Mendelian susceptibility to mycobacterial disease (MSMD) is characterized by increased susceptibility to weakly virulent mycobacteria (Bacillus Calmette-Guérin [BCG] vaccines and environmental mycobacteria), Mycobacterium tuberculosis, Candida spp. and Salmonella spp. The aim of this study is to evaluate clinical features and immunological findings of MSMD patients with interleukin 12 receptor beta 1 (IL12Rβ1) deficiency.
MethodsAmong 117 screened patients with BCG infection following vaccination, 23 suspected MSMD subjects were recruited to this study by the exclusion of severe combined immunodeficiencies and chronic granulomatous diseases. Flow cytometric assessment for surface expression of IL12Rβ1 was performed. Moreover, the clinical and immunological data from the patients was evaluated.
ResultsA significant decrease (less than 1%) in the surface expression of IL12Rβ1 was reported in six cases which showed a significant increase in the count of lymphocytes (p=0.009) and CD8+ T cells (p=0.008) as compared to MSMD subjects with normal expression of surface IL12Rβ1. The frequency of disseminated BCGosis (50% vs. 20%, p=0.29), recurrent infection (83.3% vs. 40%, p=0.14) and salmonellosis (33.3% vs. 0.0%, p=0.07) was higher in IL12Rβ1 deficient subjects than IL12Rβ1 sufficient individuals.
ConclusionMSMD patients with childhood onset of mycobacteriosis (mostly after BCG vaccination) and recurrent salmonellosis could be evaluated for IL12Rβ1 expression with flow cytometry for punctual diagnosis.
Primary immunodeficiency diseases (PIDs) are a heterogeneous group of immune disorders in which one or several components of the immune system are defective.1 Mendelian susceptibility to mycobacterial diseases (MSMD) is a subgroup of PIDs with errors of innate immunity. This disorder is a rare condition that resulted from carrying mutations in at least one of the genes encoding cytokines, receptors and/or their relative essential modulators of the interleukin 12/23-interferon gamma (IL12/23-IFN-γ) axis.1 The identified mutations were reported to be in IFNGR1, IFNGR2, IL12B, IL12Rβ1, STAT1, IKBKG, CYBB, RORc, IRF8, ISG15, TYK2, JAK1 and NEMO resulting in impaired cellular responses and susceptibility to mycobacterial infections.2 Different mutations cause heterogeneity in the clinical manifestations and prognosis of the disease and which are not well understood3. To date, it has been demonstrated that IL12Rβ1 (with 191 variants reported in 17 exons) is the most affected gene among MSMD patients.4
Deficiency in IL-12 or its receptors results in an increased susceptibility to weakly virulent mycobacteria (Bacillus Calmette-Guérin [BCG] vaccines and environmental mycobacteria), Mycobacterium tuberculosis, Salmonella spp. and Candida spp.5 These features are being shared with other PIDs including severe combined immunodeficiency (SCID) and chronic granulomatous disease (CGD).6 Similar to SCID and CGD patients, common invasive and opportunistic infections are also observed in MSMD patients due to the absence of a significant reduction in the number and/or function of T lymphocytes and defects in phagocyte respiratory burst. Moreover, different underlying causes of these disorders lead to significant differences in the course of the disease, prognosis and therapeutic approaches.7
In the current study, clinical features and immunological findings were evaluated in subjects with complications after BCG vaccination and perturbed expression of IL12Rβ1 protein. Subsequently, these findings were compared with MSMD patients with a normal level of IL12Rβ1 to identify their distinctive parameters.
Patients and methodsStudy populationA prospective study was designed to evaluate Iranian subjects with complications after BCG vaccination including unilateral or bilateral localized lymphadenitis, abscess or ulcer at the site of BCG inoculation, osteomyelitis and involvement of bone marrow as well as hepatosplenomegaly were evaluated. Among the referred 117 patients during a period of 2016–2018 to our tertiary center located at Children Medical Center Hospital (Tehran, Iran),8 subjects who were suspect to MSMD were included in the present study. According to the European Society of Immune Deficiencies (ESID) criteria,9 patients diagnosed as CGD and SCID were excluded before assigning the MSMD diagnosis. Written informed consents were obtained from subjects and/or from parents of children. This study was approved by the ethics committee of Tehran University of Medical Science, Tehran, Iran.
