House dust mite (Dermataphagoides pteronyssinus) is a widespread risk factor in the development of asthma. CD4+ T lymphocytes have an important role in the pathogenesis of allergic asthma by polarizing to Th2 cells.
ObjectiveWe aimed to evaluate the immunoregulatory effects of dental follicle mesenchymal stem cells with and without IFN-γ stimulation on peripheral blood mononuclear cells of house dust mite sensitive asthmatic patients, and compared those with Dexamethasone as a systemic steroid.
Material and methodsPBMC of asthmatic patients and healthy individuals separately cultured with or without DF-MSCs in the presence and absence of IFN-γ or Der p1 or Dexamethasone for 72h. CD4+ T proliferation, cell viability, CD4+CD25+FoxP3+ Treg cell frequency and cytokine profiles of PBMC were evaluated via flow cytometry.
ResultsDF-MSCs suppressed proliferation of CD4+ T lymphocytes (pCDmix<0.01, pDerp1<0.01, pIFN<0.005) by increasing the number of FoxP3 expressing CD4+CD25+ T regulatory cells (pCDmix<0.005, pDerp1<0.01, pIFN<0.001) and suppressed lymphocyte apoptosis (pCDmix<0.05, pDerp1<0.05, pIFN<0.05), while Dexamethasone increased the apoptosis and decreased Treg cell frequency in asthmatic patients. IFN-γ stimulation increased the suppressive effect of DF-MSCs and also enhanced the frequency of FoxP3 expressing CD4+CD25+ T regulatory cells. The cytokine levels were regulated by DF-MSCs by reducing IL-4 cytokine levels (pCDmix<0.01, pDerp1<0.05, pIFN<0.05) and upregulating IFN-γ levels (pCDmix<0.01, pDerp1<0.05, pIFN<0.005) in asthmatic patients.
ConclusionIFN-γ stimulated DF-MSCs were found to have a high modulatory effect on CD4+ T cell responses, while Dexamethasone had an apoptotic effect on CD4+ T cells in asthmatic patients. DF-MSCs may be a new cell-based therapy option for allergic diseases including asthma.
Asthma is an inflammatory disease and affects an estimated 300 million people worldwide.1 Recent studies defined asthma as an inflammatory disease in which several immune system cells are involved.2 Asthma is often associated with structural remodeling of airways characterized by airway epithelial damage, wall thickening, and sub-epithelial changes.2 There is a strong link between allergen exposure, sensitization and development of asthma.3,4 In particular, exposure to allergens can lead to allergic airway inflammation and an increased risk of developing asthma.5,6
Steroids and anti-inflammatory drugs are used in the treatment of asthma. These drugs both help to control and prevent asthma attacks. However, long-term use of high-dose corticosteroids has systemic side effects such as decreased bone mineral density, skin thinning and bruising.7 Therefore, new therapeutic approaches are needed to reduce the inflammatory response with fewer side effects. In recent years, cell-based therapy has been a new focus for researchers to treat allergic diseases.
Mesenchymal stem cells (MSCs) are multipotent cells and can be isolated from several tissues mainly from bone marrow, adipose and dental tissues and are able to differentiate into various lineages of cell types.8 Recently, several types of MSCs reserved in dental and oral tissues have been identified. MSCs originated from orafacial tissues are easy to reach and can be easily isolated.9 In addition to their differentiation potential and tissue regeneration, dental MSCs have been reported to regulate immune responses in autoimmune diseases. The most potential effect of dental MSCs on inflammatory diseases is immunosuppressive capabilities by regulating lymphocyte responses.10 In addition, it has been shown that direct contact between MSCs and CD4+ T cells is required for Treg induction.11 Cell adhesion molecules expressed by MSCs such as PDL-1 (Programmed death ligand 1), vascular cell adhesion molecule-1, could be upregulated by IFN-γ and increase the immunomodulation capacity of MSCs.12,13 The interaction between cells and the action of several factors involved in the immune function of MSCs is a cooperated work.14 Currently, the role of bone marrow and adipose tissue derived MSCs in allergic allergic diseases has also been reported. However, the regulatory role of DF-MSCs in asthma remains to be elucidated.
Interferron gamma (IFN-γ) is a regulatory cytokine in Th2 mediated immune responses and can influence plasticity of Th cells. IFN-γ is known to play a key role in reversing airway responses and inducing production of IFN-γ by Th1 cells, which further inhibit Th2-induced effector functions.15–17 In addition, IFN-γ treatment of mesenchymal stem cells enhances the production of immunosuppressive mediators such as indoleamine 2,3-dioxygenase (IDO), TGF-β or prostaglandine E2 (PGE2), thus further reducing inflammatory responses.10,18 In this study, we investigated the immunoregulatory effects of IFN-γ on T lymphocyte responses alone or in combination with DF-MSCs.
Dexamethasone is a systemic steroid commonly used in the short-term treatment of acute asthma.19 Administration of dexamethasone rarely causes side effects and still one of the options for treatment of asthma exacerbations.20 We therefore compared the effects of DF-MSCs with dexamethasone on T lymphocytes in this study.
In the present study, we focused on the immunosuppressive effects of DF-MSCs on CD4+ T helper cells in allergic asthmatic patients in vitro. At the same time, changes in cytokine levels were investigated to explain the regulatory effects of DF-MSCs on mononuclear cells in asthma. This study provides basic data for further detailed investigation of the DF-MSCs as a new and effective source for cell-based therapy in allergic diseases.
Materials and methodsStudy groupsAsthma patients were selected from Marmara University Hospital, Department of Pediatric Allergy and Immunology, based on their clinical history who had a specific antigen response to Dermatophagoides pteronyssinus (Der p1) allergen in skin prick tests. Patients were included to the study before clinical treatment. Healthy individuals were selected from those who had no response to specific antigens in skin prick tests and had no corticosteroid or immunosuppressive drug treatment within the last six months.
Peripheral blood samples were collected into heparinized tubes. Patients gave informed consent according to the guidelines of the Ethics Committee of the Marmara University Medical Faculty in Istanbul, Turkey (09.2016.197, 70737436-050.06.04).
Dental follicles (DF) were collected from Marmara University Faculty of Dentistry Oral and Maxillofacial Surgery: they were obtained from five patients age between 19 and 25 years old and selected for having no autoimmune or inflammatory diseases including allergy.
