This study assesses the temporal trend of current asthma symptoms prevalence and associated factors in Chilean adolescents from South-Santiago, considering surveys performed in 1994, 2002 and 2015.
ResultsThe prevalence of current asthma symptoms showed a trend to increase from 11.1% in 1994 to 13.4% in 2015 (p<0.001); physician-diagnosed asthma increased from 11.5% to 13.8%, (p<0.001) whereas severe asthma and asthma with exercise decreased (p<0.001). Female adolescents had a higher prevalence of current asthma in the three surveys (p<0.001), and was a risk factor for asthma in the three surveys. In 2002, frequent consumption of meat and potatoes were associated with current asthma while frequent vigorous exercise was protective. Frequent exercise and parental tobacco smoking were risk for asthma in 2015 (p<0.001). Current active tobacco smoking showed a trend to increase reaching a prevalence of 28.9% in 2015 (p<0.001). There was a consistently low proportion of adolescents with current wheezing and asthma diagnosis (32.1% in 2015) and 37.6% of them had no asthma treatment.
ConclusionThe prevalence of current asthma in adolescents from the studied area would be still increasing. As in other studies, female adolescents had a higher prevalence of current asthma. Current active tobacco smoking has strikingly increased in the studied children while indoor passive tobacco exposure remains inadmissibly high. Our findings suggest that asthma in children is underdiagnosed and undertreated. More attention should be given to female gender, tobacco exposure, air pollution and local diagnostic preferences when studying and interpreting trends of asthma prevalence in adolescents from developing localities.
Asthma in childhood causes a large health impact, with huge expenses in medical consultations and medications, and with an important impairment of the quality of life of patients and their families.1,2 The temporal trend of asthma prevalence in childhood from different regions of the world has been reported by the International Study of Asthma and Allergies in Childhood (ISAAC) comparing data obtained in Phase I (1994) and Phase III (2002) and showed an overall stabilization of prevalence and severity in affluent regions and an increase in developing ones.3–5 However, the direction of changes in the prevalence (toward a stabilization, decrease, or increase) showed considerable variation among regions, countries and localities in a same country4–6; that pattern of prevalence changes occurred independently of socioeconomic development status. In Latin America, there was a slight average increase in the prevalence of asthma symptoms between 1994 and 2002 but similarly to other regions of the world, several of its centers exhibited diverse directions in the changes of asthma prevalence.
Recent information on the temporal trend of asthma prevalence and severity in Latin American children since the last ISAAC survey is little and referred to one study in Brazil7 where authors demonstrated that the trend in the prevalence and severity of current asthma symptoms in children between 1994 and 2012 was variable among participating centers. Since then, and to the best of our knowledge, there have been no studies performed in other countries of this region using the ISAAC methodology to evaluate the temporal trend of asthma prevalence and severity in children, or in the changes of associated risk/protective factors for asthma.
This study was undertaken to assess the changes in prevalence and severity of asthma symptoms in adolescents aged 13–14 years obtained from three repeated surveys (1994, 2002 and 2015), performed at the same ISAAC official center (South Santiago) and using ISAAC methodology. The ISAAC environmental questionnaire (EQ)3 which was applied in 2002 and 2015 surveys was used to assess eventual changes in risk/protective factors for current asthma symptoms in that period.
MethodsThe present study (2015) comprises data from children aged 13–14 years and strictly followed the same methodology applied in this official ISAAC center for phases I (1994) and III (2002) in South Santiago, a low-resourced area of Santiago de Chile.
ISAAC is a well-known multi-continental program that surveyed asthma and allergies in childhood reporting prevalence, severity, risk and protective factors and assessing the temporal changes occurred between its phases I, II and III all over the world. It uses a cross-sectional design, with random samples of two age groups of school children, 13–14 years old and 6–7 years old. A comprehensive description of rationale, methodology, participating centers, registration, sampling frame, sample sizes, selection of subjects, manuals, questionnaires, and related publications, can be found at the ISAAC web site.3 The questions used by ISAAC to estimate the prevalence of asthma symptoms were: (a) wheezing ever; (b) asthma ever; (c) current symptoms of asthma defined as wheezing or whistling in the chest in the past 12 months, severe asthma episodes (wheezing affecting speech), asthma symptoms with exercise, and sleep disturbance due nocturnal dry cough. In 2015 we added the question “Have you or your parents ever been told by a doctor that you have asthma? to explore the concordance with the question used by ISAAC “Have you ever had asthma?” which is often used as surrogate of physician-diagnosed asthma5,7; we also added questions on the use of inhaled asthma medication as “Have you ever used inhaled medication for asthma (puffs)?” and “Have you used inhaled medication for asthma (puffs) in the last 12 months?”. In this study we have used the ISAAC question on “asthma ever” as surrogate of physician-diagnosed asthma and “wheezing in the last 12 months” as current asthma.
