Common variable immunodeficiency (CVID) is a diagnostic category of primary immunodeficiency (PID) which may present with heterogeneous disorders including recurrent infections, autoimmunity, granulomatous diseases, lymphoid and other types of malignancies. Generally, the incidence of malignancy in CVID patients is around 1.5–20.7% and usually occurs during the 4th–6th decade of life. Non-Hodgkin lymphoma is the most frequent malignancy, followed by epithelial tumours of stomach, breast, bladder and cervix. The exact pathological mechanisms for cancer development in CVID are not fully determined; however, several mechanisms including impaired genetic stability, genetic predisposition, immune dysregulation, impaired clearance of oncogenic viruses and bacterial infections, and iatrogenic causes have been proposed to contribute to the high susceptibility of these patients to malignancies.
Common variable immunodeficiency (CVID) is a diagnostic category of primary immunodeficiency (PID) that includes heterogeneous disorders defined by increased susceptibility to recurrent infection, low levels of immunoglobulin (Ig) in serum and impaired specific antibody responses to pathogens or vaccines.1–4 Patients may also have a wide variety of non-infectious complications, including autoimmunity and inflammatory conditions, enteropathy, granulomatous diseases, lymphoid malignancies and different types of cancers.5,6 Malignancy has been proposed as a distinct clinical phenotype of CVID due to underlying genetic predisposing factor which influences mortality and morbidity of patients, but it is argued that this phenotype could be a secondary consequence to viral infections or immune dysregulation.7
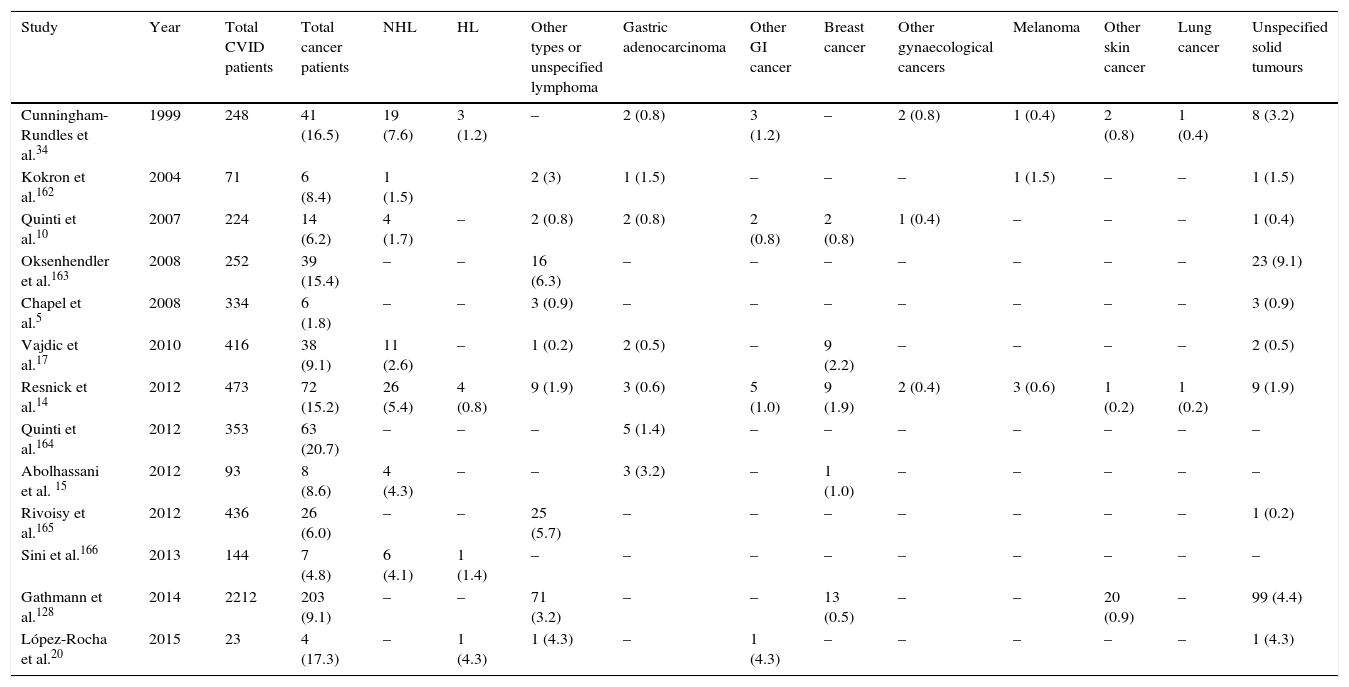
Several studies report a high frequency of malignancy in CVID patients.8–16 Generally, the incidence of cancer in these patients is around 10% (range 1.5–20.7%, Table 1) and usually occurs during the 4–6th decade of life, with a risk 5–12 times higher than in the general population.12,17 In two large cohorts in New York and Italy, the incidence of cancers was noted to be 15.2% and 20.7% in CVID patients, respectively.14,18 The most common site of malignancy is lymphoid tissues.19–21 Recent studies reported that Non-Hodgkin lymphoma (NHL) is the most frequent malignancy, followed by epithelial tumours of stomach, breast, bladder and cervix. Among epithelial tumours in CVID patients, gastric adenocarcinoma is the most prevalent cancer.14,21–23 In fact, the cumulative incidence of cancers in CVID appears to have expanded, but the data for cancers other than lymphoma are difficult to separate out.24 However, in the New York study the frequency of other malignancies was reported about 7%,14 and 3% in the European Society for immunodeficiencies study.5 In a study by the Australasian Society of Clinical Immunology and Allergy (ASCIA), the increased risks of malignancies in CVID patients compared to the population without CVID were identified as 12-fold for NHL, 7-fold for stomach cancer, 2.49-fold for leukaemia, 2.24-fold for breast cancer, and surprisingly, 146-fold for thymus cancer.17
Frequency of different types of malignancy among different cohorts of CVID patients.