Isolation of dental follicle mesenchymal stem cellsThe isolation, characterization and multipotency analysis of DF-MSCs are given in the supplementary text (Supplement 1).
Isolation of peripheral blood mononuclear cells (PBMC)Peripheral blood was collected from seven Der p1+ asthmatic patients aged from 8 to 13 years old, and seven healthy individuals without an autoimmune or inflammatory disease as control group.
PBMC were isolated from heparinized peripheral blood samples by using Ficoll-Paque (GE Healthcare Bio-Sciences) density gradient solution, as previously described.21 The cells were cultured in RPMI (Gibco, USA) supplemented with 10% FBS and 1% penicillin/streptomycin with the addition of stimulatory agents.
Coculture of PBMC with DF-MSCsDF-MSCs (5×104/well in a 48-well plate) were plated 48h prior to the addition of ten-fold of lymphocytes in the culture medium. DF-MSCs, and PBMC (1:10) were cocultured for 72h. The cultures were stimulated by using 0.5μg/mL of the CDmix (anti-CD3 and anti-CD28), or IFN-γ (0.5μg/mL, Millipore, CA, USA), Der p1 (0.5μg/mL) or Dexamethasone (10−4M). Then, lymphocyte proliferation (carboxyfluorescein succinimidyl ester, CFSE), apoptosis (Annexin V/PI), CD4+CD25+FoxP3+ Treg ratios from PBMC and cytokine levels from culture supernatants were analyzed via flow cytometry.
CFSE assay and evaluation of lymphocyte proliferationThe proliferation of lymphocytes was evaluated by labeling PBMC with 10μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, USA) before culturing. The lymphocytes were cultured unstimulated (US) or stimulated with anti-CD3/anti-CD28 (CDmix), or with CDmix and following stimulants; IFN-γ or Der p1 or Dexamethasone in the presence and absence of DF-MSCs and analyzed for CFSE signaling via flow cytometry (FACS Calibur). Flow cytometry analysis was performed by staining PBMC with anti-CD3 and anti-CD4 antibodies for CFSE signaling. Proliferative response of lymphocytes was analyzed by gating CD3+CD4+ cells via flow cytometry.
Detection of apoptosis of the lymphocytes by Annexin V/7AADAfter a 72-h incubation period, the apoptotic ratio of the lymphocytes was quantified by using Annexin V/PI kit (eBiosciences, USA), according to the manufacturer's instructions. The kit included Annexin V-PE and 7-aminoactinomycin D (7AAD). Flow cytometric analysis was performed by staining PBMC with anti-CD3 and anti-CD4 antibodies for Annexin V/7AAD detection. Apoptosis of lymphocytes was analyzed by gating CD3+CD4+ cells via flow cytometry.
CD4+CD25+FoxP3+ Tregulatory cell frequencyAfter 72h of coculture, the CD4+CD25+FoxP3+ Treg lymphocytes were quantified using Human FoxP3 Buffer Kit (eBioscience, USA). The frequency of FoxP3 expressing Tregulatory cells (CD4+CD25+FoxP3+) was analyzed in the cultured lymphocytes via flow cytometry. The kit included anti-human CD4 (FITC), anti-human CD25 (APC) and anti-human FoxP3 (PE) (eBioscience, USA). CD4+FoxP3+ cells were gated from CD25+ cells.
Analysis of cytokine levelsThe supernatants were collected after culture period and IL-10, IL-4 and IFN-γ levels were measured (pg/mL) by using cytometric bead array (CBA) human Th1/Th2 Kit (BD Biosciences, USA) via flow cytometry, according to the manufacturer's instructions.
Statistical analysisThe differences between groups were analyzed via a one-way ANOVA test using GraphPad Prism 6 software. Data was analyzed as mean±SD and p values less than 0.05 were considered significant.
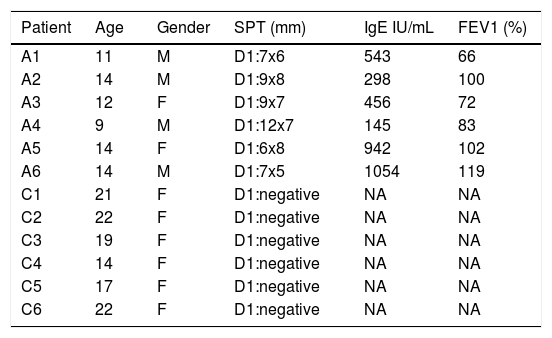
ResultsDemographic data of study participantsAsthma patients were selected from clinically diagnosed and non-treated asthmatic individuals with positive response to Der p1 antigen in skin prick test (SPT). Healthy volunteers were selected from non-asthmatic and clinically had no symptoms of allergic diseases. Clinical and demographic data of study subjects are shown in Table 1.
Demographic data. Asthmatic patients were clinically inspected and evaluated for asthma symptoms. Skin prick test (SPT), forced expiratory volume (FEV%), and serum IgE levels were recorded for patient selection criteria. A: asthmatic patients; C: healthy subjects.