The prevalence was calculated dividing the number of positive responses to each question by the number of completed questionnaires and expressed as percentage. The three studies, ISAAC phases I, III, and the present one, were approved by the Chilean Ministry of Health's Ethical Committee and full-informed signed consent for participation was obtained from parents or guardians.
Data analysisDescriptive statistics were employed for comparison of proportions among the three surveys and chi-square test for trend was used to calculate the direction and significance of changes (to an increase, decrease, or no change), from 1994 to 2015. Because the 2002 and 2015 surveys included the ISAAC EQ, multivariate logistic regression analysis, adjusted by gender, maternal tertiary education and current tobacco smoking, was used to examine associations between current asthma symptoms and factors as frequent (≥times per week) consumption of some dietary products (meat, vegetables, fruits, fast-food, among others); pets at home, frequent vigorous exercise (≥times per week), three or more hours a day spent watching television. Current asthma (wheezing in the last 12 months) was employed as dependent variable because we have shown that it correlates well with bronchial hyperresponsiveness to methacholine in adolescents.8 Proportions of frequent consumption of dietary products, active and passive tobacco exposures, pets at home, maternal tertiary education, among others, reported in 2002 and 2015 were compared using chi-square test. Concordance between the questions “Have you ever had asthma?”, “Have you or your parents ever been told by a doctor that you have asthma?”, as those on ever and current use of inhaled asthma medications was estimated using the inter-rater agreement test (kappa). MedCalc Statistical Software version 18.5, Ostend, Belgium, was employed for the analysis.
