Inhaled corticosteroids are used to treat infants with troublesome asthma-like symptoms but their effect on the lung function of these young patients is controversial.
Material and MethodsForty-four infants with recurrent wheezing (more than 3 episodes) and family history of asthma completed this randomised, parallel, double-blind, controlled trial to compare the effect on lung function (main endpoint) of once-daily inhaled fluticasone (375μg) versus placebo for 3 months. Pulmonary function was measured while infants were asymptomatic, using the raised volume rapid thoracic compression technique (spirometry-like), and values were converted to z-scores.
ResultsThe fluticasone group showed a significant increase in forced flows, (p<0.001), a lower number of physician diagnosed wheezing episodes (p<0.002), and a significant decrease in the parent-reported number of wheezing episodes per month (p<0.03), as compared to placebo. One third of parents in the placebo group reported a clinical improvement in their infants. There was no significant difference in morning plasma cortisol between groups at entry or discharge.
ConclusionsWe conclude that once-daily treatment with 375μg fluticasone increased forced flows and controlled symptoms in infants with recurrent wheezing without altering plasma cortisol levels. The spirometry-like technique is a useful tool to objectively assess the efficacy of anti-asthma medications in infants with repeated troublesome asthma-like symptoms.
Recurrent wheezing (RW) is a common health problem during infancy and probably one of the most frequent causes of medical consultation and admissions during the first two years of age. Several infants with RW are treated with regular inhaled corticosteroids (ICS) at the clinical setting even though there is controversy about their effect on symptoms and lung function in infants with recurrent asthma-like symptoms. It has been reported that inhaled fluticasone delivered to wheezy infants using pressurised metered dose inhalers (pMDI) plus holding chambers is useful to control respiratory symptoms and to improve lung function1–7. However, other randomised controlled trials have not found clinical or functional benefit when treating infants with RW with pMDI fluticasone 8 or p MDI beclomethasone 9. Regardless of discrepancies on the beneficial effects of ICS in infants with RW, those medications are being increasingly prescribed to infants with repeated asthma symptoms in the clinical practice. Recently the use of ICS in those young patients has been supported by evidence-based recommendations10,11.
Although ICS have been reported to be useful for the treatment of infants with asthma-like symptoms, the few trials which have considered lung function as endpoint reported contradicting results on the effect of ICS on lung function in wheezy infants12. The latter is likely to be the result of large methodological differences between studies regarding case definitions, age of patients, formulation of pMDI ICS, doses and delivery systems (jet nebulisers or pMDI plus spacers), aerosol quality (particle size distribution) and importantly, in the techniques employed for measuring lung function in infants.
A recent spirometric-like technique, the raised volume rapid thoracic-abdominal compression (RVRTAC), which performs measurements in a more physiological range13 and has a very low variability14, may offer significant advantages over other methods to assess the functional effect of ICS in infants with RW. This technique measures forced volumes (FVC and FEVt) and expiratory flows (FEF50 %, FEF75 %, FEF25-75 %) from a lung volume quite close to total lung capacity which makes it, as occurs with spirometry in older children, suitable to assess changes of lower airway calibre determined by treatments or diseases.
This randomised, double-blind and placebo-controlled study was undertaken to determine the effect of once-daily inhalation of 375μg of fluticasone propionate, inhaled during 12weeks, on forced expiratory flows measured by the RVRTAC technique in infants with recurrent wheezing at risk of asthma.
Material and MethodsSubjectsFifty infants with three or more troublesome episodes of physician diagnosed wheezing and at least one direct relative (parent or sibling) with physician-diagnosed asthma were recruited from our outpatient clinic at the Department of Pediatric Respiratory Medicine, Hospital El Pino in Santiago de Chile. Subjects were excluded if they had a birth-weight less than 2.5 kilograms, oxygen requirement in the neonatal period, cystic fibrosis, cardio-pulmonary malformations, neurological impairment, or other chronic diseases with eventual repercussions on the respiratory system. They were also excluded if they had been treated with systemic corticosteroids or registered hospital admission in the preceding 2months. Full written, informed and signed consent was obtained from parents and the study was approved by the Hospital's Ethics Committee.