| Patient | Age | Gender | SPT (mm) | IgE IU/mL | FEV1 (%) |
|---|---|---|---|---|---|
| A1 | 11 | M | D1:7x6 | 543 | 66 |
| A2 | 14 | M | D1:9x8 | 298 | 100 |
| A3 | 12 | F | D1:9x7 | 456 | 72 |
| A4 | 9 | M | D1:12x7 | 145 | 83 |
| A5 | 14 | F | D1:6x8 | 942 | 102 |
| A6 | 14 | M | D1:7x5 | 1054 | 119 |
| C1 | 21 | F | D1:negative | NA | NA |
| C2 | 22 | F | D1:negative | NA | NA |
| C3 | 19 | F | D1:negative | NA | NA |
| C4 | 14 | F | D1:negative | NA | NA |
| C5 | 17 | F | D1:negative | NA | NA |
| C6 | 22 | F | D1:negative | NA | NA |
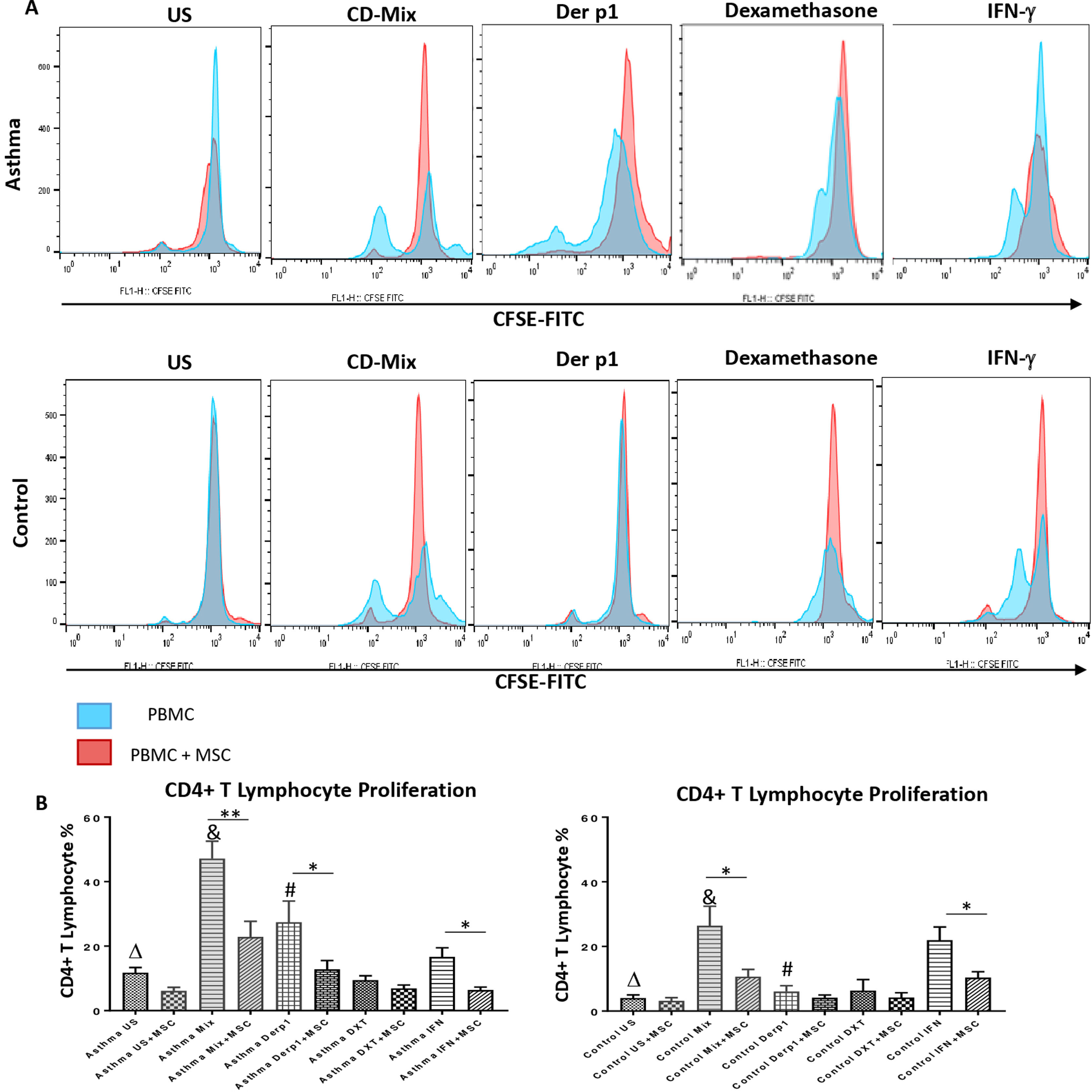
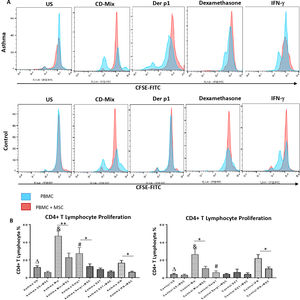
In order to evaluate the modulatory effect of DF-MSCs on proliferative response of CD4+ T lymphocytes, CD3+CD4+ cells were selected within the lymphocyte gate and CFSE labeled cells were evaluated for proliferation. The proliferative response of lymphocytes was significantly high in un-stimulated, CDmix and Der p1 stimulated PBMC cultures alone in asthmatic patients compared to healthy individuals (p<0.05, p<0.01 and p<0.01, respectively). DF-MSCs significantly suppressed the proliferation of lymphocytes in the cocultures of CDmix stimulated PBMC both in asthma patients and healthy individuals when compared with PBMC cultures alone (p<0.01 and p<0.05, respectively), whereas no significant difference was observed in healthy individuals in Der p1 stimulated cocultures (p>0.05). IFN-γ stimulation of DF-MSCs further suppressed the lymphocyte proliferation in asthma patients. Dexamethasone suppressed lymphocyte proliferation in asthma patients and healthy individuals compared to CDmix stimulated cultures (p<0.005) and there was no significant decrease in the lymphocyte proliferation in the co-cultures of DF-MSCs in the presence of Dexamethasone compared to PBMC cultures alone (p>0.05) (Fig. 1).
Lymphocyte Proliferation. (A) Flow cytometry analysis of PBMC shown with histograms. CD3+CD4+ T lymphocytes were gated from FSC-SSC area in order to analyze CFSE signaling (data not shown). (B) Proliferative response was high in CDmix and Der p1 stimulated PBMC cultures of asthma patinets compared to healthy individuals (p<0.01 and p<0.005, respectively). DF-MSCs significantly decreased lymphocyte proliferation in CDmix (p<0.01), Der p1 (p<0.05) and IFN-γ stimulated (p<0.05) PBMC cocultures of asthmatic patients and CDmix and IFN-γ stimulated cocultures of healthy individuals (p<0.05). There was a lower proliferative response to Der p1 stimulation in the healthy group, and DF-MSCs had no significant effect on Der p1 stimulated PBMC of the healthy individuals (p>0.05). The differences between groups were analyzed via a one-way ANOVA test, p<0.05 was considered significant. Statistical significance between groups (p<0.05) was shown with symbols as Δ; un-stimulated, &; CDmix stimulated, #; Der p1 stimulated cultures.
