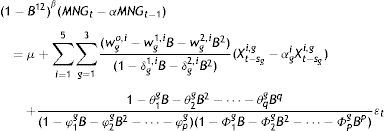Previous ecological studies have shown a temporal and spatial association between influenza epidemics and meningococcal disease (MNG); however, none have examined more than two respiratory viruses.
MethodsData were obtained in Chile between 2000 and 2005 on confirmed cases of MNG and all confirmed cases of respiratory viruses (influenza A and B; parainfluenza; adenovirus; and respiratory syncytial virus [RSV]). Both variables were divided by epidemiological weeks, age range, and regions. Models of transference functions were run for rates of MNG.
ResultsIn this period, 1022 reported cases of MNG and 34,737 cases of respiratory virus were identified (25,137 RSV; 4300 parainfluenza; 2527 influenza-A; 356 influenza-B; and 2417 adenovirus). RSV was the major independent virus temporally associated to MNG (it appears one week before MNG), followed by parainfluenza, influenza-B, adenovirus, and influenza-A.
ConclusionsThe rate of MNG in Chile is temporally associated to all of the respiratory viruses studied, but with variability according age range, and regions.
What is already known about the topic?
- •
Ecological studies worldwide show a temporal and spatial association between influenza and respiratory syncytial virus (RSV) epidemics.
What this paper adds
- •
This is the first study to consider laboratory confirmation of many respiratory virus in all age ranges. We found that the rate of meningococcal disease in Chile is temporally related to all the respiratory viruses tested (RSV, parainfluenza, influenza A and B, and adenovirus), but varied according to the combination of specific age group and region e.g. climate and latitude.
Meningococcal disease (MNG) refers to several highly lethal presentations (occult bacteraemia, meningitis, fulminate sepsis) caused by Neisseria meningitidis. Moreover, N. meningitidis is the main cause of meningitis worldwide. It is well known that around 10% of the general population become nasopharyngeal carriers of N. meningitidis. Humans are the only reservoir of N. meningitidis and transmission is by direct contact with nasopharyngeal secretion. MNG in Chile is under surveillance notifying all suspected cases to the Epidemiological Unit of The Ministry of Health and by sending N. meningitidis isolated from clinical samples in any microbiology laboratory to our National reference Laboratory. During the period of 2000–2005, MNG cases in Chile were: 41% meningitis, 40% meningococcaemia, 16% meningitis plus meningococcaemia, and 2% others (including Waterhouse–Friderichsen syndrome); no cases of bacteraemia were reported.1 Half of the MNG cases were children under the age of five.1 MNG has a universal distribution and presents itself in Chile mainly during the winter season. Since 1993, the incidence of MNG in Chile has been stable and in 2005 it was 1.3/100,000 population, with a mortality rate of 0.2–0.3/100,000 population.1
Viral respiratory tract infections also predominate in Chile during winter.1 These viruses, together with N. meningitidis, live in upper respiratory tract mucose.2 Ecological studies in Denmark,2 the UK/Wales,3 France,4 and Spain5 show a temporal and spatial association between influenza epidemics and MNG, with an increased number of MNG cases following (approximately two weeks after) influenza cases.3,4 The explanation might be alterations in the immune response of the host and/or damage to the respiratory epithelium caused by the virus.2 However, these studies used “influenza-like clinical cases” without requiring viral confirmation.2–5 Two small case–control studies with identified respiratory viruses yielded contradictory results.6,7 While Makras et al.6 reported that only influenza type A, and not type B, was a risk factor for MNG during an outbreak among 55 recruits in Greece; Dunlop et al.7 showed no association of confirmed common respiratory viruses (rhinovirus, adenovirus, parainfluenza, influenza type A, and respiratory syncytial virus [RSV]) and MNG among 104 children in the UK. Other ecological,8,9 and case–control10 studies have reported a lack of association between RSV and MNG.
There is also a lack of information in Latin America regarding the potential association between MNG and influenza or other respiratory viruses. Considering that epidemiological and laboratory surveillance of MNG and respiratory tract infections has existed in Chile for many years, we explored the hypothesis that different types of respiratory viruses (influenza, parainfluenza, adenovirus, and RSV) were associated with MNG in different age groups in Chile. Our hypothesis considers that more than one respiratory virus could be temporally associated with MNG according to different regions (climate).