ResultsGeneralThere were 8858 questionnaires for analysis: 3051, 3057 and 2750 collected in 1994, 2002 and 2015, respectively. The response rates for the three studies were 86.2%, 84.8%, and 81.9%. The proportion of girls was significantly higher than boys in 1994 (53.2%, p<0.01) and 2002 (57.8%, p<0.01), but not in the 2015 survey (50.9%, p=0.35).
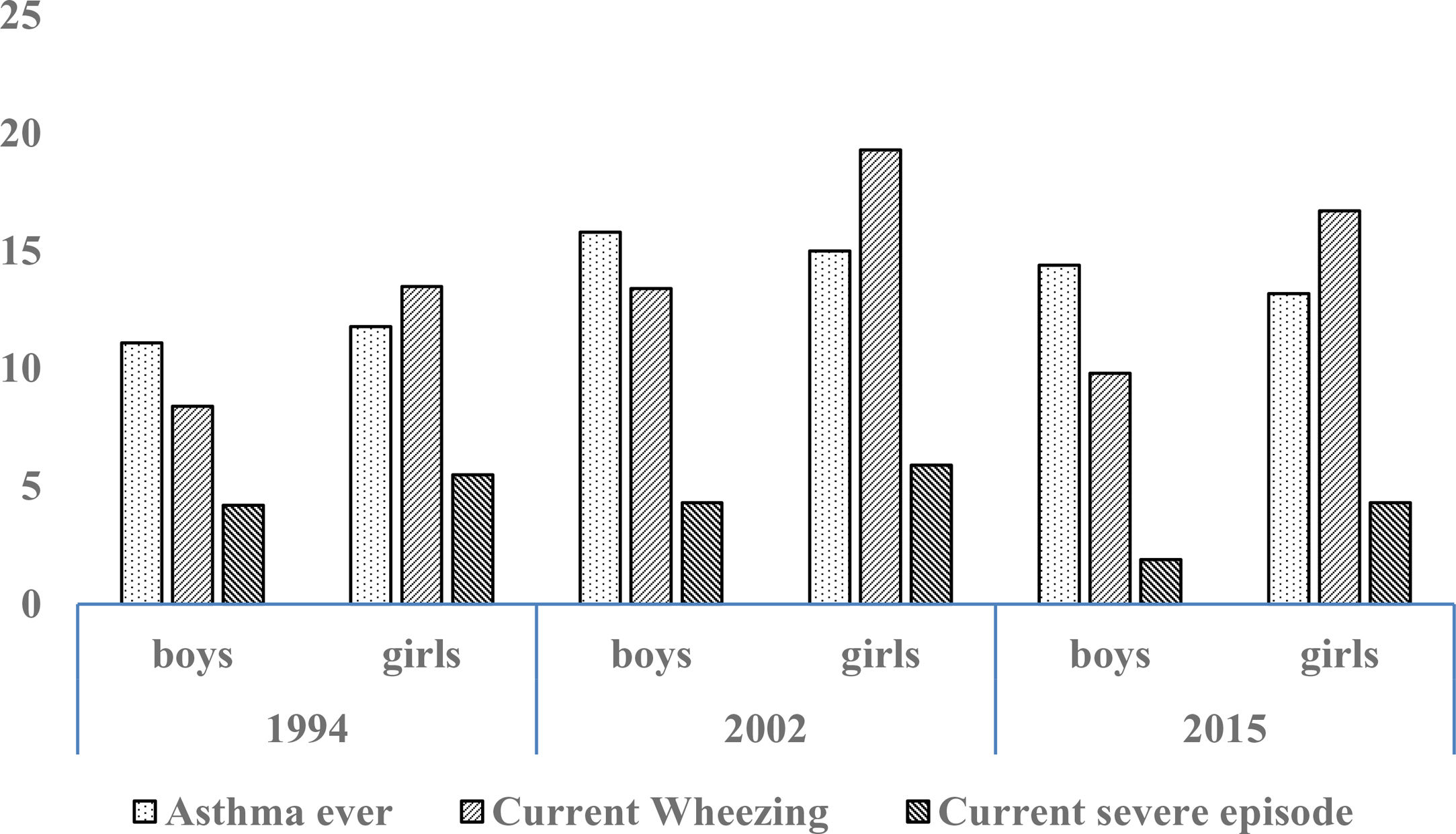
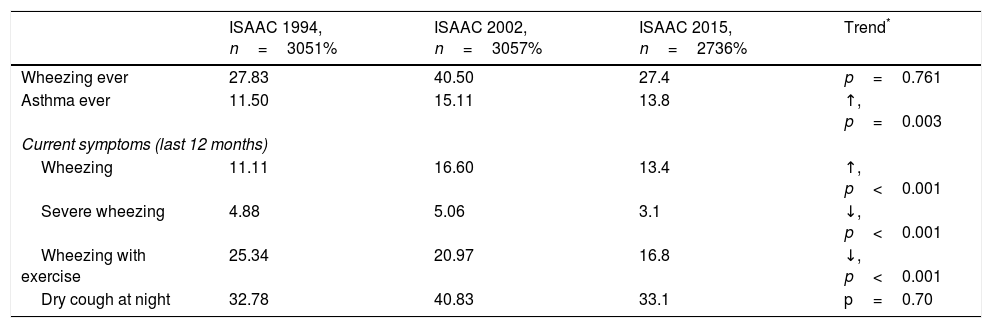
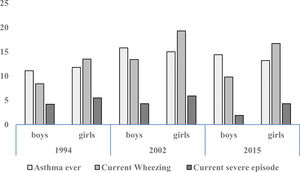
Prevalence and trendsThe prevalence and trend of asthma symptoms in the 21-year time span is in Table 1. Current asthma and physician-diagnosed asthma (asthma ever) showed a significant trend to increase whereas wheezing ever, current severe episodes of wheezing and exercise-induced wheezing had a significant trend to decrease. Girls had a significantly higher prevalence of asthma symptoms than boys in the three surveys (p<0.001) (Fig. 1) and female gender was a significant risk for current asthma in 1994 (OR 1.25; 95%CI 1.14–1.36), 2002 (OR 1.61; 95%CI 1.19–2.189 and 2015 (OR 1.71; 95%CI 1.25–2.33), (p<0.001). The prevalence of adolescents with current wheezing who had an asthma diagnosis was similar in 1994 (33.1%; 95%CI 28.3–38.3), 2002 (34.6%; 95%CI 30.0–38.1) and 2015 (32.1%; 95%CI 27.6–37.1); with a non-significant trend (p=0.77). Regarding the use of inhaled asthma medications which was only assessed in 2015, 62.4% of adolescents with current asthma symptoms and diagnosis of asthma had used inhaled asthma medication in the last 12 months. Among children with current asthma symptoms the use of inhaled asthma medications was 26.9% (95%CI 22.6–31.6), and 29.8% (19.5–42.7) in those who had four or more wheezing episodes. There was a very good agreement between the question “Have you ever had asthma?” (ISAAC) and “Have you or your parents ever been told by a doctor that you have asthma?” (kappa 0.83, p<0.0001).