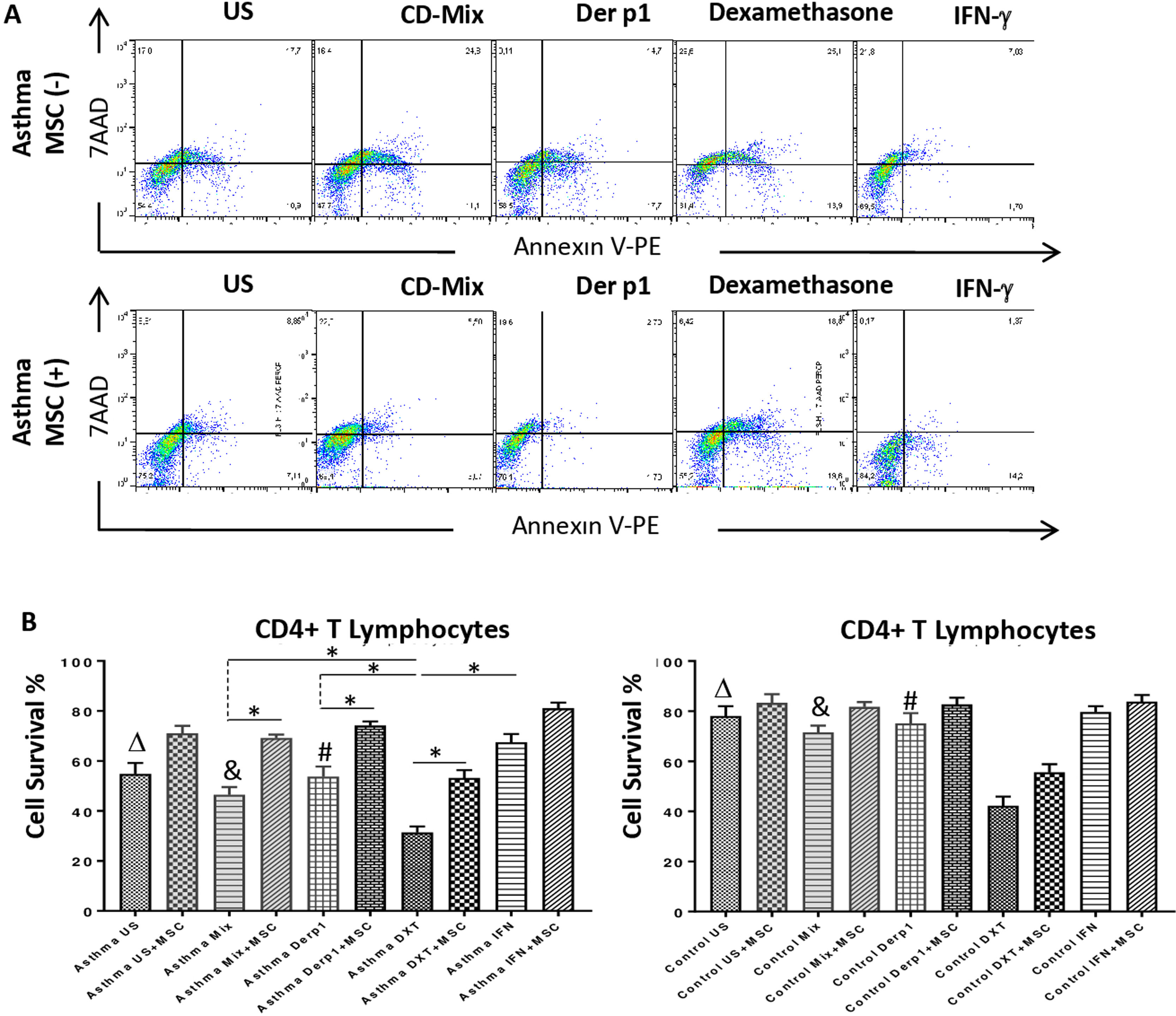
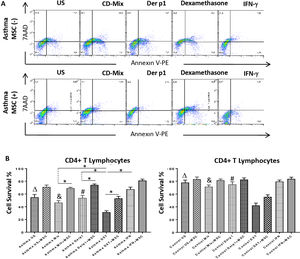
Annexin V and 7AAD rates of the lymphocytes were quantified for apoptosis and cell viability via flow cytometry. CD3+CD4+ quadrant was gated for the analysis of Annexin V/7AAD and the double negative area was quantified for cell viability. The inhibitory effect of DF-MSCs on the apoptotic signals on the lymphocytes was significant. The cell viability ratio of un-stimulated CD4+ T lymphocytes and stimulated with CDmix and Der p1 was significantly lower in asthmatic patients PBMC cultures compared to those in healthy subjects (p<0.05). The cell viability ratio of CD4+ T lymphocytes stimulated with CDmix was significantly increased when the lymphocytes were cocultured with DF-MSCs (p<0.05) and the cell viability ratio of lymphocytes was close to the ratio of PBMC cultures alone in healthy subjects. In addition, IFN-γ pre-stimulation of DF-MSCs further increased the cell viability compared to unstimulated DF-MSCs. DF-MSCs significantly increased the viability of lymphocytes in Der p1 stimulated PBMC cocultures (p<0.05), whereas there was no significant change observed in healthy subjects with the same stimulation. In Dexamethasone-stimulated PBMC cultures the viability of lymphocytes was significantly decreased compared to CDmix and Der p1 stimulation (p<0.05 and p<0.05, respectively) and DF-MSCs tended to increase the cell viability ratio of lymphocytes in dexamethasone stimulated cocultures (Fig. 2).
Lypmhocyte cell survival. (A) Flow cytometry analysis of PBMC was performed for CD3+CD4+ T lymphocytes and analyzed for Annexin V-7AAD. The lower left quadrant was quantified for viability of cells. (B) The cell survival ratio was lower in CDmix and Der p1 stimulated PBMC cultures of asthmatic patients compared to healthy individuals (p<0.05 and p<0.005). Dexamethasone induced lymphocytes to undergo apoptosis, and cell viability significantly decreased with this stimulation when compared with CDmix, Der p1 and IFN-γ stimulated PBMC cultures alone (p<0.05). DF-MSCs significantly increased viability and decreased early apoptosis of T lymphocytes in CDmix, Der p1, Dexamethasone stimulated cocultures of asthmatic patients (p<0.05). The differences between groups were analyzed via a one-way ANOVA test, p<0.05 was considered significant. Statistical significance between groups (p<0.05) was shown with symbols as Δ, un-stimulated; &, CDmix stimulated; #, Der p1 stimulated cultures. *p<0.05.