Collection of data and immunologic diagnosisA detailed questionnaire was completed by interview for all patients to record demographic data, immunological and clinical findings, a past medical history of documented recurrent and chronic infections, and other complications. Demographic data of the subjects included the sex, age at the study time, the age of onset, age at diagnosis, delay of the diagnosis, consanguinity, and family history for PID. Assessment of the immune system was performed using white blood cells count and absolute lymphocytes count, investigation of some specific markers including CD3, CD4, CD8, CD16 and CD19, and results of nitro blue tetra zolium test (NBT). History of the BCG vaccination and relative local/systemic reaction, BCGosis and related manifestations, other mycobacterial infections, salmonellosis, other complications related to PID (lymphoproliferation, allergy, autoimmunity, enteropathy and malignancy), treatment approaches (esp. IFN-γ prescription, surgery and bone marrow transplantation) were also included in the questionnaire. In patients without CGD or SCID diagnosis, IL12Rβ1 expression was assessed using flow cytometry.
Flow cytometric assessment of IL12Rβ1 expressionTwenty-one subjects suspected to MSMD and 14 age-sex matched normal subjects were investigated for measuring IL12Rβ1 expression using flow cytometry analyses. Peripheral blood mononuclear cells (PBMCs) were isolated from 5mL heparinized whole blood samples using Ficoll-Paque (Lymphoflot, BIO-RAD, Germany) density gradient centrifugation. 1×106cells/mL were cultured in RPMI 1640 with 10% fetal bovine serum (FBS) containing 2μg/mL phytohemagglutinin (PHA) (All from Sigma, Germany) in 37°C with 5% CO2 for 24h. To obtain activated cells, 20IU/mL of recombinant human IL-2 was conjugated to complete the stimulation of lymphocytes. After four days of incubation, activated cells were harvested through centrifugation for staining after washing them with RPMI 1640 (Sigma). Prepared cells were stained by phycoerythrin (PE) Mouse Anti-Human CD212 (BD Pharmingen™, USA) and evaluated according to the manufacturer's instructions. Isotype-matched control antibodies (PE Mouse IgG1, κ Isotype Control, BD Pharmingen™) were used to determine background levels of staining. Flow cytometry was carried out using a Partec flow cytometer (Partec PAS, Germany) and the results were analyzed using FlowMax software (Partec PAS).
Statistical analysisAnalyses of the results were performed using SPSS 22.0 software (IBM Corporation, USA). Values were expressed as number and percentage, mean±standard deviation and median (interquartile range, IQR). Normality of distribution for data was assessed using Kolmogorov–Smirnov and Shapiro–Wilk tests, which were followed by parametric or non-parametric evaluations of the numerical data. Independent sample t-test was used for data with normal distribution, while Mann–Whitney U and Spearman correlation tests were applied for an abnormal distribution of the data. p-Value of less than 0.05 was considered significant.
ResultsDemographic informationFrom 117 patients with BCGitis, 94 individuals had a diagnosis of SCID and CGD and subsequently, 23 individuals diagnosed as MSMD (15 males [65.2%] and eight females [34.8%]) aged from 1.0 to 16.0 years were enrolled in this study (Table 1). All of the selected patients were on routine BCG vaccination at the time of birth with lymphadenopathy as an unusual presentation. Localized or generalized involvement of distant lymph nodes (submandibular, supraclavicular or intuitive, etc.) was present in 13 and five patients, respectively. Interestingly, 11 cases (47.8%) with large, multiple or recurrent lymphadenopathies did not respond to medical or conservative treatments which were subjected for aspiration or surgery. Eleven patients had a history of hospitalization, in which 86.9% of them received anti-mycobacterial agents.
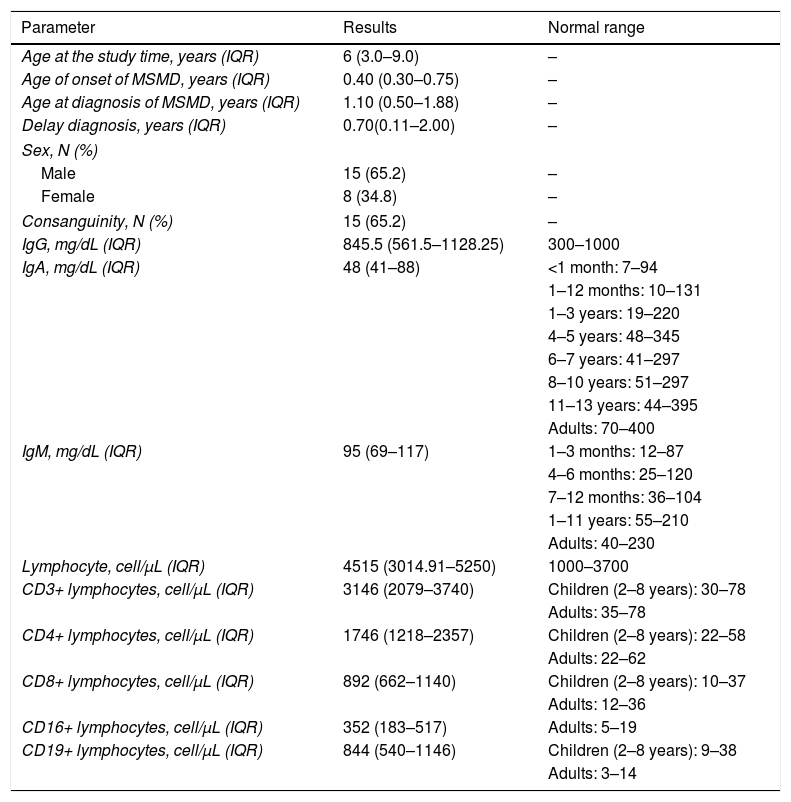
Characteristics of patients with MSMD (n=23).