| Study | Year | Total CVID patients | Total cancer patients | NHL | HL | Other types or unspecified lymphoma | Gastric adenocarcinoma | Other GI cancer | Breast cancer | Other gynaecological cancers | Melanoma | Other skin cancer | Lung cancer | Unspecified solid tumours |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cunningham-Rundles et al.34 | 1999 | 248 | 41 (16.5) | 19 (7.6) | 3 (1.2) | – | 2 (0.8) | 3 (1.2) | – | 2 (0.8) | 1 (0.4) | 2 (0.8) | 1 (0.4) | 8 (3.2) |
| Kokron et al.162 | 2004 | 71 | 6 (8.4) | 1 (1.5) | 2 (3) | 1 (1.5) | – | – | – | 1 (1.5) | – | – | 1 (1.5) | |
| Quinti et al.10 | 2007 | 224 | 14 (6.2) | 4 (1.7) | – | 2 (0.8) | 2 (0.8) | 2 (0.8) | 2 (0.8) | 1 (0.4) | – | – | – | 1 (0.4) |
| Oksenhendler et al.163 | 2008 | 252 | 39 (15.4) | – | – | 16 (6.3) | – | – | – | – | – | – | – | 23 (9.1) |
| Chapel et al.5 | 2008 | 334 | 6 (1.8) | – | – | 3 (0.9) | – | – | – | – | – | – | – | 3 (0.9) |
| Vajdic et al.17 | 2010 | 416 | 38 (9.1) | 11 (2.6) | – | 1 (0.2) | 2 (0.5) | – | 9 (2.2) | – | – | – | – | 2 (0.5) |
| Resnick et al.14 | 2012 | 473 | 72 (15.2) | 26 (5.4) | 4 (0.8) | 9 (1.9) | 3 (0.6) | 5 (1.0) | 9 (1.9) | 2 (0.4) | 3 (0.6) | 1 (0.2) | 1 (0.2) | 9 (1.9) |
| Quinti et al.164 | 2012 | 353 | 63 (20.7) | – | – | – | 5 (1.4) | – | – | – | – | – | – | – |
| Abolhassani et al. 15 | 2012 | 93 | 8 (8.6) | 4 (4.3) | – | – | 3 (3.2) | – | 1 (1.0) | – | – | – | – | – |
| Rivoisy et al.165 | 2012 | 436 | 26 (6.0) | – | – | 25 (5.7) | – | – | – | – | – | – | – | 1 (0.2) |
| Sini et al.166 | 2013 | 144 | 7 (4.8) | 6 (4.1) | 1 (1.4) | – | – | – | – | – | – | – | – | – |
| Gathmann et al.128 | 2014 | 2212 | 203 (9.1) | – | – | 71 (3.2) | – | – | 13 (0.5) | – | – | 20 (0.9) | – | 99 (4.4) |
| López-Rocha et al.20 | 2015 | 23 | 4 (17.3) | – | 1 (4.3) | 1 (4.3) | – | 1 (4.3) | – | – | – | – | – | 1 (4.3) |
In this study, we sought to demonstrate the epidemiology and aetiology of common types of malignancies in CVID patients by reviewing the most recent evidence on this topic.