The primary objective of the study was to assess the effect of once daily inhaled fluticasone propionate 375μg on forced expiratory flows (FEF50, FEF75 and FEF25-75) as compared to placebo for a period of 3months. Secondarily, we assessed the effect on some clinical variables such as reduction in the number of wheezing exacerbations, parental reporting of disease improvement and number of symptom-free days. The study was conducted during the viral season (autumn-winter) as a double-blind, randomised, placebo-controlled, parallel-group trial.
Following a run in period of at least two weeks, infants were scheduled for measuring baseline lung function and then were randomly allocated to receive either A) inhaled fluticasone propionate 375μg (3 puffs of FP 125μg; or B) placebo (3 puffs) daily in the morning for 3months using a valved holding chamber treated for static with detergent (Aerochamber Plus®). For the fluticasone group, the MDI contained 125μg per activation, Flixotide® (GSK, UK) and for the placebo group the MDI contained Placebo (GSK, UK), indistinguishable from those containing the active medication. In addition, inhaled Salbutamol 200μg (100μg per activation, Aerolin®, GSK, UK) was employed if required due to cough or wheezing.
Parental EvaluationParents were experienced in recognizing wheezing and cough and they wee explained to record wheezing or whistling sounds in the daily card as a combined categorical score (yes or no). Also, parents were requested to bring the child to our respiratory outpatient clinic if they started with wheezing or cough.
Pulmonary FunctionPulmonary function (PF) was assessed at entry (baseline) and completion of the study (between the 12th-15th week) by the raised-volume rapid thoracic-abdominal compression technique (RVRTC), under sedation with chloral hydrate (70–100mg/kg), as previously described14 and following international recommendations 13. Lung function was measured when infants were asymptomatic and salbutamol was stopped 8 hours prior to testing. All infants were tested at least 2 hours postprandial to reduce the effect of gastric contents on lung volume and pulse oxymetry was monitored during the tests. A facemask was positioned over the infant's face, covering mouth and nose, and the cervical region was maintained in a slightly overextended position. Several lung inflations at 30 cmH2O were performed until there was a clear respiratory pause, then the lung was inflated again and at this point thoracic compression was initiated and maintained until residual volume was reached. Forced expiratory manoeuvres were repeated with increases of 5 to 10 cmH2O in jacket pressure until maximum expiratory flows were obtained (no further increase in flows). The best curve was selected as that with the highest product of FVC and FEF25-75% and the best shape (without transients). The equipment for spirometry in infants employed at our laboratory in Santiago, Chile, was custom-built and tested by specialised engineers and pulmonologists from the Pediatric Pulmonology Section, Riley Hospital for Children, University of Indiana, USA.
Growth and safetyAt each scheduled visit the infant's body length was measured by the same investigator using an infantometer. In addition, morning serum fasting cortisol (between 8–9a.m.) was measured by radio-immune assay at baseline and discharge from study.
Analysis of dataLung function values, baseline and at the end of study were converted to z-scores using the regression equations of Jones et al15, and the results were compared using test for paired samples (within group) and for independent samples (between groups). For all the spirometric parameters assessed, a z-score less than —1.65 was considered as abnormal. Spirometric variables, which are dependent upon somatic size, were analysed using analysis of co-variance and adjusting for body length. Categorical variables, such as presence or absence of wheezing in the month previous to visit and tobacco exposure were compared using Fishers exact test, while continuous variables, such as number of wheezing episodes, plasmatic cortisol and somatic size were analysed by t-test and p values less than 0.05 were considered statistically significant.
ResultsOf the 50 infants who entered the study, six subjects were withdrawn: five patients because parents were unwilling to continue with the trial (three in fluticasone group and two in placebo group), and one from the placebo group because of receiving oral corticosteroids prescribed for a wheezing exacerbation soon after randomisation.