| Parameter | Results | Normal range |
|---|---|---|
| Age at the study time, years (IQR) | 6 (3.0–9.0) | – |
| Age of onset of MSMD, years (IQR) | 0.40 (0.30–0.75) | – |
| Age at diagnosis of MSMD, years (IQR) | 1.10 (0.50–1.88) | – |
| Delay diagnosis, years (IQR) | 0.70(0.11–2.00) | – |
| Sex, N (%) | ||
| Male | 15 (65.2) | – |
| Female | 8 (34.8) | – |
| Consanguinity, N (%) | 15 (65.2) | – |
| IgG, mg/dL (IQR) | 845.5 (561.5–1128.25) | 300–1000 |
| IgA, mg/dL (IQR) | 48 (41–88) | <1 month: 7–94 |
| 1–12 months: 10–131 | ||
| 1–3 years: 19–220 | ||
| 4–5 years: 48–345 | ||
| 6–7 years: 41–297 | ||
| 8–10 years: 51–297 | ||
| 11–13 years: 44–395 | ||
| Adults: 70–400 | ||
| IgM, mg/dL (IQR) | 95 (69–117) | 1–3 months: 12–87 |
| 4–6 months: 25–120 | ||
| 7–12 months: 36–104 | ||
| 1–11 years: 55–210 | ||
| Adults: 40–230 | ||
| Lymphocyte, cell/μL (IQR) | 4515 (3014.91–5250) | 1000–3700 |
| CD3+ lymphocytes, cell/μL (IQR) | 3146 (2079–3740) | Children (2–8 years): 30–78 |
| Adults: 35–78 | ||
| CD4+ lymphocytes, cell/μL (IQR) | 1746 (1218–2357) | Children (2–8 years): 22–58 |
| Adults: 22–62 | ||
| CD8+ lymphocytes, cell/μL (IQR) | 892 (662–1140) | Children (2–8 years): 10–37 |
| Adults: 12–36 | ||
| CD16+ lymphocytes, cell/μL (IQR) | 352 (183–517) | Adults: 5–19 |
| CD19+ lymphocytes, cell/μL (IQR) | 844 (540–1146) | Children (2–8 years): 9–38 |
| Adults: 3–14 | ||
Abbreviations: Ig; immunoglobulins, CD; cluster of differentiation, MSMD; Mendelian susceptibility to mycobacterial diseases.
Note: For quantities data the median is shown [with IQR, 25th and 75th percentiles]. N, count.
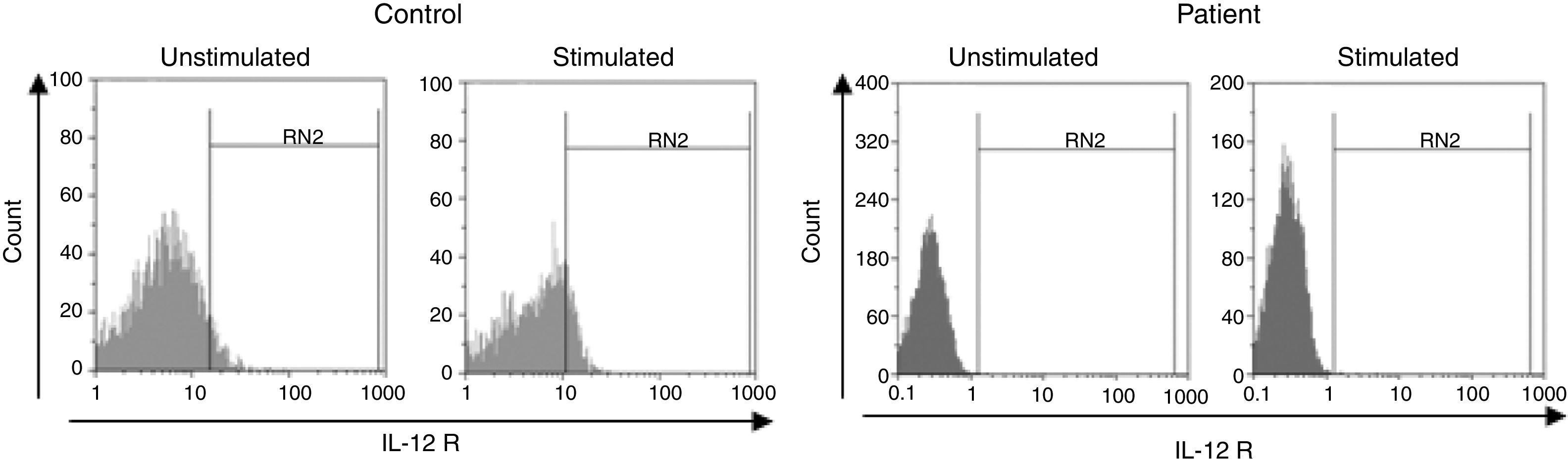
The median expression of IL12Rβ1 protein before stimulation with PHA and IL2 in lymphocytes of MSMD subjects [0.5% (0.27–3)] compared with healthy controls [1.2% (0.5–4)] was not significant (p=0.51). Moreover, after stimulation with PHA and IL2, this compression between MSMD subjects [4.2% (0.9–15.9)] and normal groups [2.1% (1.7–6.6)] was not statistically significant (p=0.85). However, the differences of IL12Rβ1 expression before and after stimulation within MSMD subjects were statistically significant (p=0.005). This difference was also significant before and after stimulation within normal groups (p=0.037).