MethodsBetween January 2000 and December 2005, data of confirmed MNG cases were obtained weekly from the Epidemiology Department of the Chilean Ministry of Health. For this same period of time, data of confirmed cases of respiratory virus: identified by nasopharyngeal aspirated for influenza (A and B), parainfluenza (1,2,3), adenovirus, and RSV by indirect immunofluorescence, from the Public Health Laboratory of the Chilean Ministry of Health as a part of the National Respiratory Virus Surveillance were obtained weekly. This surveillance was carried out in pre-established sentinel health centres throughout the country.
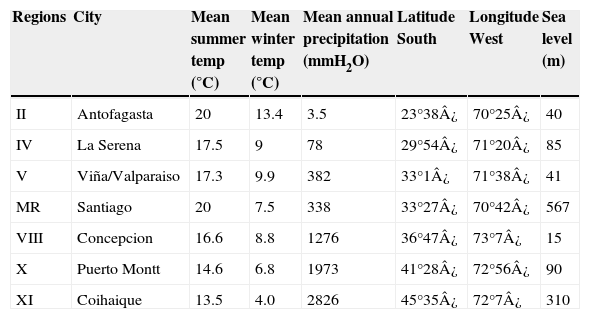
Both MNG and respiratory viruses cases were divided by epidemiological weeks during this period (2000–2005), by age range group (<1 y or infants, 1–14 y or children and >14 y of age or adolescents/adults) and by seven regions or geographical areas most representative of Chile (regions II and IV in the north, region V and the metropolitan region in the centre and regions VIII, X and XI in the south). Mean temperature, precipitation, latitude and sea level characteristics of each region are presented in Table 1. We used rates of MNG and rates of each of the respiratory viruses (number of cases per 10,000 population for each age group and each region). Population data were taken from the Chilean National Institute of Statistics.
Mean temperature, precipitation, latitude and sea level characteristics of the Chilean regions in the study (2000–2005).
| Regions | City | Mean summer temp (°C) | Mean winter temp (°C) | Mean annual precipitation (mmH2O) | Latitude South | Longitude West | Sea level (m) |
|---|---|---|---|---|---|---|---|
| II | Antofagasta | 20 | 13.4 | 3.5 | 23°38¿ | 70°25¿ | 40 |
| IV | La Serena | 17.5 | 9 | 78 | 29°54¿ | 71°20¿ | 85 |
| V | Viña/Valparaiso | 17.3 | 9.9 | 382 | 33°1¿ | 71°38¿ | 41 |
| MR | Santiago | 20 | 7.5 | 338 | 33°27¿ | 70°42¿ | 567 |
| VIII | Concepcion | 16.6 | 8.8 | 1276 | 36°47¿ | 73°7¿ | 15 |
| X | Puerto Montt | 14.6 | 6.8 | 1973 | 41°28¿ | 72°56¿ | 90 |
| XI | Coihaique | 13.5 | 4.0 | 2826 | 45°35¿ | 72°7¿ | 310 |
MR=metropolitan region.
Models of transference functions for the rates of MNG by each epidemiological week and age range in each region had the following general formula:
where g=1,2,3 is the three age groups (infants, children and adolescents/adults); i=1,2,3,4,5 corresponds to the five respiratory viruses considered (RSV, adenovirus, parainfluenza, influenza A and influenza B). B is the backward operator; BXt=Xt−1β is 1 or 0, depending whether stationary differentiation is applied or not to the weekly MNG rates of the regional and age groups. α is 1 or 0, depending whether we model the original series of MNG or the differential series of the regional and age groups. μ is a specific constant for each region and age group. αg,ri is 1 or 0, depending whether it is necessary to differentiate the ith predictor of age group gwgs,i and δgs,i (with s=0,1,2) correspond to the coefficients of the numerator and denominator, respectively, of the transference function to the ith predictor of the regional and age group g. θjgj=1,…,q is the coefficient of the moving average (MA) process. φkg and Φlg with k=1,…,pl=1,…,P are the coefficients for the autoregressive and stationary autoregressive component, respectivelyWe considered stationary R-squared and normalised Bayesian information criteria together to select the final model. The analysis was made using the SPSS, v17 software.
ResultsBetween January 2000 and December 2005, 1762 cases of MNG were reported in the seven Chilean regions studied. Among the cases, 1022 (58%) had serotype determination (81% serotype B, 16.1% serotype C, 1.5% serotype W, and 1.3% serotype Y). No cases of N.meningitidis serotype A were found. During the same period 34,737 cases of respiratory viruses were identified. Of these, 25,137 cases were RSV, 2527 influenza-A, 356 influenza-B, 4300 parainfluenza, and 2417 adenovirus.