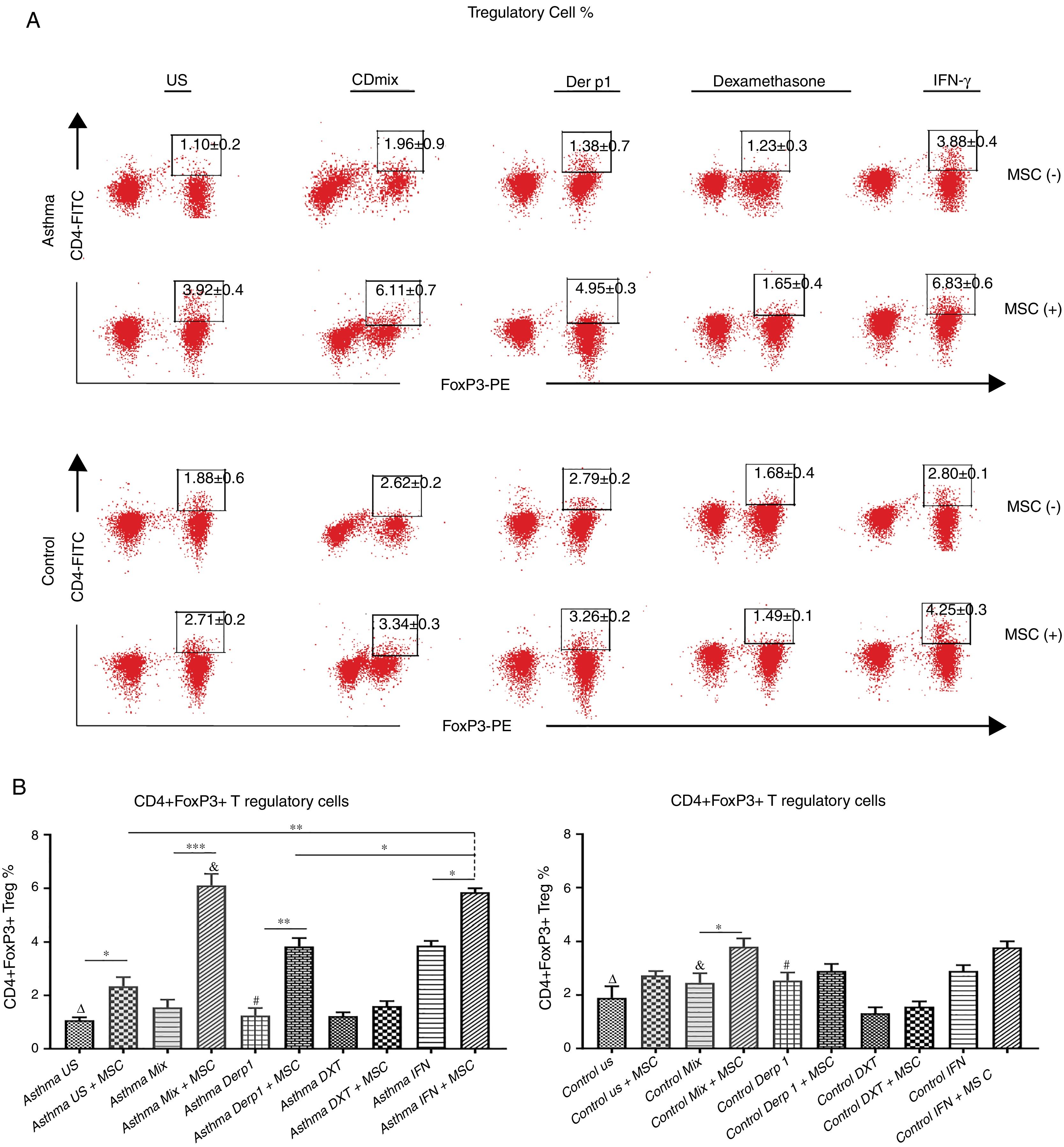
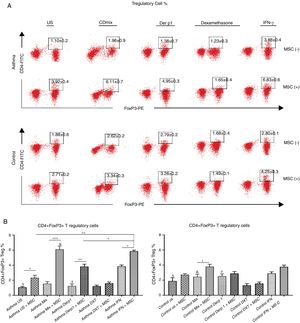
We evaluated the effects of DF-MSCs on the Treg cell ratio in asthmatic patients’ PBMC. CD4+CD25+FoxP3+ Treg cell ratio was significantly lower in un-stimulated, CDmix and Der p1 stimulated PBMC cultures compared to healthy individuals (p<0.05). DF-MSCs increased CD4+CD25+FoxP3+ Treg cell ratio significantly in un-stimulated, CDmix and Der p1 stimulated cocultures compared to PBMC cultures alone (p<0.05, p<0.005 and p<0.01, respectively). IFN-γ stimulation of DF-MSCs further increased FoxP3 expressing Treg cells compared to unstimulated and Der p1 stimulated DF-MSCs in asthmatic patients (p<0.01 and p<0.05, respectively). CD4+CD25+FoxP3+ Treg ratio was lower in unstimulated and Der p1 stimulated PBMC cultures of asthmatic patients than in healthy individuals (p<0.05). This data may be evidence that DF-MSCs can regulate immune responses by increasing the amount of FoxP3 expressing Treg cells (Fig. 3).
CD4+CD25+FoxP3+ T regulatory cell ratio. (A) Flow cytometry analysis was performed by gating CD25+ lymphocytes for CD4+FoxP+ cells. B) FoxP3 expressing CD4+CD25+ Tregulatory cell ratio was lower in PBMC cultures of asthma patients compared to healthy individuals (p<0.05). DF-MSCs significantly increased Treg ratio in unstimulated (p<0.05), CDmix (p<0.005), Der p1 (p<0.01) and IFN-γ stimulated (p<0.05) PBMC cocultures of asthmatic patients compared to PBMC cultures without stem cells, while significant increase in Treg ratio observed only in CDmix cocultures in healthy individuals (p<0.05). The differences between groups were analyzed via a one-way ANOVA test, p<0.05 was considered significant. Statistical significance between groups (p<0.05) was shown with symbols as Δ, un-stimulated; &, CDmix stimulated; #, Der p1 stimulated cultures. *p<0.05, **p<0.01, ***p<0.005.