Surface expressions of IL12Rβ1 were found to be less than 1% on lymphocytes of six subjects (26.0%) with MSMD (Fig. 1). Patients showing less than 1% surface expression for IL12Rβ1 were considered as IL12Rβ1 deficient and were compared with the remaining MSMD patients (n=15).
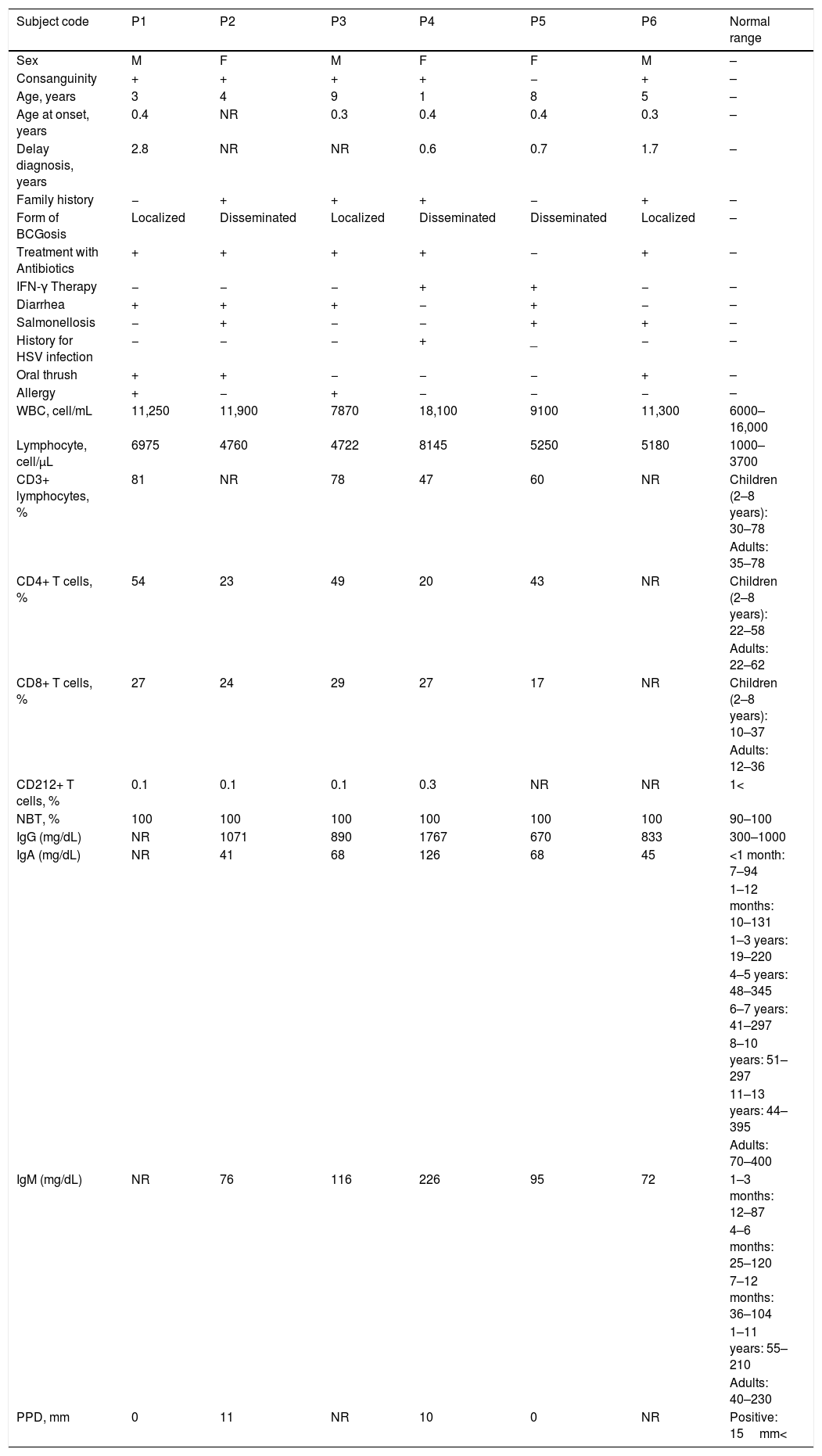
Clinical and immunological findings of IL12Rβ1 deficient subjectsSix patients showing less than 1% surface expression for IL12Rβ1 were considered as IL12Rβ1 deficient (three males and three females). Except for one patient (P5), the other IL12Rβ1 deficient subjects had consanguinity and were treated with antimycobacterial antibiotics. Only two patients (P4 and P5) had IFN-γ therapy. Four patients had a family history (P2, P3, P4 and P6). Three patients had a localized form of BCGosis (P1, P3 and P6) and other IL12Rβ1 deficient subjects had a disseminated BCGosis (P2, P4 and P5). Four patients had diarrhea (P1, P2, P3 and P5). The salmonellosis was in two patients (P5 and P6). Another IL12Rβ1 deficient subject (P4) with disseminated BCGosis had a history of herpes simplex virus (HSV) infection. Three patients had oral thrush (P1, P2 and P6). The allergic manifestations were seen in two subjects (P1 and P3). All clinical and immunological findings in these IL12Rβ1 deficient MSMD subjects are mentioned in Table 2.
Clinical and immunological findings in IL12Rβ1 deficient MSMD subjects.
| Subject code | P1 | P2 | P3 | P4 | P5 | P6 | Normal range |
|---|---|---|---|---|---|---|---|
| Sex | M | F | M | F | F | M | – |
| Consanguinity | + | + | + | + | − | + | – |
| Age, years | 3 | 4 | 9 | 1 | 8 | 5 | – |
| Age at onset, years | 0.4 | NR | 0.3 | 0.4 | 0.4 | 0.3 | – |
| Delay diagnosis, years | 2.8 | NR | NR | 0.6 | 0.7 | 1.7 | – |
| Family history | − | + | + | + | − | + | – |