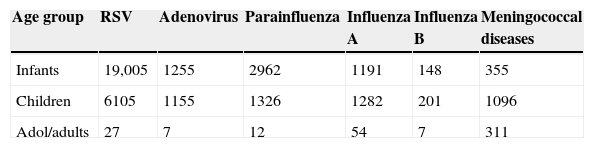
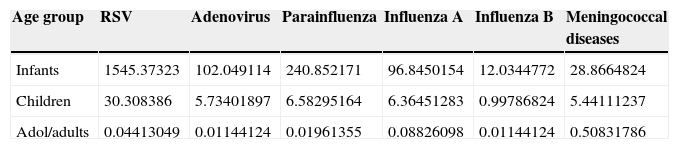
The main respiratory virus identified was RSV followed by parainfluenza among infants; and the majority cases of MNG occurred among children (Table 2). The prevalence of MNG and respiratory viruses is presented in Table 3, where the highest incidence rate of MNG and respiratory viruses (mainly RSV) occurred among infants.
Number of cases of respiratory viruses and meningococcal diseases in Chile (2000–2005).
| Age group | RSV | Adenovirus | Parainfluenza | Influenza A | Influenza B | Meningococcal diseases |
|---|---|---|---|---|---|---|
| Infants | 19,005 | 1255 | 2962 | 1191 | 148 | 355 |
| Children | 6105 | 1155 | 1326 | 1282 | 201 | 1096 |
| Adol/adults | 27 | 7 | 12 | 54 | 7 | 311 |
Adol/adults=adolescent/adults, RSV=respiratory syncytial virus.
Prevalence (rate per 10,000 population) of respiratory viruses and meningococcal diseases in Chile.
| Age group | RSV | Adenovirus | Parainfluenza | Influenza A | Influenza B | Meningococcal diseases |
|---|---|---|---|---|---|---|
| Infants | 1545.37323 | 102.049114 | 240.852171 | 96.8450154 | 12.0344772 | 28.8664824 |
| Children | 30.308386 | 5.73401897 | 6.58295164 | 6.36451283 | 0.99786824 | 5.44111237 |
| Adol/adults | 0.04413049 | 0.01144124 | 0.01961355 | 0.08826098 | 0.01144124 | 0.50831786 |
Adol/adults=adolescent/adults, RSV=respiratory syncytial virus.
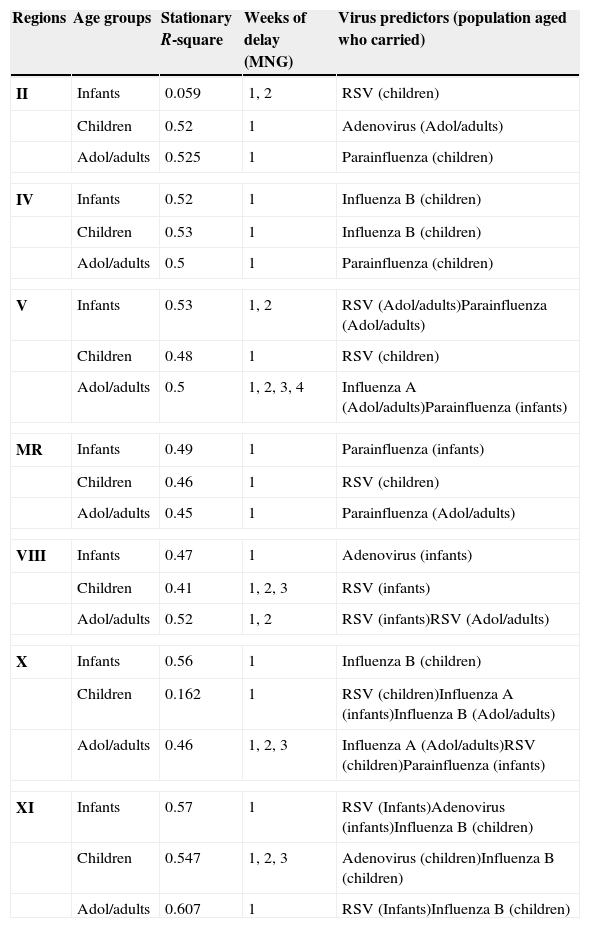
The weekly rate of MNG in each region and age range is strongly related (auto-regression) to the MNG in the previous week plus one or more particular virus some weeks before in the same or a different age group. All models include one term of moving average (MA) for each age group and region (with additional terms of MA for some age groups in regions II, V, VIII, X and XI). The relevant virus temporally related of each model, with their respective delays in the appearance of the dependent variable (rate of MNG) and corresponding stationary R-square are presented in Table 4.