Trend for cumulative (ever) and current (last 12 months) prevalence of asthma symptoms in Chilean adolescents aged 13–14 years between 1994 and 2015.
| ISAAC 1994, n=3051% | ISAAC 2002, n=3057% | ISAAC 2015, n=2736% | Trend* | |
|---|---|---|---|---|
| Wheezing ever | 27.83 | 40.50 | 27.4 | p=0.761 |
| Asthma ever | 11.50 | 15.11 | 13.8 | ↑, p=0.003 |
| Current symptoms (last 12 months) | ||||
| Wheezing | 11.11 | 16.60 | 13.4 | ↑, p<0.001 |
| Severe wheezing | 4.88 | 5.06 | 3.1 | ↓, p<0.001 |
| Wheezing with exercise | 25.34 | 20.97 | 16.8 | ↓, p<0.001 |
| Dry cough at night | 32.78 | 40.83 | 33.1 | p=0.70 |
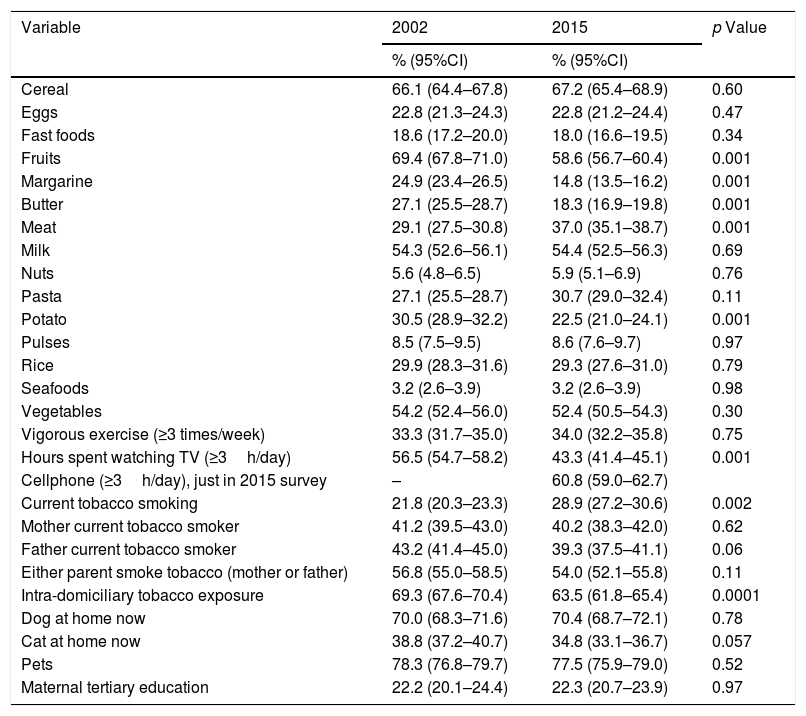
The changes occurred in the proportion of children who responded the EQ in 2002 and 2015 are in Table 2. There was a significant trend to the increase in the proportion of children who reported current tobacco smoking (16.2% in 1994, 21.8% in 2002 and 28.9% in 2015, p<0.0001) and the trend of intra-domiciliary tobacco prevalence decreased between 1994 and 2015 (67.3% in 1994, 70% in 2002 and 63.5% in 2015; p<0.004). There was a significant increase in the frequent consumption of meat between 2002 and 2015 while there was a significant decrease in frequent consumption of fruits, potatoes, butter/margarine, TV watching three or more hours a day; tertiary maternal education remained about 22%. In 2015 we asked for frequent use of mobile phone/internet and 60.8% of the adolescents responded they spent three or more hours a day using their mobile phones.
