The precise pathogenesis of chronic spontaneous urticaria (CSU) remains unknown. However, an important association between CSU and autoimmune disorders such as Hashimoto's disease (HD) has been reported.
We investigated the frequency of HD as a comorbidity of CSU and the prevalence rate of autoreactivity among CSU patients with HD.
Patients and methodsThe presence of thyroid autoantibodies and the levels of thyroid hormones were examined in 40 CSU patients who showed urticaria symptoms for >4 weeks. Patients who were diagnosed with HD, including subclinical ones, and were in need of treatment received thyroid therapy, and the changes in their urticarial symptoms were observed. An autologous serum skin test (ASST) was also performed to examine the relation of CSU with autoreactivity.
ResultsEleven of the 40 CSU patients were diagnosed with HD, and 4 of the 5 patients who received and completed thyroid therapy showed considerable remission of urticarial symptoms during and after treatment. In addition, the rate of positive ASST results tended to be higher in CSU patients with HD (5 of 7) than in those without HD (2 of 6).
ConclusionsThe comorbidity rate of HD in CSU patients was high, and such patients tended to have a positive ASST. Thyroid therapy in CSU patients with HD can lead to a considerable remission of urticarial symptoms, which may suggest that HD is possibly involved in the aetiology of CSU, or is at least a potential exacerbating factor for CSU.
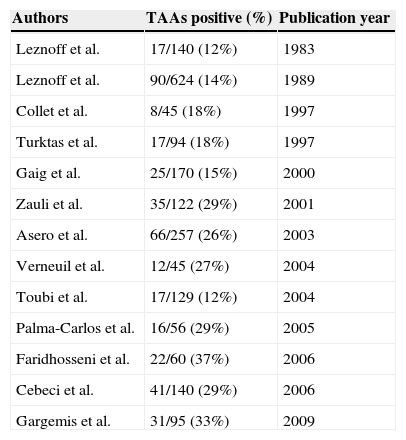
Chronic spontaneous urticaria1 (CSU) is a common disorder; however, its precise pathogenesis remains unknown. Some reports have suggested that approximately 30–50% of CSU shows autoreactivity.2–6 More interestingly, various autoimmune disorders have been associated with CSU,7 such as Hashimoto's disease (HD), one of the most frequent forms of autoimmune thyroiditis. According to previous reports,8–21 12–37% (median, 26%) of chronic urticaria patients have thyroid autoantibodies (see Table 1). In some cases, the existence of thyroid disease can be identified simply by the presence of aberrant thyroid hormone levels. However, it is necessary to examine thyroid autoantibody and thyroid hormone levels to fully detect HD because many patients with HD have normal hormone levels without legible clinical symptoms except for the presence of thyroid autoantibodies and slight goitre (swelling of the thyroid gland). HD develops gradually over several years and the patient's levels of thyroid hormones gradually decrease from the baseline levels in each individual. Thus, many HD patients have no symptoms and normal hormone level in their early disease stage and do not have diagnosis of HD among general population, but such subclinical HD patients are recommended watchful waiting.
Reported positive rates of thyroid autoantibodies among CSU patients.
| Authors | TAAs positive (%) | Publication year |
|---|---|---|
| Leznoff et al. | 17/140 (12%) | 1983 |
| Leznoff et al. | 90/624 (14%) | 1989 |
| Collet et al. | 8/45 (18%) | 1997 |
| Turktas et al. | 17/94 (18%) | 1997 |
| Gaig et al. | 25/170 (15%) | 2000 |
| Zauli et al. | 35/122 (29%) | 2001 |
| Asero et al. | 66/257 (26%) | 2003 |
| Verneuil et al. | 12/45 (27%) | 2004 |
| Toubi et al. | 17/129 (12%) | 2004 |
| Palma-Carlos et al. | 16/56 (29%) | 2005 |
| Faridhosseni et al. | 22/60 (37%) | 2006 |
| Cebeci et al. | 41/140 (29%) | 2006 |
| Gargemis et al. | 31/95 (33%) | 2009 |
TAAs, thyroid autoantibodies.
In this study, the comorbidity rate of HD (or thyroid autoimmunity) in CSU patients and the possible influence of thyroid therapy on urticarial symptoms in Department of Dermatology, Fukuoka National Hospital, National Hospital Organization were examined. The frequency of autoreactivity among CSU patients with HD was also investigated.