| Form of BCGosis | Localized | Disseminated | Localized | Disseminated | Disseminated | Localized | – |
| Treatment with Antibiotics | + | + | + | + | − | + | – |
| IFN-γ Therapy | − | − | − | + | + | − | – |
| Diarrhea | + | + | + | − | + | − | – |
| Salmonellosis | − | + | − | − | + | + | – |
| History for HSV infection | − | − | − | + | _ | − | – |
| Oral thrush | + | + | − | − | − | + | – |
| Allergy | + | − | + | − | − | − | – |
| WBC, cell/mL | 11,250 | 11,900 | 7870 | 18,100 | 9100 | 11,300 | 6000–16,000 |
| Lymphocyte, cell/μL | 6975 | 4760 | 4722 | 8145 | 5250 | 5180 | 1000–3700 |
| CD3+ lymphocytes, % | 81 | NR | 78 | 47 | 60 | NR | Children (2–8 years): 30–78 |
| Adults: 35–78 | |||||||
| CD4+ T cells, % | 54 | 23 | 49 | 20 | 43 | NR | Children (2–8 years): 22–58 |
| Adults: 22–62 | |||||||
| CD8+ T cells, % | 27 | 24 | 29 | 27 | 17 | NR | Children (2–8 years): 10–37 |
| Adults: 12–36 | |||||||
| CD212+ T cells, % | 0.1 | 0.1 | 0.1 | 0.3 | NR | NR | 1< |
| NBT, % | 100 | 100 | 100 | 100 | 100 | 100 | 90–100 |
| IgG (mg/dL) | NR | 1071 | 890 | 1767 | 670 | 833 | 300–1000 |
| IgA (mg/dL) | NR | 41 | 68 | 126 | 68 | 45 | <1 month: 7–94 |
| 1–12 months: 10–131 | |||||||
| 1–3 years: 19–220 | |||||||
| 4–5 years: 48–345 | |||||||
| 6–7 years: 41–297 | |||||||
| 8–10 years: 51–297 | |||||||
| 11–13 years: 44–395 | |||||||
| Adults: 70–400 | |||||||
| IgM (mg/dL) | NR | 76 | 116 | 226 | 95 | 72 | 1–3 months: 12–87 |
| 4–6 months: 25–120 | |||||||
| 7–12 months: 36–104 | |||||||
| 1–11 years: 55–210 | |||||||
| Adults: 40–230 | |||||||
| PPD, mm | 0 | 11 | NR | 10 | 0 | NR | Positive: 15mm< |
Abbreviations: F, female; M, male; HSV, herpes simplex virus; WBC, white blood cells; PPD, purified protein derivative; NR, not recorded; NBT, nitroblue-tetrazolium.