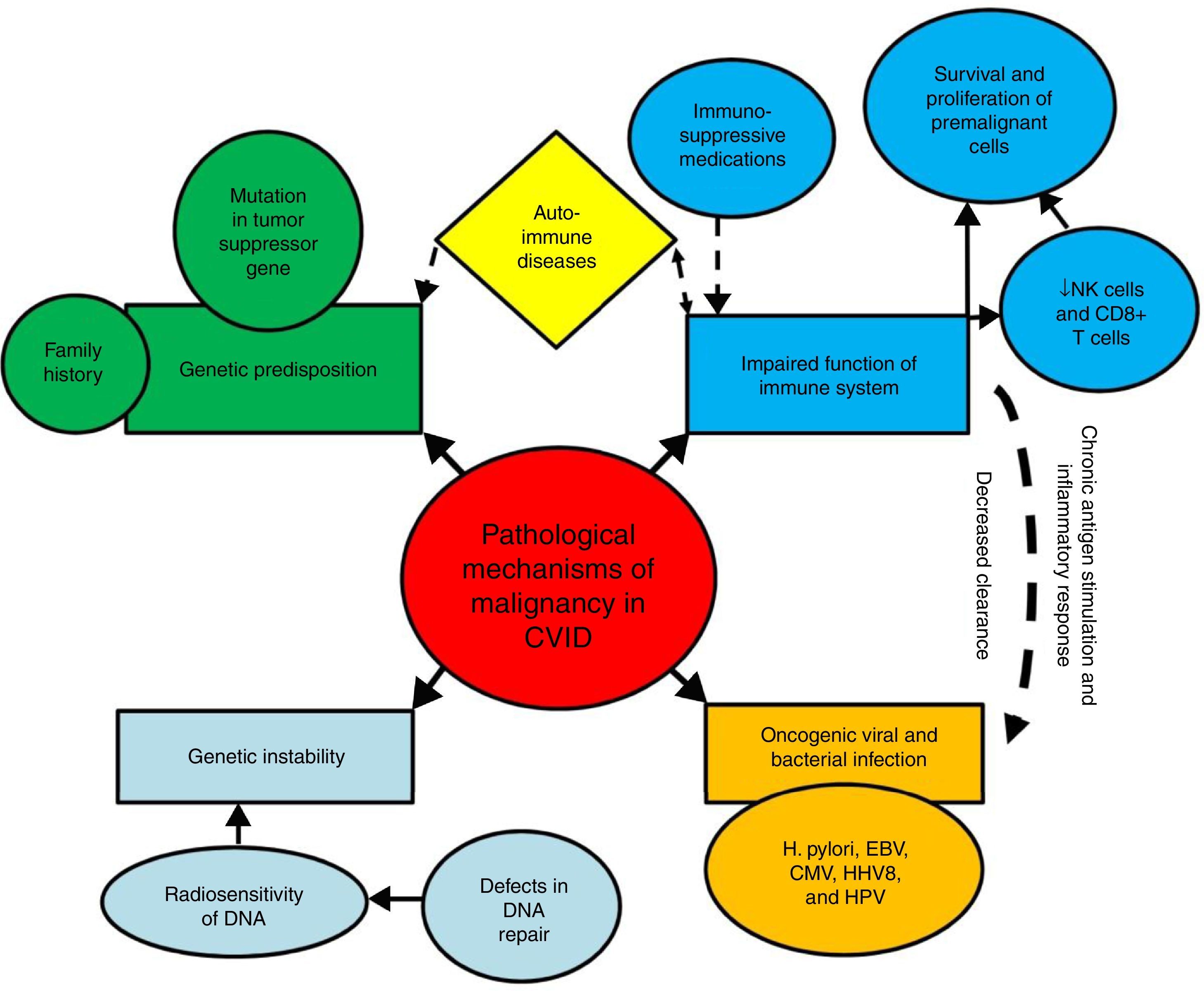
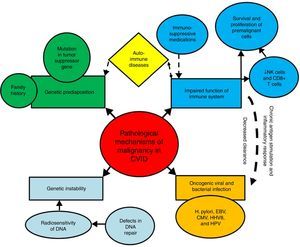
Mechanisms of increased susceptibility of CVID patients to malignancyThe exact pathological mechanisms of malignancy in CVID are not fully determined; although several mechanisms have been suggested to contribute to the high susceptibility of these patients to specific types of malignancies.16 These mechanisms include innate genetic instability and genetic predisposition, persistent activation and proliferation of the lymphoid cells during the course of infections, impaired clearance of oncogenic viruses and bacterial infections (Fig. 1).
Mechanisms of susceptibility of CVID patients to malignancy. Several mechanisms have been suggested to contribute to the high susceptibility of CVID patients to malignancies. These mechanisms include genetic instability and genetic predisposition, persistent activation and proliferation of the lymphoid cells during the course of infections, impaired clearance of oncogenic viruses and bacterial infections.