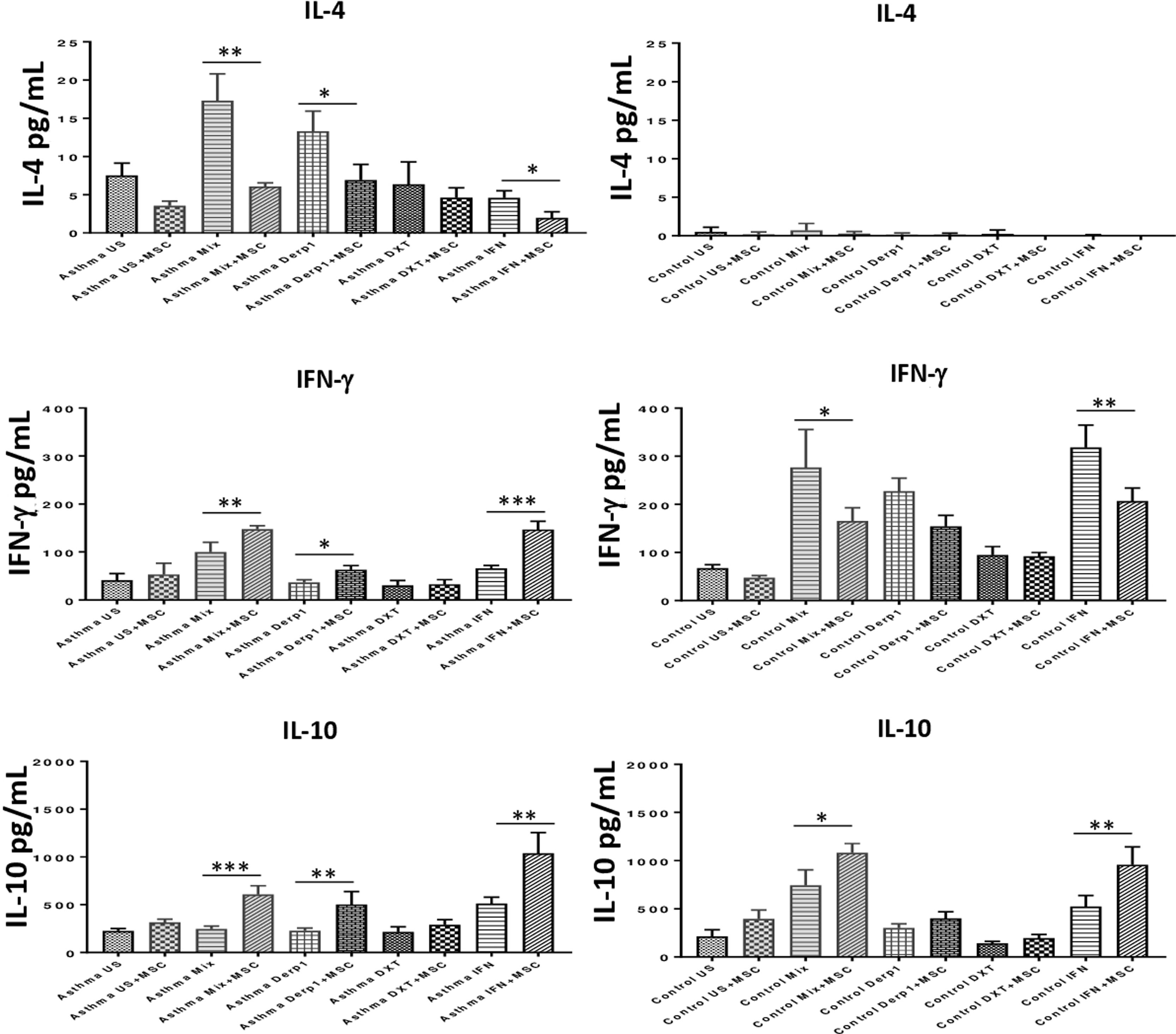
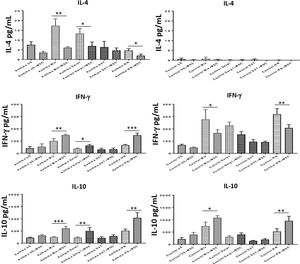
To assess the immunomodulatory effects of DF-MSCs on lymphocyte phenotypes we evaluated Th1 and Th2 cytokine levels in PBMC of asthma patients. Both Th1 and Th2 signature cytokines were analyzed in PBMC cultures in the presence and absence of DF-MSCs. PBMC cultures of asthma patients showed high levels of IL-4, which is the signature cytokine of Th2 lymphocytes, and lower levels of IFN-γ and IL-10 compared to healthy individuals. This data indicates that Th2 polarization was significant in Der p1+ asthma patients. IL-4 levels were significantly decreased (p<0.01) and IFN-γ and IL-10 levels were significantly increased (p<0.05) when cultured with DF-MSCs compared to PBMC cultures alone in asthmatic patients, whereas no significant change in cocultures observed in IL-4 levels in healthy individuals. Interestingly, DF-MSCs significantly increased the level of IFN-γ levels in CDmix (p<0.01), Der p1 (p<0.05) and IFN-γ stimulated (p<0.005) cocultures compared to PBMC cultures alone in asthmatic patients. In contrast, DF-MSCs decreased the level of IFN-γ in PBMC of healthy individuals in CDmix and IFN-γ stimulated cocultures (p<0.05 and p<0.01, respectively). DF-MSCs increased IL-10 levels in all stimulated cocultures except for dexamethasone, but the increase in CDmix, Der p1 and IFN-γ stimulated cocultures showed significant rise in IL-10 levels in PBMC of asthmatic patients (p<0.005, p<0.01 and p<0.005, respectively). These data suggest that DF-MSCs can reduce Th2 mediated responses and regulate Th1/Th2 cytokine secretion in PBMC of Der p1+ asthmatic patients (Fig. 4).