Therefore, we are reporting the results of lung function measurements in the 44 infants (21 in the fluticasone group and 23 in placebo group) who completed the study.
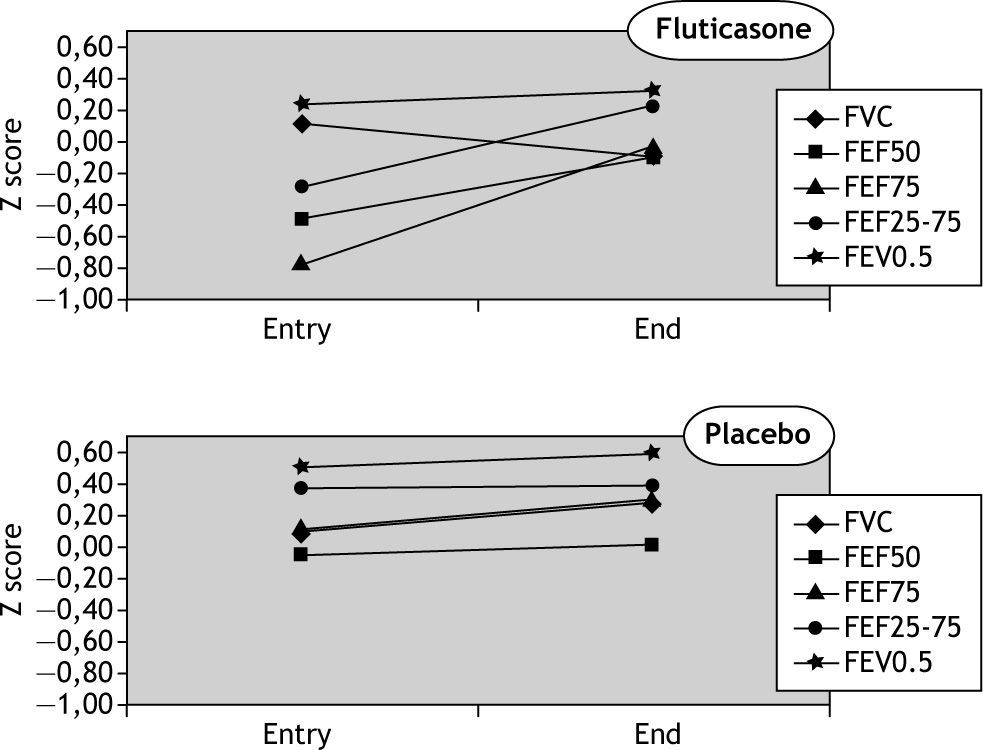
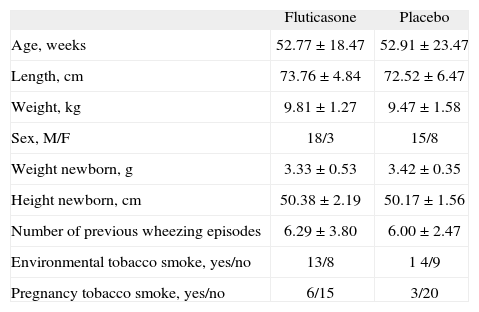
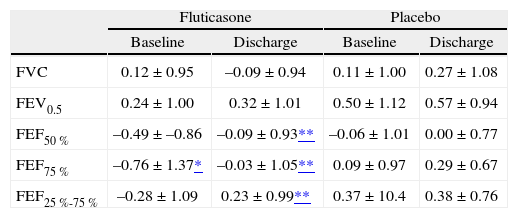
Upon entry the two groups did not significantly differ with respect to gender, age, somatic size, history of exposure to indoor tobacco smoke, maternal smoking during pregnancy or the number of previous episodes of wheezing, admissions, and emergency room visits (Table I). However, the fluticasone group had a slight but significantly lower FEF75 % than the placebo group (Table II). Mean values of lung function in both groups, measured baseline and at discharge were within the normal range (Table II).