Impaired immune response results in decreased clearance of oncogenic viruses and bacteria, as well as chronic antigen stimulation, chronic inflammatory response, and survival and proliferation of premalignant and malignant cells, all of which can predispose CVID patients to oncogenic mutations and malignant transformations.25–29 The key cells of the immune system for tumour surveillance are natural killer (NK) cells and CD8+ T cells, which are parts of the innate and adaptive immune response. After recognition of a tumour antigen via the T cell receptor (TCR), activated CD8+ cytotoxic T cells can kill the tumour target cells. Moreover, CD4+ T cells, especially T helper cell type 1 (Th1), provide “help” for the activation of CD8+ T cells, and can display cytotoxic activity in some situations.30–32 However, defects in CD4+ T cells and CD8+ T cells immunity, which occur in approximately one-third of CVID patients, possibly contribute to their susceptibility to malignant complications.33–35 In addition, the frequency of NK cells, as other tumour killer cells has been reported to be lower in CVID.36,37 However, data of 801 CVID patients from the national UK Primary Immune Deficiency (UKPID) registry showed that, overt cancer is associated with significantly lower absolute CD8+ T cell but not NK cell numbers, raising the question as to what extent immune senescence, especially of CD8+ T cells, might contribute to the increased risk of cancers.38
There are multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases, and cancer development.39 It is demonstrated that the risks of all NHL increases in association with rheumatoid arthritis, Sjögren syndrome, systemic lupus erythematosus, and celiac disease.40 All of these conditions are also associated with CVID.41,42 In addition, the use of nonsteroidal anti-inflammatory drugs, systemic corticosteroids, and immunosuppressive medications was shown to be associated with risk of NHL in rheumatoid arthritis patients.40
Viral and bacterial infectionsInfections with certain type of viruses and bacteria have been recognised as risk factors for several types of cancer in different ways; when some viruses directly affect the genes inside cells and cause them to grow out of control, other infectious organisms can cause long-term inflammation which leads to changes in the infected cells and in nearby immune cells, and eventually lead to cancer.43–45 It is demonstrated that chronic inflammation can promote all stages of tumorigenesis including apoptosis evasion, sustained angiogenesis, DNA damage, limitless replication, self-sufficiency in growth signalling, and insensitivity to anti-growth signalling, as well as tissue invasion and/or metastasis.46 In addition, some types of bacterial and viral infections can suppress patient immune system, and therefore help to the development of cancers.47,48
Although immunoglobulin therapy greatly reduces the episodes of infections and enhances patients’ survival, it does not appear to address the development of cancer, especially lymphoma. The definite reason for the increased susceptibility to lymphoid malignancies is unclear. The proposed mechanism includes genetics, radiosensitivity, immune dysregulation, and chronic infections such as Helicobacter pylori, Epstein–Barr virus (EBV), human herpes virus type 8 (HHV8) and cytomegalovirus (CMV). Up to now, the strongest association between chronic bacterial infection and the development of cancer involves H. pylori, which is associated with increased risk of adenocarcinoma of the stomach and mucosa-associated lymphoid tissue (MALT) lymphoma.49–52 It is suggested that the robust immune response generally fails to clear the H. pylori infection, leading to a chronic inflammatory response which is thought to be a key element of the carcinogenic activity of the bacterium. H. pylori infection is commonly found in CVID patients with chronic gastritis complication10,53,54; therefore, it may account for the increased incidence of gastric cancer and extra-nodal marginal zone lymphoma in these patients.55–57
Many different types of viruses may cause an increased risk of cancer by directly transforming the cells they infect, or by causing a chronic inflammatory condition, or both. Those most commonly associated with chronic inflammation are the hepatitis B and C viruses, which result in chronic active hepatitis and hepatocellular carcinoma.58,59 The prevalence of hepatitis is also increased in CVID, occurring in nearly 12% of patients.60–62 Among viral infections, EBV is associated with nasopharyngeal cancer and certain types of lymphomas such as Hodgkin and NHL, and may also contain a chronic inflammatory component.63,64 It is revealed that EBV causes sustained proliferation of peripheral B cells, but when coupled with a secondary mutation led to malignant transformation; such as the occurrence with chromosomal translocations that activate the c-myc oncogene in Burkitt's lymphoma.65 Other viral infections such as the CMV, human papillomavirus (HPV) and herpes simplex virus have been implicated in the increased incidence of cervical and other carcinomas.66,67 Moreover, HHV8 has been found in nearly all tumours in patients with Kaposi sarcoma.68 However, there are few reports of viral infections such as EBV,69 HHV8,70 HPV71 and CMV72,73 to induce an increased risk of malignancy in CVID patients.33,55
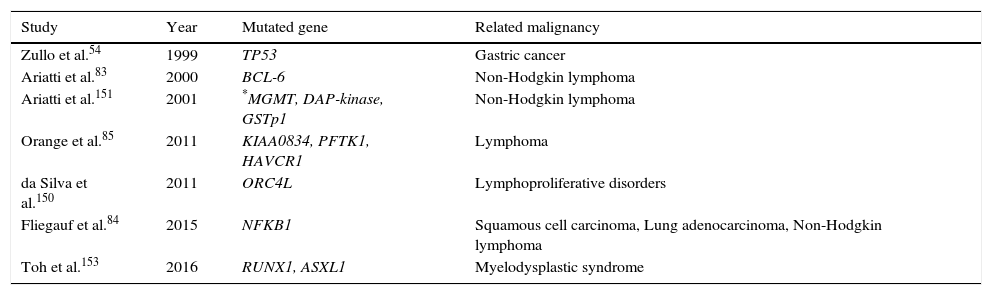
Genetic predispositionA genetic predisposition to some types of malignancies is a probable aetiology of cancer in some PID patients, although not usually as a result of oncogenic genes. Instead, other mechanisms such as the presence of certain mutations or defective tumour suppressor genes appear to be involved.74,75 Certain mutations in the specific genes such as BRCA1, BRCA2, BARD1 and BRIP1 greatly increase a person's risk of developing breast cancer and ovarian cancer, but the contribution of these genetic changes to CVID patients’ overall risk is not investigated. Mutations in a main tumour suppressor gene, tumour protein p53 (TP53) encoding for p53 protein, are one of the most common genetic variations in human cancers, and contribute to the complex network of molecular events leading to tumour formation.76 In numerous studies, defects in p53 have been reported in gastric cancer from Japan,77 Portugal76 and Germany.78 Zullo et al.54 hypothesised that p53 alterations can play a role in the gastric carcinogenesis of patients with CVID. However, in a study by Kilic et al. they found no statistically significant correlation between the presences of TP53 mutations in CVID patients, also none of the 20 CVID patients were found to have TP53 gene mutations. In addition, TP53 mutations were not detected in tumour biopsy. However, during nine years follow-up, one patient developed non-Hodgkin lymphoma.79 Genetic variation in other genes including B-cell lymphoma 2 (BCL2), B-cell lymphoma 6 (BCL6),80,81TNF, LTA, NFKB1,82 and PFTK1 (Table 2) which are reported in some types of malignancy are also associated with CVID.83–85
The most common genetic mutations associated with malignancies in CVID patients.