Comparative prevalence (%) of frequent intake (≥3 times a week) of dietary products and other environmental variables between 2002 and 2015.
| Variable | 2002 | 2015 | p Value |
|---|---|---|---|
| % (95%CI) | % (95%CI) | ||
| Cereal | 66.1 (64.4–67.8) | 67.2 (65.4–68.9) | 0.60 |
| Eggs | 22.8 (21.3–24.3) | 22.8 (21.2–24.4) | 0.47 |
| Fast foods | 18.6 (17.2–20.0) | 18.0 (16.6–19.5) | 0.34 |
| Fruits | 69.4 (67.8–71.0) | 58.6 (56.7–60.4) | 0.001 |
| Margarine | 24.9 (23.4–26.5) | 14.8 (13.5–16.2) | 0.001 |
| Butter | 27.1 (25.5–28.7) | 18.3 (16.9–19.8) | 0.001 |
| Meat | 29.1 (27.5–30.8) | 37.0 (35.1–38.7) | 0.001 |
| Milk | 54.3 (52.6–56.1) | 54.4 (52.5–56.3) | 0.69 |
| Nuts | 5.6 (4.8–6.5) | 5.9 (5.1–6.9) | 0.76 |
| Pasta | 27.1 (25.5–28.7) | 30.7 (29.0–32.4) | 0.11 |
| Potato | 30.5 (28.9–32.2) | 22.5 (21.0–24.1) | 0.001 |
| Pulses | 8.5 (7.5–9.5) | 8.6 (7.6–9.7) | 0.97 |
| Rice | 29.9 (28.3–31.6) | 29.3 (27.6–31.0) | 0.79 |
| Seafoods | 3.2 (2.6–3.9) | 3.2 (2.6–3.9) | 0.98 |
| Vegetables | 54.2 (52.4–56.0) | 52.4 (50.5–54.3) | 0.30 |
| Vigorous exercise (≥3 times/week) | 33.3 (31.7–35.0) | 34.0 (32.2–35.8) | 0.75 |
| Hours spent watching TV (≥3h/day) | 56.5 (54.7–58.2) | 43.3 (41.4–45.1) | 0.001 |
| Cellphone (≥3h/day), just in 2015 survey | – | 60.8 (59.0–62.7) | |
| Current tobacco smoking | 21.8 (20.3–23.3) | 28.9 (27.2–30.6) | 0.002 |
| Mother current tobacco smoker | 41.2 (39.5–43.0) | 40.2 (38.3–42.0) | 0.62 |
| Father current tobacco smoker | 43.2 (41.4–45.0) | 39.3 (37.5–41.1) | 0.06 |
| Either parent smoke tobacco (mother or father) | 56.8 (55.0–58.5) | 54.0 (52.1–55.8) | 0.11 |