Baseline characteristics of infants with RW in both study groups
| Fluticasone | Placebo | |
| Age, weeks | 52.77 ± 18.47 | 52.91 ± 23.47 |
| Length, cm | 73.76 ± 4.84 | 72.52 ± 6.47 |
| Weight, kg | 9.81 ± 1.27 | 9.47 ± 1.58 |
| Sex, M/F | 18/3 | 15/8 |
| Weight newborn, g | 3.33 ± 0.53 | 3.42 ± 0.35 |
| Height newborn, cm | 50.38 ± 2.19 | 50.17 ± 1.56 |
| Number of previous wheezing episodes | 6.29 ± 3.80 | 6.00 ± 2.47 |
| Environmental tobacco smoke, yes/no | 13/8 | 1 4/9 |
| Pregnancy tobacco smoke, yes/no | 6/15 | 3/20 |
Values are expressed as mean ± SD
Lung function values (mean ± SD) in infants with RW treated with inhaled fluticasone or placebo by 3months
| Fluticasone | Placebo | |||
| Baseline | Discharge | Baseline | Discharge | |
| FVC | 0.12 ± 0.95 | –0.09 ± 0.94 | 0.11 ± 1.00 | 0.27 ± 1.08 |
| FEV0.5 | 0.24 ± 1.00 | 0.32 ± 1.01 | 0.50 ± 1.12 | 0.57 ± 0.94 |
| FEF50 % | –0.49 ± –0.86 | –0.09 ± 0.93** | –0.06 ± 1.01 | 0.00 ± 0.77 |
| FEF75 % | –0.76 ± 1.37* | –0.03 ± 1.05** | 0.09 ± 0.97 | 0.29 ± 0.67 |
| FEF25 %-75 % | –0.28 ± 1.09 | 0.23 ± 0.99** | 0.37 ± 10.4 | 0.38 ± 0.76 |
In the fluticasone group there was a significant increase in the mean values of forced flow parameters but the change in forced volumes (FVC and FEV0.5) was not significant. Also, no significant changes occurred in the placebo group for any of the spirometric parameters (Fig. 1). There was no association between change in lung function and the number of previous episodes of wheezing, exposure to tobacco, sex, length and weight. No correlation was found between changes in symptoms and in lung function.
Parental AssessmentWheezing and cough in the four weeks prior to entering the study were reported by parents in almost all infants in both groups (22/23 in fluticasone group and 22/24 in the placebo group). At discharge from the study, the proportion of mothers who reported their children were “definitely better than when they entered the study” was significantly greater for the fl uticasone (95.2 %) than for the placebo group (30.4 %),p < 0.001).
Physician AssessmentThe number of infants who had wheezing on physical examination by the paediatric respiratory physician at randomisation was not significantly different between groups: 16/25 in the ICS group and 15/24 in placebo group (Fisher, p = 0.75).At the end of the study the number of infants with wheezing as found by the same respiratory physician (blind to treatments) was 2/21 in the ICS group and 11/23 in placebo group (Fisher, p < 0.001).
The mean number of physician-diagnosed wheezing episodes per group during the study was significantly lower in the fluticasone group (0.35; 95 %CI, 0.10-0.60) as compared to placebo (1.08; 95 %CI, 0.71-1.46). In addition, the mean number of physician diagnosed URTI episodes per group during the study was significantly lower in fluticasone as compared to placebo (0.3; 95 %CI, 0.1-0.5 vs. 1.0; 95 %CI, 0.6-1.4; p < 0.002), respectively.