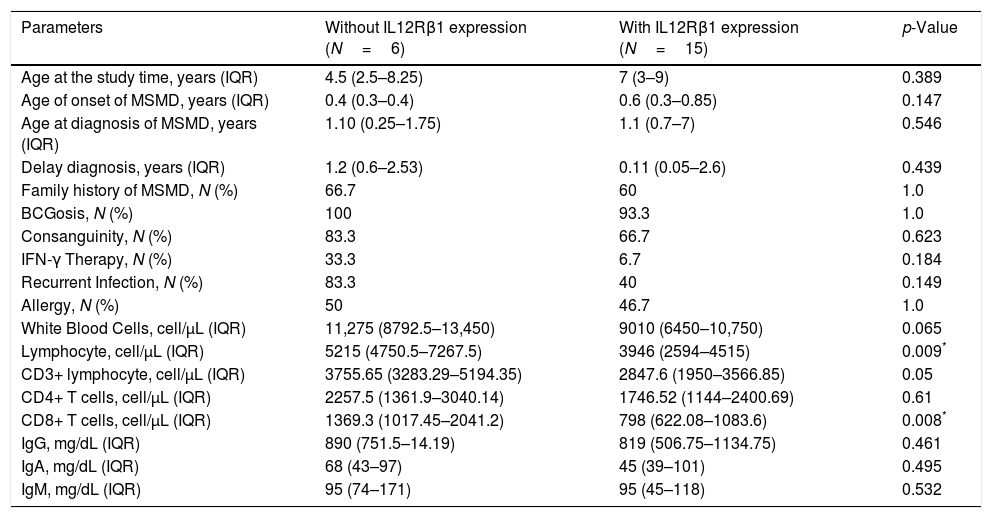
All of the IL12Rβ1 deficient subjects had a history of recurrent lymphadenitis with improvements following medical or surgical treatments. The frequencies of IFN-γ therapy (33.3% vs. 6.7%, p=0.18), disseminated BCGosis (50% vs. 20%, p=0.29), recurrent infection (83.3% vs. 40%, p=0.14) and failure to thrive (FTT, 33.3% vs. 6.7%, p=0.18) were higher in IL12Rβ1 deficient versus IL12Rβ1 sufficient subjects. Also, the affected patients with salmonellosis in the IL12Rβ1 deficient group were 33.3%, but there was no history of salmonellosis in IL12Rβ1 sufficient subjects (p=0.071). Clinical and immunological findings of the patients with IL12Rβ1 deficiency and normal IL12Rβ1 are presented in Table 3.
Comparison of MSMD subjects with and without IL12Rβ1 deficiency.
| Parameters | Without IL12Rβ1 expression (N=6) | With IL12Rβ1 expression (N=15) | p-Value |
|---|---|---|---|
| Age at the study time, years (IQR) | 4.5 (2.5–8.25) | 7 (3–9) | 0.389 |
| Age of onset of MSMD, years (IQR) | 0.4 (0.3–0.4) | 0.6 (0.3–0.85) | 0.147 |
| Age at diagnosis of MSMD, years (IQR) | 1.10 (0.25–1.75) | 1.1 (0.7–7) | 0.546 |
| Delay diagnosis, years (IQR) | 1.2 (0.6–2.53) | 0.11 (0.05–2.6) | 0.439 |
| Family history of MSMD, N (%) | 66.7 | 60 | 1.0 |
| BCGosis, N (%) | 100 | 93.3 | 1.0 |
| Consanguinity, N (%) | 83.3 | 66.7 | 0.623 |
| IFN-γ Therapy, N (%) | 33.3 | 6.7 | 0.184 |
| Recurrent Infection, N (%) | 83.3 | 40 | 0.149 |
| Allergy, N (%) | 50 | 46.7 | 1.0 |
| White Blood Cells, cell/μL (IQR) | 11,275 (8792.5–13,450) | 9010 (6450–10,750) | 0.065 |
| Lymphocyte, cell/μL (IQR) | 5215 (4750.5–7267.5) | 3946 (2594–4515) | 0.009* |
| CD3+ lymphocyte, cell/μL (IQR) | 3755.65 (3283.29–5194.35) | 2847.6 (1950–3566.85) | 0.05 |
| CD4+ T cells, cell/μL (IQR) | 2257.5 (1361.9–3040.14) | 1746.52 (1144–2400.69) | 0.61 |
| CD8+ T cells, cell/μL (IQR) | 1369.3 (1017.45–2041.2) | 798 (622.08–1083.6) | 0.008* |
| IgG, mg/dL (IQR) | 890 (751.5–14.19) | 819 (506.75–1134.75) | 0.461 |
| IgA, mg/dL (IQR) | 68 (43–97) | 45 (39–101) | 0.495 |
| IgM, mg/dL (IQR) | 95 (74–171) | 95 (45–118) | 0.532 |
The numbers of lymphocytes and CD8+ T cells were significantly increased in MSMD subjects with deficient expression of IL12Rβ1 compared with MSMD patients with normal levels of IL12Rβ1 (p=0.009 and p=0.008, respectively). There was an increase in the numbers of leukocytes, CD3+ lymphocytes and CD4+ T cells in MSMD patients with deficient expression of IL12Rβ1 compared with the remaining MSMD patients, however, the differences were not statistically significant (p=0.065, p=0.05 and p=0.610, respectively). There was an increase in the numbers of CD16+ NK cells and CD56+ NK cells in MSMD patients with deficient expression of IL12Rβ1 compared with MSMD patients with normal levels of IL12Rβ1, however, the differences were not statistically significant (p=0.182 and p=0.053, respectively).