| Intra-domiciliary tobacco exposure | 69.3 (67.6–70.4) | 63.5 (61.8–65.4) | 0.0001 |
| Dog at home now | 70.0 (68.3–71.6) | 70.4 (68.7–72.1) | 0.78 |
| Cat at home now | 38.8 (37.2–40.7) | 34.8 (33.1–36.7) | 0.057 |
| Pets | 78.3 (76.8–79.7) | 77.5 (75.9–79.0) | 0.52 |
| Maternal tertiary education | 22.2 (20.1–24.4) | 22.3 (20.7–23.9) | 0.97 |
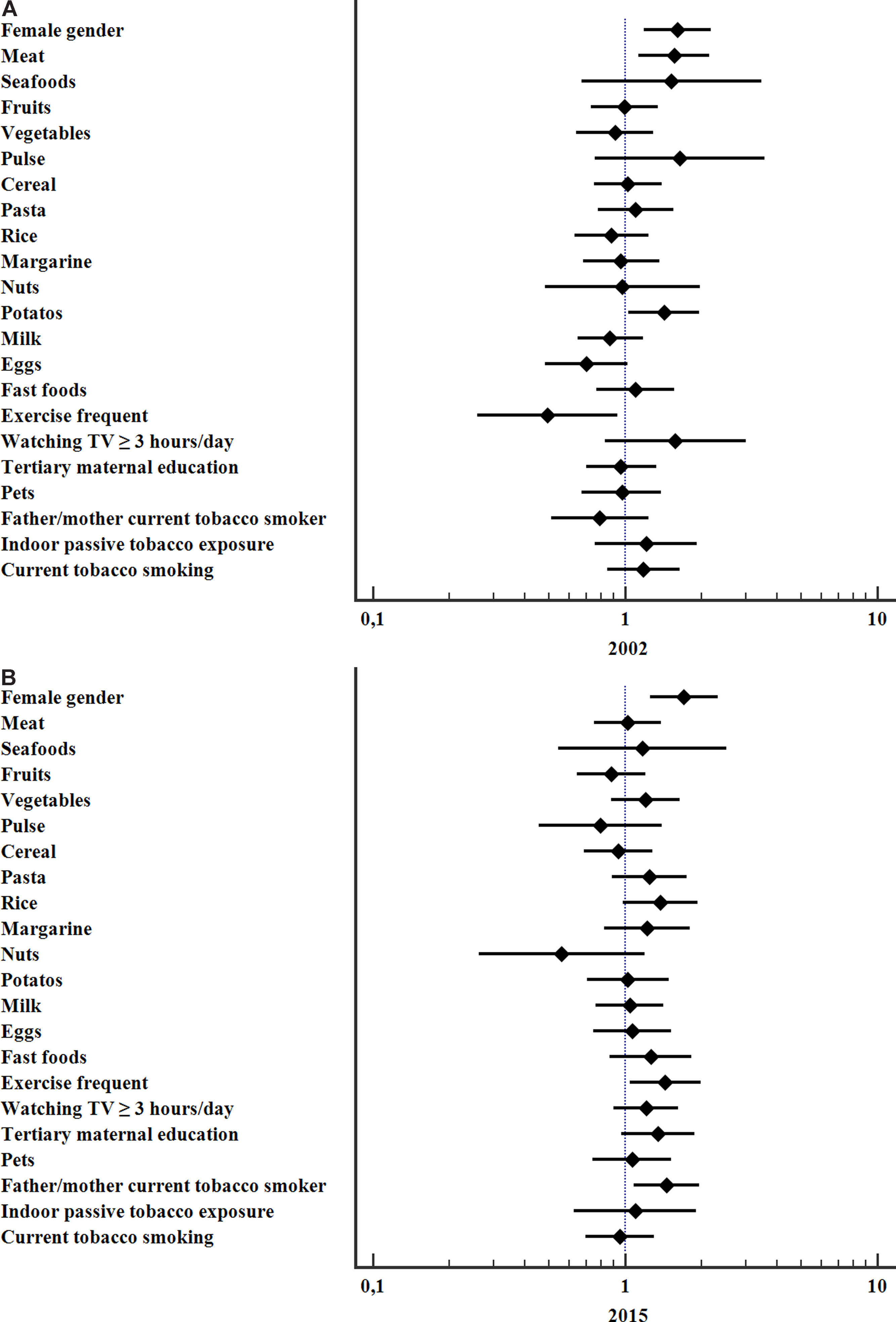
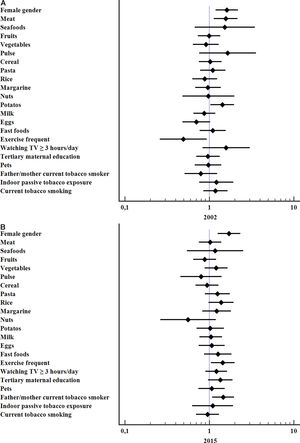
The variables significantly associated with a higher risk for current asthma in 2002 were frequent consumption of meat (OR 1.56; 95%CI 1.12–2.18, p=0.006), potatoes (OR 1.43; 95%CI 1.03–1.98, p=0.031) and female gender (OR 1.61; 95%CI 1.19–2.18, p=0.002) whereas frequent vigorous exercise was protective (OR 0.49; 95%CI 0.26–0.93, p=0.03). In 2015 the significant associations with current asthma were: female gender (OR 1.71; 95%CI 1.25–2.33, p=0.001), parental current smoking (OR 1.46; 95% CI 1.09–1.97, p=0.013), and frequent vigorous exercise (OR 1.44; 95%CI 1.04–2.00, p=0.027), Fig. 2.
DiscussionChanges in prevalenceThis study shows an overall increasing trend in the prevalence of reported current asthma and physician-diagnosed asthma while the current prevalence of severe asthma episodes and exercise-induced asthma decreased from 1994 to 2015. Noticeably, the prevalence of current asthma and physician-diagnosed asthma markedly increased between 1994 and 2002 but show a decrease between 2002 and 2015; although the trend considering the 21 years was still to increase, this finding might suggest that asthma prevalence is stabilizing in the adolescents from the studied area.