Growth and safetyThere was no significant difference in longitudinal growth between fluticasone (1.2cm; 95 %CI, 1.0cm-1.4cm) and placebo (1.3cm; 95 %CI, 1.1cm-1.5cm) groups. In both fluticasone and placebo groups, the plasma cortisol level at entry and after ending the study was within the normal range and the difference within or between groups was not significant. Values for serum cortisol in fluticasone and placebo groups at the end of the study were 13.3 (95 %CI, 10.8-15.8) μg/dL and 15.2 (95 %CI, 11.4-18.7) μg/dL, respectively.
The average number of salbutamol doses (puffs) administered daily by parents to infants in the fluticasone group tended to be lower than the number of puffs administered to the placebo group but the difference was not statistically significant (2.5 [95 %CI, 2.0-3.0] vs. 3.0 [95 %CI, 2.6-3.5]; p = 0.10).
DiscussionThis study found that once-daily inhalation of 375μg fluticasone during 3months caused a modest yet significant increase of forced expiratory flows (FEF50, FEF75 and FEF25–75) measured by the RVRTAC technique in infants with RW at risk of asthma, without significant changes in FVC and FEV0.5. This finding would suggest an underlying airway inflammatory defect as responsible for the bronchial narrowing in these infants, which could be better detected by measures of forced expiratory flow than forced expiratory volumes (FVC, FEV0.5) as previously reported16. At the same time this study suggests that spirometric-like measurements using the RVRTC technique can be used at clinical level for the follow-up of infants with troublesome and recurrent asthma symptoms as a direct and objective assessment of the response to treatments.
The group treated with inhaled fluticasone also showed a significant clinical improvement, in terms of parent-reported control of symptoms and fewer physician diagnosed wheezing episodes during the study period as compared to placebo group. The parental estimation of changes induced by medications in infants with recurrent asthma-like symptoms, although used in several studies, may be deeply influenced by cultural and other factors that would decrease the objectivity of parental assessment of symptoms in their infants. This seems to be supported by the finding of this and other studies using randomised, double-blind and placebo-controlled designs, since an important number of infants in the placebo groups are reported to improve their symptoms8,9. In our study 32.4 % of parents of infants in the placebo group reported their children were “definitely better” than when they entered the study. It has been reported that parent-reported symptoms are not sufficiently reliable, and the agreement between parents' and clinicians' reports of wheeze is less than 50 %17.
In the present study lung function was measured when infants were asymptomatic and that would explain the normal spirometric values obtained in both groups. However, the significant increase of forced expiratory flows (FEF50, FEF75 and FEF25-75) and FEV0.5/FVC in the group treated with fluticasone would suggest an underlying sub-clinical airway inflammation in infants with RW as previously found using airway endoscopic methods 18,19. The normal lung function found in our infants when they were free of symptoms reinforces previous findings by other authors who, using the same technique (RVRTC), demonstrated that when asymptomatic, RW infants have spirometric lung function within the normal range16.
The results from studies regarding the effect of ICS on the lung function of wheezy infants are rather conflicting. Moeller et al5, in infants with recurrent wheezing and increased exhaled nitric oxide, found that 100μg twice-daily fluticasone during 4weeks significantly decreased the level of nitric oxide but had no significant effect on FEV0.5 and symptoms. On the other hand, Teper et al 3 reported that inhaled fluticasone 125 ug twice daily for 6months in infants and young children with RW resulted in a significant increase in VmaxFRC and better control of symptoms than placebo. However, Hofhuis et al7, reported that after 3months of treatment with inhaled fluticasone propionate, 200 ug daily in wheezy infants, there was no improvement in lung function (VmaxFRC) and no reduction in respiratory symptoms compared with placebo. Similarly, Schokker S et al 8 in a six-month study about the effect of 100μg of inhaled fluticasone twice daily in wheezy preschool children aged 1–5years, found that symptoms improved in both fluticasone and placebo groups, and there was no significant difference between ICS and placebo, nor in lung function between groups assessed by the interrupter technique and forced oscillation technique.