| Study | Year | Mutated gene | Related malignancy |
|---|---|---|---|
| Zullo et al.54 | 1999 | TP53 | Gastric cancer |
| Ariatti et al.83 | 2000 | BCL-6 | Non-Hodgkin lymphoma |
| Ariatti et al.151 | 2001 | *MGMT, DAP-kinase, GSTp1 | Non-Hodgkin lymphoma |
| Orange et al.85 | 2011 | KIAA0834, PFTK1, HAVCR1 | Lymphoma |
| da Silva et al.150 | 2011 | ORC4L | Lymphoproliferative disorders |
| Fliegauf et al.84 | 2015 | NFKB1 | Squamous cell carcinoma, Lung adenocarcinoma, Non-Hodgkin lymphoma |
| Toh et al.153 | 2016 | RUNX1, ASXL1 | Myelodysplastic syndrome |
Evidence demonstrates that family history of malignancy35 as well as some autoimmune and chronic inflammatory diseases including celiac disease, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, psoriasis and autoimmune cytopenias are associated with the development of malignancy.39,84,86–88 The prevalence of autoimmune diseases in patients with CVID is higher than 20% reported in the literature.89 Therefore haematological malignancies especially NHL and Hodgkin lymphomas should be suspected in CVID patients, since half of CVID patients with autoimmunity presented autoimmune haematological diseases including autoimmune haemolytic anaemia (AIHA) and immune thrombocytopenic purpura (ITP).90–93 However, there has been no report of a long-term follow-up cohort study in CVID patients to confirm the incidence of haematological malignancies following autoimmune disorders.
Genetic instabilityThe higher incidence of cancer in CVID cases has also been explained by genomic instability. It is manifested by an increased level of chromosomal damage after mutagenic stresses like radiation or chemical agents in the environment, as well as from endogenous DNA-damaging agents that arise by-products of cellular metabolism.94–97 There are several reports which indicate radiosensitivity of DNA in CVID patients after exposure to radiation98–100 and its involvement with malignancy progress.55,95,101
In CVID patients, a dose-dependent chromosomal instability was demonstrated by in vitro irradiation of lymphocytes.98 Irradiated lymphocytes show higher chromosomal aberrations and lower mitotic indices than lymphocytes of control, indicating an increased chromosomal radiosensitivity in some CVID patients.99,102 The impact of these findings could be greatly improved by identifying the underlying genetic cause of radiation sensitivity in CVID patients. Recently, the cause of radiosensitivity in CVID patients is explained as defects in DNA repair pathways, which acts as the primary response to ionising radiation in healthy individuals.95,102 It is demonstrated that ionising radiation induces a broad spectrum of lesions in DNA, and double strand breaks (DSBs) represent the most significant lethal lesions.97 The main mechanism for the repair of DSBs in DNA is non-homologous end-joining (NHEJ), a process that rejoins breaks with the use of little or no homology. The main proteins that operate in NHEJ are Ku70, Ku80 and DNA-dependent protein kinase (DNA-PK) complex, as well as XRCC4 and DNA ligase IV.97,103,104
In a recent study, Van Schouwenburg et al.105 identified five CVID patients with heterozygous variants in genes which are involved in NHEJ. They also found variants in DCLRE1C, PRKDC, RAG2, NHEJ1, MRE11A, ATM and NLRP2 genes in CVID patients. All these genes are important in the initiation of V(D)J recombination or DSB repair by NHEJ. As NHEJ is essential in DNA repair, inefficient NHEJ may lead to malignant conditions in a subgroup of CVID patients.106 In 2011, Duvvuri et al. provided evidence that some CVID patients have defective repair of activation-induced cytidine deaminase (AID)-induced mutations, which is directed by the DNA mismatch repair machinery including mutS homolog 2 and 6 (MSH2/MSH6), Exonuclease 1 (EXO1), and MutL homolog 1 (MLH1)/PMS2.107 Moreover, some mutations in genes of the Mre11-Rad50-Nbs1 (MRN) complex and the homologous recombination pathway as well as MSH5 gene have been reported in CVID.101,108 Paradoxically, Nemati et al.109 did not confirm the previously observed association between the RAD50 and development of CVID in an Iranian population. However, a growing body of evidence shows the importance of DNA mismatch repair in the pathogenesis of CVID, at least in subgroups of these patients.110 Altogether, defective repair of AID-induced mutations with reports of increased chromosomal radiation sensitivity and associated lymphoproliferative disorders in CVID patients, suggest that altered DNA damage repair may be a cause of malignancy in CVID.107
Most common malignancies in CVIDGastric cancerBased on an epidemiological data from the American Cancer Society, gastric cancer is the fourth most common type of cancer in males and the fifth most common form in females, accounting for 6.8% of the total cancer cases and 8.8% of total cancer-related deaths in 2012 globally.111–113 Several studies reported increased risk of gastric cancer (7–47-fold) in CVID patients.12,17,114,115 In a large study during 1990–2008 by ASCIA registry, of 416 studied CVID patients from 79 centres in Australia, a seven-fold increase in the risk of gastric cancer has been reported.17 In other studies, Bonilla et al. in 2005 and Mellemkjaer et al. in 2002 reported a 10-fold increased risk of gastric cancer and more precisely gastric carcinoma in patients with CVID.12,115 However, an old study in 1985 showed surprisingly higher results. During 11 years follow-up of 220 CVID patients, a 47-fold increase in the risk of gastric cancer was reported.114 Moreover, the Standardised Incidence Ratio (SIR) of gastric adenocarcinoma in CVID patients in Sweden and Denmark was equal to 10.3.12