DiscussionAutosomal recessive IL12βR1 deficiency, as the most common cause of MSMD, results in the absence of a functional receptor for both IL12 and IL23 cytokines.10 IL12 plays a crucial role in stimulating T cells and NK cells to produce IFN-γ against intracellular pathogens.11 IL23 is a key mediator in employing different components of innate immunity through the induction of IL17 production. IL17 induces the production of pro- inflammatory cytokines (IL6, IL1β, TNFα, etc.), chemokines (IL8, MCP-1, etc.), and prostaglandins.12 Thus, any impairment in the IL-12 receptor could be correlated with increased susceptibility to poorly pathogenic non-tuberculous mycobacterial infections and non-typhoidal salmonellae.13 In the current study, 117 Iranian subjects showing complications resulted from the routine BCG vaccination were screened. Among these patients, 23 cases had MSMD diagnosis from which only six patients with defective IL12Rβ1 protein expression had significantly increased lymphocytes and CD8+ T cells.
Lichtenauer-Kaligis et al.14 indicated that among 11 patients suffering from unusual Mycobacterium BCG infections following vaccination eight patients had mutations in the IL12Rβ1gene. They confirmed a deficiency in the IL12Rβ1 molecule through assessment of the IL12Rβ1 protein by flow cytometry. In another study, Tan et al.15 found 18 patients with MSMD, in whom the percentage of activated lymphocytes with surface expression of IL12Rβ1 was under 1%, suggesting that this value could be considered as cut-off for the screening of these patients. These data were in consistent with our findings. These studies demonstrate that flow cytometric analysis for assessment of the expression of IL12Rβ1 in subjects suspecting for MSMD is an effective and reliable method for initial screening. In this regard, the results of Ramirez et al.16 also confirmed the concordance of the flow cytometric assessment for surface expression of IL12Rβ1evel with the type of the mutation. In fact, they reported a surface IL12Rβ1 less than 1% in subjects with biallelic null mutations and decreased expression of IL12Rβ1 in subjects with mutations resulting in conformational changes.
In the 15 patients with normal expression of IL12Rβ1 in our cohort, the IL12Rβ1 deficiency cannot be excluded by obtained data from IL-12RB1 expression protein. It is evident that the assessment of surface expression for IL12Rβ1 does not ensure the proper function of the sufficiently expressed chains.17 In fact, among missense, nonsense, frame shift and splice site mutations reported in the IL12Rβ1 gene, most of the mutations directly preclude the expression of IL12Rβ1 but there were exceptions resulting in diminished expression or in the expression of shortened IL12Rβ.14,17–21 However, our results highlight the severity of clinical and immunologic profiles in those patients with completely abolished protein expression. To further investigate to insure the hypomorphic mutations, genetic analyses are highly recommended.22–24
Overall good prognosis in IL12Rβ1 deficient MSMD subjects could be correlated with the low penetrance of mycobacterial infection and resulted in acquired immunity preventing the recurrence of secondary mycobacterial or non-mycobacterial infections.25 Different effects of possible compensatory mechanisms for impairments of IL12/23 induced signaling also affect the prognosis depending on the mutation causing the defect.26 This selective susceptibility to mycobacterial disease with low penetrance might be as a result of the presence of IFNs other except IFN-γ and sufficiency of IL12Rβ2 surface expression for development of Th1 cell population. This may also be regarded as the main cause for the lymphadenopathy in IL12Rβ1, IL12p40 and IFN-γ deficient subjects in contrast to those MSMD subjects with IFN-γR deficiency.27 Besides the above mentioned reasons, results of this study were indicative of significant increase in the number of lymphocytes and CD8+ T cells along with insignificant increase in the numbers of leukocytes, total and CD4+ T cells in IL12Rβ1 deficient MSMD subjects which may be regarded as an underlying reason for milder first presentations of the disease in these subjects leading to a slightly longer delay diagnosis. Mild clinical phenotypes have been reported in MSMD subjects with IL12Rβ1 deficiency which can be controlled with antibiotics and may lead to postponing required molecular diagnosis and supplementation of IFN-γ.27