The differing results of studies that have assessed the effect of ICS in infants with recurrent wheezing, either using clinical score systems or lung function measurements, are likely to be the result of the marked differences in methodology among those studies. The different case definitions (wheezy infants, recurrent wheezing, persistent wheezing, asthmatics), the large variations in the age of the study groups (between 1–5years) and variation in treatments (treatment period, ICS formulations, doses and different delivering systems) may influence the results thereby precluding valid comparisons among such studies.
The fluticasone dose we used (375μg) could appear as high, however, several factors inherent to aerosol characteristics and delivery system (type of valved holding chamber, static charge, mask seal, etc), as well as to those related to infant peculiarities (bad cooperation, changing pattern of breathing and behaviour while inhaling aerosols, and infant airways which favours nasal or upper airway deposition), could markedly decrease the final dose of medication that reach the infants' lower respiratory tract. Under those conditions the dose/effect relationship for ICS administered to infants by pMDI plus spacer, at the commonly prescribed doses and at the daily practice would predictably be suboptimal. It is well known that a very low proportion of the dose delivered will finally reaches the infant lower airway, and particularly the peripheral airway, either when using pMDI with holding chamber20 or jet nebulisers21. Dubus et al22 reported that a markedly low percentage (3 %) of the fluticasone dose is delivered from plastic holding chambers to the respiratory tract of infants and toddlers. It is likely that modern pMDI corticosteroids formulations (as HFA solutions, not HFA suspensions) that produce an aerosol with a very large proportion of its mass contained in extra-fine particles (< 2.1μm) and ultra-fine (< 1.2μm) are more appropriate to reach the infant bronchi and bronchioli than aerosols from conventional pMDI corticosteroids (CFC and HFA suspensions)23. These HFA extra-fine ICS deliver a substantially higher dose to the lungs because extra-fine aerosols are independent from breathing pattern and can efficiently bypass the nose, and this favours the aerosol entry and peripheral airway deposition. It has been reported that lung doses for pMDI HFA beclomethasone solution in infants are between 3.5 to 10-fold greater than for pMDI CFC beclomethasone24. However, whether the extra-fine ICS are able to determine a better effect on improving lung function or symptoms in infants with RW remains to be proven.
The interpretation of the lower occurrence of physician diagnosed URTI (colds) in the group treated with fluticasone as compared to placebo is somehow limited by the small sample size of this study and because it was not considered as an endpoint in the study design. However, it has been shown that high doses of inhaled budesonide at the onset of upper respiratory symptoms reduced the severity of wheezing and the number of documented episodes of URTI25. Furthermore, a recent study in preschool-age children with moderate-to-severe virus-induced wheezing shows that preemptive treatment with high-dose fluticasone, as compared with placebo, reduced the use of rescue oral corticosteroids26.
In the present study, which included only infants less than 2years of age, the once daily use of inhaled fluticasone 375μg for 3months had no adverse effects upon plasma cortisol level or on linear growth. These findings are in agreement with previous studies, which have used lower doses of fluticasone twice-daily but for a longer time3.
Although recognizing the value of other techniques employed for assessing lung function in infants, it seems that RVRTC (spirometry-like) would be better to evaluate changes in airway function determined by ICS or other broncho-active agents. Apart from its very low variability this method has a demonstrated good sensitivity to detect infant airway dysfunction, and would be the method of election to follow-up airway functional impairment from infancy to late childhood using spirometric parameters.
ConclusionsThis randomised, double-blind, placebo-controlled study in infants with RW at risk of asthma demonstrated that treatment with once-daily inhaled 375μg of fluticasone during 3months improves forced expiratory flows (FEF50 %, FEF75 % and FEF25 %-75 %) and controlled symptoms without altering plasma cortisol levels. A clinical improvement reported by parents also occurred in one third of infants from the placebo group. The spirometry-like technique is a useful and objective tool to assess the effect of treatments or the disease progression in infants with repeated troublesome asthma-like symptoms.
Conflict of interestThe University of Santiago de Chile (USACH) was the sponsor for this research. The authors have no conflict of interest to declare.