There are modifiable and non-modifiable risk factors for gastric cancer in the general population. Non-modifiable include advanced age, male gender, genetic predisposition, blood group A, radiation, lower socio-economic status, gastric surgery and a history of EBV infection. Modifiable risk factors include H. pylori infection, smoking, pernicious anaemia, diet (consumption of salt-preserved foods and N-nitroso compounds), and geography.116,117 Other than those mentioned, there are some risk factors which are more relevant to gastric cancer in CVID. These CVID-associated factors include pernicious anaemia, gastric atrophy, achlorhydria, decreased gastric IgA, and chronic H. pylori infection.17,118 Moreover, mutations in tumour suppressor TP53 gene have been reported to be related to an increased risk of gastric cancer in CVID. In a study by Zullo et al. six (18%) of 34 CVID patients had overexpression of p53.54 However, in another study, no significant relation was found between TP53 mutation and gastric cancer in CVID patients.79
For many years it has been demonstrated that infection with H. pylori causes chronic inflammation and significantly increases the risk of developing duodenal and gastric ulcer disease which is associated with a two- to nine-fold increased risk of gastric cancer.119–122 Although such a precise study has not been conducted in CVID patients, some gastrointestinal alterations such as decreased production of gastric IgA and hydrochloric acid have been proposed to predispose CVID patients to H. pylori infection that causes more gastric inflammation and increased carcinogenesis.123,124 On the other hand, infection with H. pylori causes chronic gastritis and gastric atrophy that leads to a higher gastric PH and it permits the proliferation of nitrate-reducing anaerobic bacteria which are involved in the pathogenesis of gastric cancers through the generation of carcinogenic N-nitrosamines.125 Considering the high-risk factors for gastric cancer in CVID, periodic screening for H. pylori infection and endoscopic follow-up is recommended for CVID patients.
Breast cancerBreast cancer is the most common cancer in women worldwide, and second most common cancer overall after lung cancer.126 ASCIA registry in Australia reported a significant increase of breast cancer in female CVID patients with a SIR of 2.24 (95% CI 1.02–4.24), and nine observed cases from more than 400 patients with CVID.17 Similar data was shown in a long-term cohort study of 224 CVID patients, who were followed for a mean time of 11.5 years in Italy. In this cohort, two patients with breast cancer and a Standardised Prevalence Ratio (SPR) of 2.02 (95% CI 0.22–7.29) was reported.127 Finally, in the largest cohort by European Society for Immunodeficiencies, 13 cases of breast tumours were identified among 2212 patients with CVID.128 Immune impairment may also have a role in the development of breast cancer in CVID patients by increasing susceptibility to some infections such as the mouse mammary tumour virus, which has been found in 38% of human breast cancers and shared DNA repair gene defects.15
Thymus cancerThymoma is a rare malignancy with unknown aetiology. According to cancer registry data, the overall incidence of thymoma in the U.S. is 0.13 per 100,000 person-years. Thymoma is very uncommon in children and young adults.129 Several studies have suggested that patients with thymoma have an increased risk for other malignancies, especially for developing B-cell non-Hodgkin lymphoma, consistent with an effect of immune disturbance arising from the thymoma or its treatment. Also, patients with thymoma may have an elevated risk of developing soft tissue sarcomas.130,131 Hypogammaglobulinaemia could be associated with 6–11% of thymoma patients mimicking CVID, known as Good's syndrome.132,133 This subgroup of patients potentially suffers from a cell-mediated immune defect due to an increased risk of opportunistic viral infections including herpes simplex virus (HSV), varicella zoster virus (VZV), CMV and HHV8 and autoimmune conditions (myasthenia gravis, pure red cell aplasia and pernicious anaemia).133 An enormous 146-fold increased risk of thymus cancer in CVID patients has been reported in 416 patients in the ASCIA registry.17 In another study, a large excess risk for thymoma was observed on the basis of two thymoma cases occurred in CVID patients.126