Patients and methodsThe levels of free thyroxine (T4), thyroid-stimulating hormone (TSH), anti-thyroglobulin (Tg) antibody, and anti-thyroid peroxidase (TPO) antibody were examined in serum samples from 40 CSU patients (6 men and 34 women; age range, 17–67 years; median, 55 years), including the cases 1 and 2 described below, who had visited the Department of Dermatology, Fukuoka National Hospital, National Hospital Organization during a period from June, 2010 to March, 2012 and agreed to the tests.
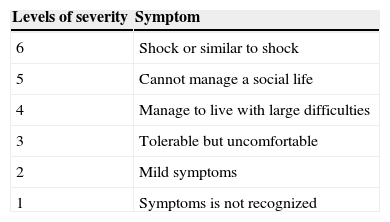
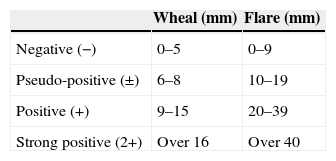
When either abnormal hormone levels or antithyroid antibodies were detected, subjects were sent to endocrinologists for the diagnosis of HD. HD was diagnosed if subjects were antithyroid antibody positive and had goitre of any degree according to the diagnosis criteria of HD.22 HD patients with normal thyroid hormone levels do not necessarily become a target of the treatment. However, thyroid therapy (oral thyroid hormone replacement therapy for three months) was offered for such HD patients as well as those with abnormal hormone levels according to their consent since we previously experienced a CSU patient with highly suspected HD (as being negative for conventional thyroid autoantibodies but with significantly abnormal hormone levels) who showed remarkable improvement of his intractable CSU symptoms after thyroid therapy (data not shown). Meanwhile, HD patients did not receive thyroid therapy when the endocrinologists judged that thyroid therapy was not required. Triiodothyronine was used as treatment for HD since it acts on the thyroid gland directly and shows therapeutic effects in a shorter time than thyroxine.23 The changes in their urticarial symptoms were carefully observed during and after the thyroid therapy. We assessed the severity of CSU symptoms using Japanese Guidelines for Diagnosis and Treatment of Urticaria24,25 (Table 2). An autologous serum skin test (ASST) was also performed according to standard methodology7 on 13 of the 40 CSU patients who provided consent for checking if their intractable urticaria was of the autoreactive type. Diagnosis criteria of ASST in the current study were based on those of the National Hospital Organization Sagamihara National Hospital, as shown in Table 3. ASST was basically conducted under treatment with antihistamines; however, medication of antihistamines was temporarily suspended for more than three days prior to retest for those with negative results according to the subject consent.
Diagnosis criteria of autologous serum skin test from “The practical side of allergy test and immunotherapy” compiled under the supervision of Dr. Kazuo Akiyama at National Hospital Organization Sagamihara National Hospital.
| Wheal (mm) | Flare (mm) | |
|---|---|---|
| Negative (−) | 0–5 | 0–9 |
| Pseudo-positive (±) | 6–8 | 10–19 |
| Positive (+) | 9–15 | 20–39 |
| Strong positive (2+) | Over 16 | Over 40 |
This study was approved by the Ethics Committee of Fukuoka National Hospital, National Hospital Organization, and all subjects provided informed consent for participation.
Case 1An 18-year-old woman had been experiencing urticaria for >7 years. She had been treated with various antihistamines to control her urticarial symptoms, for >4 years after her first visit to our clinic. Several of those antihistamines were temporarily effective, but their effects did not last long. Ebastine, a second-generation antihistamine, was fairly effective, but it gradually became ineffective in controlling her urticarial symptoms with its usual dose in combination with a histamine H2 blocker (i.e., 5mg/day ebastine and 50mg/day ranitidine). Doubling the dose of ebastine (10mg/day) suppressed her urticarial symptoms but resulted in the adverse effect of strong sleepiness, which substantially affected her school life and daily activities (note that 5–10mg/day ebastine is the regular dose for adults in Japan).
A positive result was obtained for anti-Tg antibody and the thyroid hormone levels were normal; the patient was diagnosed with HD. One month after the start of the daily administration of 25μg triiodothyronine, she noticed some relief in her symptoms. The dose of triiodothyronine was then increased to 50μg and was continued for two more months together with the treatment for CSU (5mg/day ebastine and 50mg/day ranitidine). After a total of three months of this thyroid therapy, her urticarial symptoms showed considerable remission and became very well controlled with ebastine alone at 5mg/day.