Cytokine levels in culture supernatants. IL-4 level was high and IFN-γ and IL-10 levels were low in CDmix and Der p1 stimulated PBMC cultures of asthma patients compared to healthy individuals. DF-MSCs significantly decreased IL-4 levels in CDmix (p<0.01), Der p1 (p<0.05) and IFN-γ stimulated (p<0.05) cocultures in asthmatic patients and increased IFN-γ (p<0.01, p<0.05, and p<0.005, respectively) and IL-10 levels (p<0.005, p<0.01, and p<0.01, respectively). In contrast, DF-MSCs decreased IFN-γ levels in PBMC cocultures with CDmix (p<0.05) and IFN-γ stimulation (p<0.01) in healthy subjects compared to PBMC cultures alone. The differences between groups were analyzed via a one-way ANOVA test, p<0.05 was considered significant. *p<0.05, **p<0.01, ***p<0.005.
In the current study, DF-MSCs were evaluated for their modulatory effects on CD4+ T lymphocytes of asthmatic subjects and compared with an immunosuppressive agent “Dexamethasone”. The aim of this study was to investigate the regulatory efficacy of a new and an easily accessible stem cell source of dental follicles, in order to suggest it as a cell-based therapy option for allergic diseases. We demonstrated the anti-proliferative, anti-apoptotic and modulatory effects of DF-MSCs on activated CD4+ T lymphocytes of asthmatic patients by modulating Th2 response with the induction of Treg cells and shifting Th2 cells toward Th1 cells.
Asthma occurs as an airway obstruction, bronchial hyperreactivity and chronic inflammation resulted with remodeling of airways. Current treatments with corticosteroids should relieve symptoms, attenuate inflammation but also have side effects.19 Dexamethasone has been proposed as a pharmaceutical therapy for acute asthma patients. Current treatment options consist of prednisolone or dexamethasone,20,22 but also have side effects. To compare the modulatory effects of DF-MSCs we used dexamethasone, which is a well-known immunosuppressive drug. The need for new options for therapies come into question. The exciting development in recent years of cellular therapy allows many adventages such as anti-inflammatory features and accelarating tissue regeneration damaged with inflammation. The interest in stem cells from extrapulmonary sources is increasing in the cellular therapy of asthma with their modulatory effects in the inflammatory niche.23,24 The immunomodulatory effects of MSCs derived from bone marrow and adipose tissue have been reported in allergic diseases, but the effects of dental tissue mesenchymal stem cells as an extrapulmonary source are still unknown. Dental mesenchymal stem cells are interesting because the oral region is a source of large amounts of stem cells for therapeutic procedures, and it does not require additional surgery on the patient during tissue extraction.25,26
Additionally, the immunosuppressive effects of dental mesencymal stem cells (DPSCs, SHED and DFSCs) have been proved with our previous studies.27 In this study, we isolated and used DF-MSCs because of their potential immunosuppressive activity. We investigated the immunoregulatory effects of DF-MSCs on CD4+ T lymphocytes in PBMCs isolated from house dust mite sensitive (Der p1+) asthmatic patients.
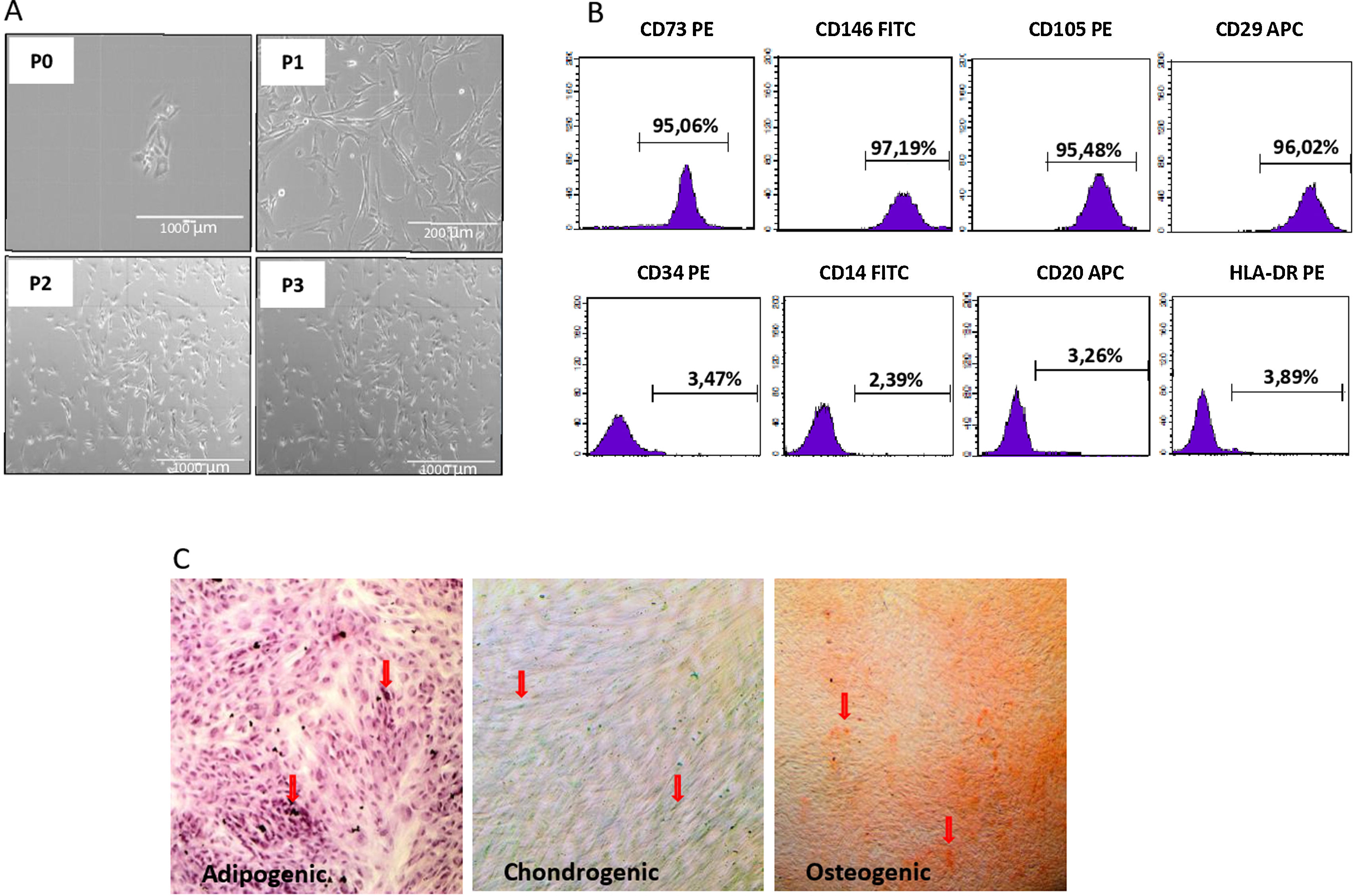
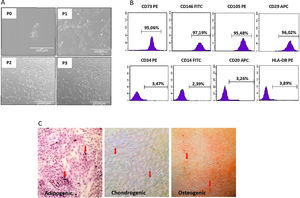
We isolated DF-MSCs and they remained adherent to a plastic surface and seem as fibroblast-like cells. The expressions of CD29, CD73, CD105 and CD146 were over 95% and lack the expression of CD14, CD34, CD20, and HLA-DR. They were differentiated into osteogenic, chondrogenic and adipogenic lineages in vitro.
