7 | 20.6 |
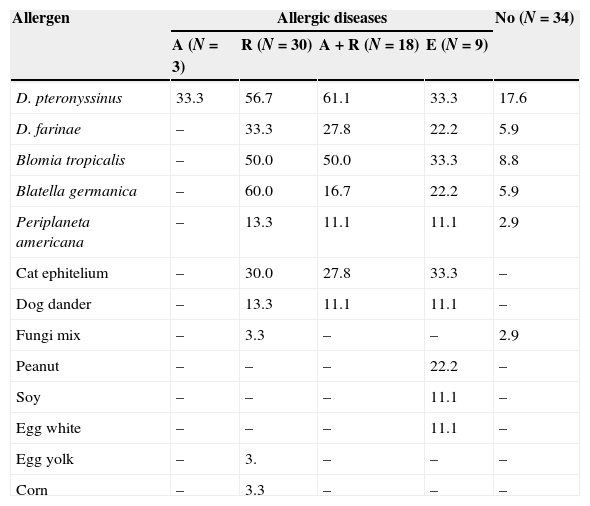
Sensitisation to house dust mite and cockroach was more prevalent independently of the allergic disease presented. There was no association between severe asthma (4/7 or 57.1%) and higher frequency of allergic sensitisation (OR=0.89, 95%CI: 0.14–5.48; p=1.0). Sensitisation to food allergens was low (Table 3).
Frequency of positive skin prick test to different allergens in diabetes mellitus type I patients according to allergic disease presented: asthma (A), rhinitis (R), asthma and rhinitis (A+R), eczema (E) or not.
| Allergen | Allergic diseases | No (N=34) | |||
|---|---|---|---|---|---|
| A (N=3) | R (N=30) | A+R (N=18) | E (N=9) | ||
| D. pteronyssinus | 33.3 | 56.7 | 61.1 | 33.3 | 17.6 |
| D. farinae | – | 33.3 | 27.8 | 22.2 | 5.9 |
| Blomia tropicalis | – | 50.0 | 50.0 | 33.3 | 8.8 |
| Blatella germanica | – | 60.0 | 16.7 | 22.2 | 5.9 |
| Periplaneta americana | – | 13.3 | 11.1 | 11.1 | 2.9 |
| Cat ephitelium | – | 30.0 | 27.8 | 33.3 | – |
| Dog dander | – | 13.3 | 11.1 | 11.1 | – |
| Fungi mix | – | 3.3 | – | – | 2.9 |
| Peanut | – | – | – | 22.2 | – |
| Soy | – | – | – | 11.1 | – |
| Egg white | – | – | – | 11.1 | – |
| Egg yolk | – | 3. | – | – | – |
| Corn | – | 3.3 | – | – | – |
Cow's milk, wheat and fish – all negative.
The prevalence of allergic diseases has increased in the world as a whole, particularly in developing countries.8–10 In addition, a similar trend has been observed with respect to DM1 in children11,12 but with a very wide range on the disease prevalence, from 0.1 per 100,000 inhabitants/year in China and Venezuela to 37 per 100,000 inhabitants/year in Finland and Sardinia.11 In Brazil, data on DM1 prevalence shows the same features, with a variation from 7.6/100,000inhabitants/year in São Paulo13 to 12/100,000inhabitants/year in Rio Grande do Sul.14
The prevalence of allergic symptoms reported in our patients with DM1 was elevated as follows: rhinitis in 52.1% (alone 20.8%; associated 31.8%), asthma in 22.9% and atopic eczema by 9.4%. Although there is no matched control group in this study, the prevalence of allergic rhinitis observed was higher than that documented among non-diabetic children (28.2%) and adolescents (27.4%) living in the south-central city of São Paulo, evaluated through the same instrument of evaluation, ISAAC WQ.15
Regarding asthma and AD, the differences were not as intense: 22.9% (6–7 years) and 18.7% (adolescents) for asthma and 11.0% (6–7 years) and 7.1% (adolescents) for AD.15 These data are opposed to those reported by other studies, which documented DM1 as a protective factor against the development of allergic diseases.16–18 A meta-analysis conducted to assess the association between atopic diseases and DM1 evaluated 25 studies and concluded by a small but significant reduction in the prevalence of asthma in children with DM1 but without other influences on the other allergic diseases.3
In a recent study, the prevalence values of asthma, rhinoconjunctivitis and atopic eczema obtained by ISAAC phase 1 were compared with the incidence of DM1 (per 100,000/year; Diabetes Mondiale Project Group study) from 31 countries participating simultaneously in both studies. A positive correlation between the incidence of DM1 and the prevalence of wheezing and atopic eczema was observed. There was no correlation between the incidence of DM1 and the prevalence of rhinitis or rhinoconjunctivitis. These data question the role of the environment on the expression of these diseases.19