LymphomaThe incidence rate of lymphoma in CVID patient is approximately 7–10%, which appears to be higher than in the normal population.34,128 For the first time Kinlen et al.,114 in 1985 reported a 30-fold increase in the risk of lymphoma by performing a prospective study of 220 CVID patients with 11 years’ follow-up. Two years later, Cunningham-Rundles134 reported a 259-fold increase in lymphoma risk. Quinti et al.127 in 2007 reported a 12–18-fold increased incidence of lymphoma development in CVID patients. Despite advances in immunoglobulin replacement, infection surveillance and antibiotic administration, lymphoma remains the most common severe complication of CVID, which can progress even with no prior history or symptoms of lymphoid hyperplasia.135
NHL is the most frequent CVID-associated malignancy and several studies have shown that NHL had estimates ranging from 3 to 8%.5,17,34 In a long-term study, 38 of 416 patients with CVID had malignancy, which NHL represented 29% of the total.17 However, there are much fewer reports of Hodgkin lymphoma in CVID, compared to NHL. In one cohort of 473 patients with CVID, there were only four cases (0.8%) of Hodgkin lymphoma, while 32 patients (6.7%) developed NHL.14
Lymphomas in CVID are usually extranodal, B cell in type, more common in adult patients and are usually EBV-negative.34,136,137 In the US report with 1–25 years’ follow-up of cases, 8.2% of 248 CVID patients had lymphoid malignancies which were all B cell in type. NHL was the most frequent diagnosis,19 with some of these being further classified into specific B-cell phenotypes, including MALT, marginal zone lymphoma, and T cell-rich B-cell EBV-associated lymphoma.33 MALT lymphomas are the most common NHL subtype in CVID patients. In a review of MALT lymphomas, extranodal marginal zone were among the most frequently reported lymphomas.21,54,138 Several other cases of MALT lymphomas, localised in the lung and salivary glands have also been published.11,139,140
There are some proposed risk factors for increased of lymphoma in CVID patients, these include chronic infections (such as HHV8, CMV, EBV and H. pylori), persistent inflammatory autoimmune disease, immune dysregulation and radiosensitivity,11 but their relative contribution and exact mechanism in CVID is still unknown.
Several studies show that defective immune responses may influence susceptibility to lymphoma due to complicated underlying mechanisms. Importantly, the majority of patients with CVID typically have a defective T-cell response to mitogens and microbial antigens141,142 which has been proposed as an explanation for the increased risk of malignancy especially in those with late-onset combined immunodeficiency (LOCID) presenting with CVID-like phenotype. It is reported that 29% of patients with LOCID developed lymphoma versus 4% of patients with CVID without associated T cell defects.143 There is an association between the number of invariant natural killer T (iNKT) cells in CVID patients with the percentage of class-switched memory B cells and propensity to lymphoproliferation.144 Moreover, it has been demonstrated that CVID patients have reduced numbers of T helper-17 (Th17) cells in their circulation.145 According to the role of interleukin 9 (IL-9) in Th17 cells expansion, one can reasonably suppose that decreased number of Th17 cells in CVID patients may lead to an increase in the IL-9 level as a compensatory effect.146 On the contrary, the studies demonstrating effective interventions of IL-9 in causing Hodgkin lymphoma,147 through the potential role of this cytokine as a tumorigenesis factor in growth/proliferative and anti-apoptotic activities on the different transformed cells.148 These findings are consistent with studies showing that IL-9 induces thymic lymphomas in mice, and IL-9 production is associated with the Hodgkin disease and human T-lymphotropic virus type-1 transformed T-cells in human.149
Recently, it has become clear that some genes including HAVCR1, PFTK1 and KIAA0834 play an important role in the development of lymphoma in CVID patients.85 The vast majority of patients with CVID were identified with duplications in a gene for initiation of DNA replication, namely ORC4L, which is related to the B cell lymphoproliferative disorders.150 In addition, there are few studies showing that chromosomal rearrangements of BCL6 were detected in two-thirds of patients with CVID and NHL.83,151 In addition, mutations in BCL6 have been suggested as a genetic marker for explaining the histogenesis of B-cell lymphoproliferation in CVID patients. In one study by Ariatti et al.151 a panel of five CVID-related NHL was used to accurate analysis of histogenetic markers which disclose that somatic hypermutation of IgVH and BCL-6 genes occurred in 5/5 cases and mutations were stable in all cases, with no evidence of ongoing mutation processes. Promoter hypermethylation of O6-methylguanine-DNA-methyltransferase (MGMT), glutathione S-transferase (GST) p1, as well as of death-associated protein (DAP)-kinase, was detected in 2/5, 3/5 and 3/5 CVID related NHL, respectively. Hypermethylation of the MGMT, DAP-kinase and GSTp1 genes occurs at sustained frequencies in CVID-related NHL and may give novel therapeutic targets and prognostic markers for the clinical management of these lymphomas.151 Also, one patient, who had TP53 gene mutations, developed NHL during nine years’ follow-up in the Kilic et al. study in 2012.