Interferon-gamma (IFN-γ) is known to enhance the immunosuppressive properties of MSCs. IFN-γ stimulation of MSCs modulates T cell responses by increasing the expression of anti-inflammatory mediators of MSCs. The suppressive effect of IFN-γ is related with its ability on the secretion of indoleamine 2,3-dioxygenase by MSCs, which is a mediator in the downregulation of activated T cells and shift them into an anti-inflammatory phenotype as regulatory T cells.28,29 Studies showed that MSCs down-regulate Fas-Fas Ligand expression and inhibit endogenous apoptotic proteases, thus, promoting T cell survival.30,31 In the present study, DF-MSCs showed a remarkable reduction in the proliferative response of CD4+ T lymphocytes of asthmatic subjects by enhancing CD4+CD25+FoxP3+ T regulatory cell frequency. Dexamethasone similarly suppressed proliferation of CD4+ T lymphocytes; however, it decreased the T regulatory cell ratio and at the same time reduced viability of lymphocytes. In contrast, DF-MSCs showed an anti-apoptotic effect on CD4+ T lymphocytes of asthmatic patients. These data demostrate that DF-MSCs may have an anti-proliferative effect on CD+ T lymphocytes by strengthening FoxP3 expressing T regulatory cells in asthmatic patients, while Dexamethasone causes apoptosis of lymphocytes in either asthmatic or healthy subjects, which indicates that dexamethasone induces cell death pathways. In addition, IFN-γ stimulation of DF-MSCs further decreased the lymphocyte proliferation by increasing CD4+CD25+FoxP3+ T regulatory cell frequency, and streghtened the anti-apoptotic effect of DF-MSCs by further increasing the viability of CD4+ T lymphocytes in asthmatic patients. Therefore, IFN-γ stimulation of DF-MSCs enhances the immunomodulatory effect of these cells.
The major proteolytic allergen derived from the house dust mite is D. pteronyssinus, which is one of the most clinically relevant allergens causing asthma, and activates memory Th2 cells.32 In the present study, antigen-specific stimulation with Der p1 antigen was performed in order to create an antigen specific response of T helper cells in PBMC of asthmatic patients. We questioned whether DF-MSCs were able to modulate Der p1 stimulated lymphocytes, which were isolated from Der p1(+) asthma patients, in vitro. Der p1 stimulation of mononuclear cells resulted with CD4+ T cell proliferation in Der p1 (+) asthmatic patients whereas there was no response to Der p1 allergen in PBMC cultures of healthy individuals. This result indicates that activated, especially allergen specific responding CD4+ T cells were present in asthma patients. DF-MSCs suppressed the Der p1 stimulated CD4+ T cell proliferation and increased CD4+CD25+FoxP3+ T regulatory cell ratio in HDM-sensitive asthma patients significantly.
IL-4 is the signature cytokine secreted by Th2 cells. Previous studies showed that tymus stromal lymphopoietin (TSLP) treated peripheral dendritic cells prime naive CD4+ T cells and shifted them to Th2 phenotype to produce the cytokines IL-4, IL-5 and IL-13 after stimulation.33,34 In the animal model of asthma administration of MSCs, reduced IL-4, IL-5 and IL-13 cytokine levels in bronchoalveolar lavages and lungs inhibited the Th2 inflammatory response and shifting of Th2 toward IFN-γ secreting Th1 cells which resulted with potential therapeutic effects.35–37 In our study, we investigated the effects of DF-MSCs on T helper lymphocyte phenotypes by the evaluation of cytokine levels in the culture supernatants. Elevated IL-4 levels in culture supernatants of asthmatic patients were notably decreased and IFN-γ levels were increased with DF-MSCs when stimulated with CDmix and Der p1, while the IFN-γ levels reduced in healthy individuals. Additionally, IFN-γ pre-stimulation of DF-MSCs further decreased IL-4 and increased IFN-γ levels in the cocultures of asthma patients. These data showed that DF-MSCs shifted Th2 cells toward Th1 cells and reduced Th2 response. At the same time, we analyzed IL-10 levels in order to evaluate the regulatory effects of DF-MSCs by inducing CD4+CD25+FoxP3+ Tregulatory cells. IL-10 levels were significantly lower in asthma patients in unstimulated, CDmix and Der p1 stimulated cultures compared to healthy individuals. DF-MSCs remarkably increased IL-10 levels both unstimulated and stimulated cultures of PBMC in asthmatic patients. However, no significant difference was seen in healthy individuals in the cocultures with the stimulation of Der p1. This result indicates that DF-MSCs preferentially activate FoxP3 expressing CD4+CD25+ T cells, which have immunosuppressive effects in the inflammatory response of Th lymphocytes.
ConclusionThe present study demonstrated that DF-MSCs reduces Th2 response in allergic asthma via enhancing T regulatory cell activation. In addition, DF-MSCs can regulate Th1/Th2 imbalance with the reduction of Th2 polarization, in vitro. We conclude that DF-MSCs can be a useful cell-based therapeutic tool and further in vivo studies can be achieved on Th2-mediated allergic diseases, including asthma.
Conflict of interestAuthors declare no conflict of interest.
This project was funded by TUBITAK (Turkish Scientific and Technological Research Council) with the project number of 214S262.