Production of IFN-γ in IL12Rβ1 deficient MSMD subjects is lower than those with a deficiency in IL12p40, because, in addition to the impairment of IL12, independent signaling of IL23 for the production of IFN-γ is also impaired.28 This impairment in the IL23 signaling as a result of IL12Rβ1 deficiency is the underlying cause of impairment in the development of IL17 producing T cells.29 This decrease in the levels of IL17 may account for the greater susceptibility to Candida spp., Klebsiella and Salmonella30 as were reported to be manifested in two subjects in our cohort (P1 and P2) and also salmonellosis in two patients (P5 and P6). On the other hand, allergic manifestations in two subjects (P1 and P3) can be attributed to the imbalance of the Th17 population in favor of Th2 cytokines as an underlying cause for allergic disorders. In line with this notion, the association of IL12Rβ1 gene polymorphisms has been previously confirmed with allergic rhinitis, atopic dermatitis and other allergic phenotypes.31,32 Allergic manifestations in IL12Rβ1 deficient subjects may be caused of impairments in dendritic cells (DCs) maturation since reduced migration and frequency in the number of CD11+ DCs which prime T cells after presenting alternatively spliced IL12Rβ1 have been reported in IL12Rβ1 knockout mice33.
Since some IL12Rβ1 deficient subjects were shown to present salmonellosis as the only infection in Fieschi et al.’s study,25 increased susceptibility to salmonella infection in MSMD subjects with defects in IL12 or its receptor may be suggestive of an immune pathway distinct from IFN-γ. Two of the IL12Rβ1 deficient subjects with disseminated BCGosis in the current study had a history of salmonellosis which also had a history of oral candidiasis. Another IL12Rβ1 deficient subject (P4) with disseminated BCGosis had a history of herpes simplex virus (HSV) infection. Interestingly, CD4+ T cells of these three patients were almost half of the other three IL12Rβ1 deficient cases presenting the localized BCGosis which can be resulted from a further reduction in Th1 cytokines in these patients.34,35 Impairment of NK dependent immunity has also been described in viral infections including herpes family viruses.36 In this regard, normal cellularity and phenotype of hyporesponsive NK cells along with a severe increase in the naturally reactive NK cells and CD56+ effector memory T cells (with prominent IFN-γ response) have been reported in MSMD subjects with deficient IL12/23 axis.4,37–39 This may be an explanation for the P4 case with a history of HSV infection who had undergone IFN-γ therapy after the diagnosis of infection with HSV which resulted in an increase in CD4+ CD16+CD56+ population and consequent treatment of HSV infection. In this regard, the positive effects of treatment with IFN-γ on the improvements of T cell proliferation and clinical manifestations of IL12Rβ1 deficient MSMD subjects have been previously demonstrated. Antimycobacterial antibiotic, IFN-γ therapy, surgery for removal of lymph nodes and also hematopoietic stem cell transplantation (HSCT) are useful treatments in MSMD cases. However, the molecular diagnosis has an important role in the decision regarding appropriate therapeutic modality. Despite universal treatment with prolonged and aggressive antimycobacterial antibiotics (particularly, maintained on antibiotic prophylaxis), cytokine replacement therapy is an additional option with most defects (except IFN-γ receptor deficiencies). Although some patients with partial defects in IL12Rβ1 defects do not require treatment with IFN-γ, however for complete abolished protein expression higher doses are often needed based upon the patient's tolerance of and response to therapy. HSCT has been recommended more in several patients with the more severe forms of MSMD; therefore very few studies addressed the efficacy in IL12Rβ1 deficiency. However, the overall mortality of 26% in these patients suggests that antibiotics and cytokine replacement therapy cannot control all patients and HSCT should be considered.40
It can be concluded that IL12Rβ1 deficiency must be regarded as a possible defect in patients suffering from unusual sensitivity to intracellular bacteria, which can be easily assessed primarily using flow cytometry. It is worth noting that more comprehensive investigations on MSMD subjects can pave the way deep into the involved cellular and molecular mechanisms, providing a better outcome for diagnosis and treatment.
FundingThis work was supported by Vice Chancellor for Research, Tehran University of Medical Sciences, No. 94-04-154-30827.
Conflict of interestThe authors declare no conflict of interest.