LeukaemiaAlthough NHL is the most common haematological malignancy seen in CVID, Myelodysplastic Syndrome (MDS) and acute leukaemia can also be seen in these patients. In 2006 Ayyildiz et al.152 reported an aggressive natural killer cell leukaemia (ANKL) in a woman with CVID. In the recent study Toh et al. in 2015 shown that three CVID patients have been diagnosed with MDS and acute leukaemias. The first case was a 60-year-old male with asymptomatic CVID and acute lymphocytic leukaemia (ALL). The second case was a 78-year-old male with CVID and AIHA. He was diagnosed with high-risk MDS with complex cytogenetics and an RUNX1 mutation. The third case was a 74-year-old male with CVID complicated by pulmonary hypertension, neutropenia, ITP and AIHA which was recently diagnosed with MDS with ASXL1 mutation.153
Prevention, screening, treatment and prognosis of malignancies in CVIDEpidemiological studies have suggested an increased risk of cancers in CVID patients with female gender, cases with higher levels of serum IgM and polyclonal lymphocytic infiltration phenotype.14,154 Furthermore, family histories of neoplasia could be a hint for the presence of genetic predisposing factor (genes involved in DNA repair and cancer immunosurveillance) in a selected group of patients helping towards finding a cause of immunodeficiency and also for implementation of preventive cares.75,155
For prevention of malignancy in CVID, the known vulnerability factors contributed to the development of malignancies should be considered in routine follow-up evaluation including H. pylori screening and eradication; monitoring of overgrowth of nitrosamine-producing bacteria due to hypochlorhydria in cases with autoimmune pernicious anaemia; decreasing unnecessary irradiation particularly in cases with defined chromosomal radiosensitivity.156 In addition to autoimmune and rheumatological disorder, CVID patients suffer from severe respiratory infections, therefore they may frequently undergo medical imaging that exposes them to irradiation. Since these patients might be sensitive to radiation, they should be protected from unnecessary medical techniques that incorporate radiation; substitution with alternative imaging including ultrasonography or magnetic resonance imaging is suggested for these patients.92,157 Beside punctual age-appropriate cancer screening such as gastric cancer (upper endoscopy), cervical cancer (Pap test and HPV tests), colorectal cancer (finding of precancerous polyps by sigmoidoscopy or colonoscopy), breast cancer (clinical breast examinations and mammograms) and lung cancer (yearly screening with low-dose computed tomography), in patients with high risk factors and exposed to oncogenes, screening by complete blood counts (white blood cell counts and differential), histopathological investigation (bone marrow aspiration and persistent enlarged lymph nodes) and endoscopy (for finding mucosal changes) should be considered if it is indicated medically.156
Treatment of malignancies in CVID is similar to routine chemotherapy protocols and surgical modalities for immunocompetent cancerous individuals but there is controversy for the use of radiotherapy due to the lack of an established threshold for radiation-induced aberrations in radiosensitive patients. Adjutant therapy could be considered in a selected group of patients using rituximab (excluding CD20 deficient patients and in patients with activated AKT antiapoptotic survival), rapamycin (in patients with activated PI3K pathway signalling) and haematopoietic stem cell transplantation (in patients associated with impaired T-cell immunity).156,158,159 Of note the dose adjustment for immunoglobulin replacement is necessary during treatment of malignancies (e.g. lymphoma and leukaemia) in patients with CVID due to a secondary cause of hypogammaglobulinemia.160,161
Not surprisingly, several prognostic studies have shown that CVID patients with cancer phenotype (particularly lymphoid malignancy) had the highest mortality rate (relative risk of 5.5 to infections only phenotype patients).7 Death due to malignancy accounts for 5.7–10.2% of mortality causality in different CVID cohorts14,18 and these patients have the overall survival over 15 years of 28% in Iranian patients and over 40 years approximately 36% in an Italian cohort.15,18
ConclusionAligned with developing different therapeutic modalities for severe infectious and non-infectious complications of CVID patients and improvement of their life expectancy, neoplasia (particularly lymphoid malignancies in younger patients and gastrointestinal tract malignancies in elder patients) become a major medical concern for treating physicians and clinical immunologists which needs intensive screening evaluations and consequently preventive interventions and timely treatment. Similar to the general population, in CVID patients the cancer risk can be reduced with changes in diet, lifestyle, and finding of precancerous conditions early as well as accurate and timely diagnosis of lymphoproliferative, infectious and autoimmune diseases. Screening for common cancers helps find these diseases at an early stage, when treatment works best. Chemoprevention and avoidance of unnecessary medical radiation are another approach. Protection from cancer-causing bacteria especially H. pylori, and certain viral infections (transforming viruses) by quick medical diagnosis and treatment is also recommended.
Financial and conflict of interest's disclosureThe authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.