Case 2A 63-year-old man had been experiencing urticaria for >6 years. His symptoms were fairly well controlled with the usual dose of an antihistamine (olopatadine, 5mg/day); however, wheal and erythema reappeared within three days whenever he forgot to take the medicine. A positive result was obtained for anti-TPO antibody, and the thyroid hormone levels were normal; the patient was diagnosed with HD. He was treated with a daily dose of 25μg triiodothyronine for three months, while olopatadine (5mg/day) was continued for the treatment of CSU. After three months, he noticed that urticaria did not reappear even after he completely stopped taking the antihistamine. No recurrence of urticarial symptoms had occurred in the absence of CSU medication for >10 months. Although his CSU recurred eventually, apparently triggered by an upper respiratory infection, the symptoms have been well controlled with 2.5mg/day olopatadine, half the dose previously used.
ResultsEleven of the 40 CSU patients examined showed positive results for thyroid autoantibodies: five for anti-Tg antibody alone, three for anti-TPO antibody alone, and three for both autoantibodies. Nine of these 11 thyroid autoantibody-positive CSU patients had normal thyroid hormone levels. HD, including subclinical ones, was diagnosed in these 11 patients (11 of 40 [27.5%]) by endocrinologists.
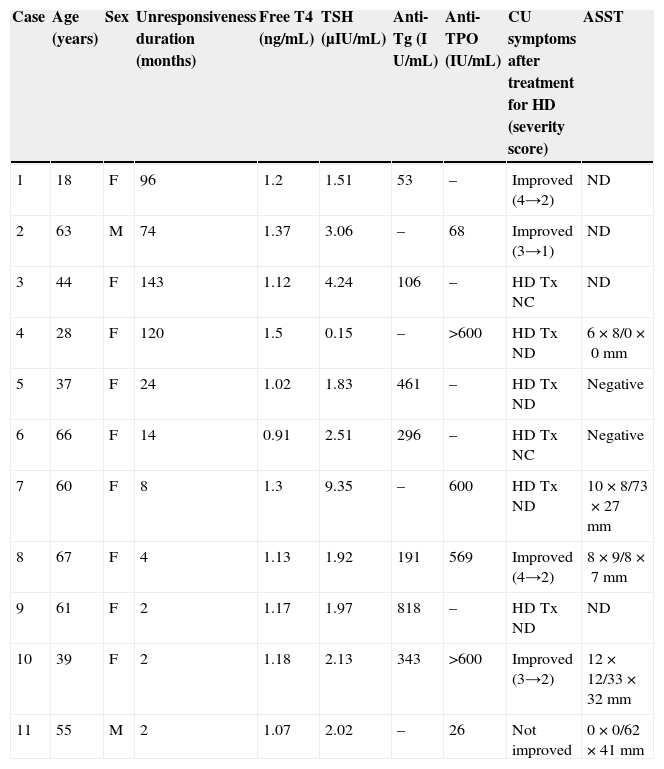
Seven of the 11 CSU patients with HD received thyroid therapy, and five of them completed their therapeutic regimen. The endocrinologists judged that thyroid therapy was not required in cases 4, 7, and 9, and case 5 was lost to follow-up because the patient never revisited the endocrinologists and our hospital. Four of the five CSU patients with HD who completed thyroid therapy (cases 1, 2, 8, and 10) showed remarkable improvement in their urticarial symptoms, while one patient (case 11) showed no apparent changes (Table 4).
Results of examinations and changes in urticaria symptoms.