The prevalence of sensitisation (positive SPT) among DM1 patients identified as having allergic disease by ISAAC WQ was 72.6% distributed as follow: rhinitis – 68.0%, asthma – 59.1%, and atopic eczema – 44.4%. Among those with no allergic manifestation it was 20.6%. Analysing the aetiology between those with respiratory symptoms we observed higher frequency of sensitisation to dust mites (61.1%), followed by cat dander (27.8%) and Blattella germanica (16.7%) (Table 3). These results are in contrast with those from Erdenen et al.20 Although they observed high frequency of symptoms related to respiratory allergy (asthma and/or rhinitis) in DM1 (56%), the prevalence of positive SPT was lower than controls.20
These results are higher than those previously observed among adolescents living in São Paulo and Nova Iguaçu identified as having asthma and/or rhinitis by ISAAC WQ.21 In that study the prevalence of sensitisation was found to be significantly higher among patients with symptoms compared with those without symptoms to D. pteronyssinus (45.5% versus 29.3%, respectively), followed by P. americana (20.0% versus 12.0%, respectively) and Blattella germanica (17.2% versus 10.1%, respectively).21 In another study with children and adolescents aged from three to 19 years living in Belo Horizonte, there were significantly high prevalence of sensitisation among allergic patients in comparison to non-allergic to: D. pteronyssinus (78.7% vs. 11.3%, respectively), D. farinae (74% vs. 9.1%, respectively), B. tropicalis (64% vs. 13%, respectively), P. americana (40% vs. 16.9%, respectively) and cat dander (18% vs. 3.9%).22 These data were corroborated by a study of total and specific serum levels of IgE (ImmunoCap) to inhalant and food allergens performed in five regions of Brazil, revealing that patients followed in allergy services have high frequency of elevated specific IgE in comparison to normal controls.23
Although food allergens – mainly cow's milk – have been studied as possible triggers for autoimmune disorder in DM1, this subject is still controversial.24,25 In the present study we found that food allergens had less relevance in the sensitisation of our patients, particularly among those with eczema: one patient was sensitive to soy and egg white and two patients were sensitive to peanuts.
The sensitisation to cockroach allergens is a marker of severe asthma, more acute attacks, more hospitalisations and more frequent nocturnal symptoms.26 Only 15.6% (15/96) of our patients were sensitised to cockroach; the figure was 14.2% (1/7) for those with severe asthma. Similar data were obtained in a study of 106 children living in central-south region of São Paulo where the prevalence observed was 21.6% of asthmatic patients.27 In this study it was not possible to confirm these data due to the small number of patients that presented severe asthma.
In conclusion, this study drew our attention to the high prevalence of allergic diseases and sensitisation to inhalant allergens observed among patients with DM1. In a first moment Th1/Th2 duality seems to have no relevance in those patients in whom the predominant Th1 composes the pathophysiology of DM1 and Th2 predominance participates in the pathophysiology of allergic diseases. It should be noted that environmental factors interacting with the genetic profile of each patient may be related to the natural history of both the DM1 as allergic diseases and, therefore, may be involved in some way in the coexistence of these diseases.28–31 Moreover, it should not be forgotten that a small proportion of patients develop DM1 by unknown mechanism and with no participation of autoantibodies.32 In these cases, the mechanism of Th1 would be minor.
Thus the need for future investigations becomes important, to enable to better evaluate the possible inter-relationships between DM1 and allergy.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionConfidentiality of data – The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.