Summary of the models of meningococcal diseases rates (per 10,000 persons) by regions and age groups.
| Regions | Age groups | Stationary R-square | Weeks of delay (MNG) | Virus predictors (population aged who carried) |
|---|---|---|---|---|
| II | Infants | 0.059 | 1, 2 | RSV (children) |
| Children | 0.52 | 1 | Adenovirus (Adol/adults) | |
| Adol/adults | 0.525 | 1 | Parainfluenza (children) | |
| IV | Infants | 0.52 | 1 | Influenza B (children) |
| Children | 0.53 | 1 | Influenza B (children) | |
| Adol/adults | 0.5 | 1 | Parainfluenza (children) | |
| V | Infants | 0.53 | 1, 2 | RSV (Adol/adults)Parainfluenza (Adol/adults) |
| Children | 0.48 | 1 | RSV (children) | |
| Adol/adults | 0.5 | 1, 2, 3, 4 | Influenza A (Adol/adults)Parainfluenza (infants) | |
| MR | Infants | 0.49 | 1 | Parainfluenza (infants) |
| Children | 0.46 | 1 | RSV (children) | |
| Adol/adults | 0.45 | 1 | Parainfluenza (Adol/adults) | |
| VIII | Infants | 0.47 | 1 | Adenovirus (infants) |
| Children | 0.41 | 1, 2, 3 | RSV (infants) | |
| Adol/adults | 0.52 | 1, 2 | RSV (infants)RSV (Adol/adults) | |
| X | Infants | 0.56 | 1 | Influenza B (children) |
| Children | 0.162 | 1 | RSV (children)Influenza A (infants)Influenza B (Adol/adults) | |
| Adol/adults | 0.46 | 1, 2, 3 | Influenza A (Adol/adults)RSV (children)Parainfluenza (infants) | |
| XI | Infants | 0.57 | 1 | RSV (Infants)Adenovirus (infants)Influenza B (children) |
| Children | 0.547 | 1, 2, 3 | Adenovirus (children)Influenza B (children) | |
| Adol/adults | 0.607 | 1 | RSV (Infants)Influenza B (children) | |
MNG=meningococcal diseases. MR=metropolitan region. RSV=respiratory syncytial virus.
The weekly rate of MNG among infants depends on the rate of RSV (in regions II, V and XI), influenza-B (regions IV, X and XI), parainfluenza (regions V and metropolitan), and adenovirus (regions VIII and XI). Among children it depends on the rate of adenovirus (in regions II and XI), influenza-B (regions IV, X and XI), RSV (regions V, metropolitan, VIII, and X); while among adolescents/adults it depends on the rate of parainfluenza (in regions II, IV, V, metropolitan, and X), influenza-A (regions V and X), influenza-B (region XI) and RSV (regions VIII, IX and X). The delay (in weeks) between viruses and MNG varies for each virus and region (Table 4). The population who carried those specific viruses also varies for each region (Table 4). For example in region II the MNG cases appear among infants 1 or 2 weeks after the RSV infection (carried by children).
DiscussionIn this ecological study, which to our knowledge is the first to consider laboratory confirmation of many respiratory viruses in all age ranges, we found that the rate of MNG in Chile is temporally related to all the respiratory viruses tested (RSV, parainfluenza, influenza A and B, and adenovirus), but varied according to the combination of specific age group and region (climate and latitude).
Almost all of the models (19/21) showed stationary R-square close to 0.5. Among the 21 models runs, RSV was the most important independent virus temporally associated with MNG cases (appearing in 10 models one week before the MNG cases), followed by parainfluenza (in seven models within the same week), influenza-B (in six models eight weeks before), adenovirus (in five models), and influenza-A (in three models with one week before). The models from the southern and coldest regions (X and XI) required more predictors, possibly because fewer MNG cases occurred in those regions or due to other factors not studied.