| Case | Age (years) | Sex | Unresponsiveness duration (months) | Free T4 (ng/mL) | TSH (μIU/mL) | Anti-Tg (I U/mL) | Anti-TPO (IU/mL) | CU symptoms after treatment for HD (severity score) | ASST |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | F | 96 | 1.2 | 1.51 | 53 | – | Improved (4→2) | ND |
| 2 | 63 | M | 74 | 1.37 | 3.06 | – | 68 | Improved (3→1) | ND |
| 3 | 44 | F | 143 | 1.12 | 4.24 | 106 | – | HD Tx NC | ND |
| 4 | 28 | F | 120 | 1.5 | 0.15 | – | >600 | HD Tx ND | 6×8/0×0mm |
| 5 | 37 | F | 24 | 1.02 | 1.83 | 461 | – | HD Tx ND | Negative |
| 6 | 66 | F | 14 | 0.91 | 2.51 | 296 | – | HD Tx NC | Negative |
| 7 | 60 | F | 8 | 1.3 | 9.35 | – | 600 | HD Tx ND | 10×8/73×27mm |
| 8 | 67 | F | 4 | 1.13 | 1.92 | 191 | 569 | Improved (4→2) | 8×9/8×7mm |
| 9 | 61 | F | 2 | 1.17 | 1.97 | 818 | – | HD Tx ND | ND |
| 10 | 39 | F | 2 | 1.18 | 2.13 | 343 | >600 | Improved (3→2) | 12×12/33×32mm |
| 11 | 55 | M | 2 | 1.07 | 2.02 | – | 26 | Not improved | 0×0/62×41mm |
HD, Hashimoto's disease; Tx, treatment; NC, not completed; ND, not done; ASST, autologous serum skin test.
Free T4 reference value: 0.90–1.70ng/dL.
TSH reference value: 0.500–5.00μIU/mL.
Anti-Tg reference value: <28IU/mL.
Anti-TPO reference value: <16IU/mL.
A total of 13 CSU patients (seven CSU patients with HD and six CSU patients without HD) underwent ASST, and five of seven CSU patients with HD (Table 2) and two of six CSU patients without HD showed positive results.
We have described two CSU cases with long disease duration wherein the urticarial symptoms improved considerably after thyroid therapy (cases 1 and 2) as described in patients and method.
DiscussionHD is the first autoimmune disease to be recognised26 and is thought to be the most common cause of primary hypothyroidism. The prevalence rate of HD is one in 30 in the general population, including the latent type, and up to one in 5–10 among women aged 40 years or older.27 It is said that the ratio of the patients who showed hormone level abnormality and need treatment is 20–30% among all the HD patients. The onset of HD is thought to be caused by a combination of genetic and environmental factors,28–30 similar to many other autoimmune diseases. Since the first report of the association between thyroid autoimmunity and CSU by Leznoff et al. in 1983, several papers have reported a high prevalence of thyroid autoimmunity among CSU patients. In our study, 27.5% of the CSU patients examined had positive results for thyroid autoantibodies and were diagnosed with HD, including subclinical HD. This result is consistent with previous studies8–21 where around 27% of the CSU patients were found to have thyroid autoantibodies. Although the clinical significance of thyroid autoantibodies as a diagnostic marker is beyond doubt, we would like to note that the fundamental mechanism of HD is thought to be thyroid tissue destruction with T cells31 and that these thyroid autoantibodies are seen to result from the exacerbation of the autoimmune reaction. Here, we have described two CSU cases with long disease duration wherein the patients showed remarkable improvement in urticarial symptoms to such a degree as to not find unpleasantness in everyday life, after thyroid hormone replacement therapy. These findings indicate that normalization of thyroid function is important in controlling urticarial symptoms, at least in some cases, and that autoimmune conditions accompanied by the presence of autoantibodies might be a possible cause or an exacerbating factor for CSU. Previous studies reported that approximately 30–50% of the CSU cases with unidentified causes are of the autoreactive type, related to IgG autoantibodies that bind to mast cell-bound IgE molecules or surface IgE receptors2–5 to stimulate and eventually degranulate the cells. Indeed, seven of the 13 CSU patients (53.8%) examined in our study had positive results for ASST, and the positive rate tended to be higher in CSU patients with HD (71.4%) than in those without HD (33.3%). Although the number of patients who underwent ASST was too small to establish accurate tendencies, this might suggest that CSU patients with HD are more likely to have autoreactivity. Interestingly, these thyroidal autoantibodies themselves do not seem to have a direct influence on mast cell degranulation32; therefore, we speculate that the addition of a thyroid hormone might possibly attenuate urticarial symptoms through its immune-modulating effects that have been shown in in vitro or in vivo studies.33,34 However, the mechanism underlying the presence of long-lasting CSU suppression even after hormone replacement therapy is discontinued remains to be elucidated.
In conclusion, our study demonstrated that many CSU cases showed autoreactivity, particularly in the case of patients with HD, and that thyroid replacement therapy in such CSU patients could largely improve urticaria symptoms. These results suggest that the presence of HD as a comorbidity might be a possible aetiological or at least an exacerbating factor for CSU.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.