The main importance of the present study is to describe, for the first time, that not only is influenza related to MNG but also RSV, parainfluenza, and adenovirus can be temporally related to MNG rates as well. This finding contrasts with the results of other ecological studies,8,9 a case–control study,10 and a series report.6 A potential explanation could be that those studies considered only one respiratory virus (either RSV,8–10 or influenza6) or by other local differences not studied across countries. Also, another case–control study failed to relate the majority of respiratory viruses to MNG, maybe because only children were included and their sample was small.7 Since RSV is the most prevalent respiratory virus among children11,12 and influenza-A H1N1 pandemic was declared in 2009 future studies seeking to establish an association with MNG should include most of the common respiratory viruses. Indeed, there were 12,302 cases of influenza-A H1N1 confirmed by PCR and 368,118 reported cases during the pandemic in Chile in 2009,13 while the rate of MNG in the same year was 0.6/100,000. Recently, a case-crossover (n=240) study carried out in Canada reported that increasing influenza A and RSV activity was associated with an acute increase in the risk of invasive MNG.14
It is not totally clear why respiratory viral infections precede MNG by some weeks. It is hypothesised that alterations in the immune response of the host (neutrophil chemotaxic and phagocytes’ alterations) and/or damage to respiratory epithelium directly caused by the viruses and/or increased rates of meningococcal transmission are responsible.2 Respiratory tract viral infections commonly result in coughing among older children and adults who are likely to have high meningococcal carriage rates.15 In vitro experimentation has demonstrated enhancement of adhesion of N. meningitides capsule to cultured epithelial cells when influenza A neuraminidase is present.16 It has been shown that RSV infection significantly increased the expression of CD14 and CD18 among surface molecules native to HEp-2 cells, which increases binding of N. meningitidis to cells.17
The present study has contributed by showing that influenza-A is not the only virus involved in this process. Rather, RSV was the principal virus associated with MNG cases; with associations as well with parainfluenza, influenza-B and adenovirus, with degrees of association varying according to age range, and geographic region. However, maybe a statistical more than a biological explanation could be plausible for those viruses found to be temporally associated several weeks before the MNG cases. Another interesting association, which needs to be studied, is the temporal relation between pneumococcal disease and influenza epidemic; as was recently described in Canada by Kuster et al.18 Unfortunately our public system does not have a systematic surveillance of pneumococcal invasive infections between the period 2000 to 2005.
The main strengths of our study are the uniformly organised health care system for systematic weekly notifying of all MNG cases and the capacity to analyse most of the common respiratory viruses (with the exception of rhinovirus, methapneumovirus, and bocavirus) occurring throughout the country; and having very good data base systems for these variables according to age groups. However, it also has some limitations. First, as with all ecological studies, it is always possible that there are fallacious statistics and this is the principal limitation of the study. Second, other factors such as temperature, precipitation and latitude were not specifically analysed in this model. Third, the temporally association between MNG cases and respiratory infection could be related to season (e.g. winter). However, the temporal and spatial association between viral infection (mainly influenza) preceding MNG cases has been previously described in ecological studies in Denmark,2 UK/Wales,3 France,4 and Spain;5 and confirmed in a case–control study,6 and recently in a case-crossover study in Canada.14
Half the MNG cases in Chile are among children under the age of five, mainly in those under the age of one (42/100,000 population between 2000 and 2001, and 14/100,000 in 2005) followed by those under two years of age (10/100,000 in 2005). Among the 13 subgroups of N. meningitidis, types B and C have been predominant in the last 10 years in Chile; appearing mainly during the winter season (June–September).1 Since 1993 the incidence of MNG (1.3/100,000 population in 2005) and the mortality rate (0.2–0.3/100,000 population) have been stable in our country.1 However, we do not have a national programme including for meningococcal vaccinations. Since type B (81%) was the main serotype locally isolated in the studied five-year period, we have to wait until a vaccine against meningococcal type B is developed.19 In contrast to parainfluenza and adenovirus infections, influenza, and RSV infections have a clear seasonal pattern in our country. Therefore, a practical application of the present study could be to include a meningococcal vaccination before those viral epidemics. This idea should be tested in the future.
In conclusion, the rate of MNG in Chile is temporally associated on all the respiratory viruses studied (RSV, parainfluenza, influenza-A and B, and adenovirus), but varies according to age and region (climate and latitude). Future studies done in order to correlate MNG should consider these factors together with at least all of these respiratory viruses.
Conflict of interestNone to declare.
FundingNone.
Ethical DisclosuresProtection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.