In Brazil, Solé et al.,7 in a study comparing the prevalence of asthma in children found in 1994, 2003 and 2012 in three ISAAC centers which participated in phases I and III, found that current asthma symptoms remained without significant changes in Curitiba and Recife, but showed a trend to decrease in Sao Paulo. However, the prevalence of physician-diagnosed asthma increased in the three cities, suggesting that a growing number of children who reported current wheezing would be diagnosed as asthma at those centers. The same explanation would apply for the findings of Brozek et al.,9 in a study performed in Poland in 1993, 2002, 2007 and 2014, in schoolchildren and those authors found a significant increase in the prevalence of physician-diagnosed asthma from 3.4% in 1993 to 12.6% in 2014, but not in the prevalence of current wheezing, which remained around 20% in the four surveys. A similar increase was found in Norway where the prevalence of asthma in schoolchildren went from 7.3% in 1985 to 16.6% in 2008.10 Longitudinal studies in other developed countries (UK) found a decrease in the prevalence of current wheeze between 2001 and 2012, from 18.9% to 15.0%.11 In USA, Akinbami et al.,12 using 2001–2013 National Health Interview Survey data, reported that asthma prevalence ceased to increase by 2013; however, there was an increase in asthma prevalence in children among 10–17-year-olds, poor children and those living in the South of the country. Recently, Zahran et al.13 using the same data from the National Health Interview Survey up to 2016, reported a further decrease of asthma prevalence in US children from 9.4% in 2010 to 8.3% in 2016, excepting for Mexican/Mexican-American children in whom asthma prevalence increased from 5.1% in 2001 to 6.5% in 2016. Thus, evidence from studies performed after ISAAC III in 2001–2002 suggests there is a non-uniform trend in the prevalence of asthma symptoms with some populations of children showing an increase, stabilization, or decrease. The observed variation in temporal trend among localities, is likely related with local environmental changes of climate, socioeconomic status, education, among others, occurred through the observational time. In addition, local physicians’ diagnostic preferences could also play a role influencing the prevalence of asthma (label) by establishing the diagnosis of asthma in patients suffering from asthma symptoms, which certainly has implications for proper asthma treatment. The present study showed a low prevalence of adolescents with current asthma symptoms who had a physician-diagnosis of asthma in the three surveys, which would reflect an under-diagnosis of asthma but also an undertreatment of the condition. The latter is evidenced because 38.6% of them did not receive inhaled asthma medication, even when those medications (bronchodilators and corticosteroids) are fully available and for free, at the primary care centers of the surveyed area. Our findings agree with those from international studies that show underestimation and undertreatment of asthma in adolescents.14
Risk/protective factorsThe information on the temporal changes of risk factors for childhood asthma occurred in a same area is scarce. Recently, Barnish et al.15 in Aberdeen, Scotland, surveyed schoolchildren on seven occasions between 1964 and 2014 and found changes in the association of determined risk factors with asthma prevalence in that period, which were mainly related, but not always, to gender, deprivation, parental asthma, and tobacco smoke exposure. We found few factors associated with current asthma in 2002 and 2015, and the only factor consistently associated with higher asthma prevalence in both surveys was female gender, which agrees with other studies reporting that female gender is a risk for development of asthma in adolescence.16 Although several potential explanations have been explored for the higher prevalence of asthma in adolescent girls, the knowledge regarding gender-related functional, environmental and hormonal aspects, among others, remains uncomplete.17–20 In 2002 frequent intake of meat and potatoes was associated with current asthma whereas frequent vigorous exercise protected against asthma; also, in 2015 there was no risk/protective association of dietary product with current asthma, but parental tobacco smoking and frequent vigorous exercise were risk factors for asthma. The finding that frequent vigorous exercise was a protective factor in 2002 and a risk factor in 2015 is difficult to explain because the similar proportion of adolescents reporting frequent exercise in 2015. The latter is concordant with the high number of hours per day spent in sedentary time (TV watching and cellphone-internet use) as reported by our adolescents in 2015, showing that sedentary behavior is likely replacing vigorous physical activity in adolescents, as reported by other authors21; furthermore; sedentarism is associated to obesity which is a well-known risk for developing asthma and severe asthma in childhood.22 An additional point for the positive association found between frequent vigorous physical activity and asthma in 2015 might be related with the high outdoor air pollution occurred in Santiago, declared as a saturated particulate matter 2.5μm zone in 2014.23 The deleterious effect of high air pollution on respiratory health, particularly during physical activity is well-known and that is why vigorous exercising is not recommended in days of high atmospheric pollution; in asthmatics children, outdoor pollution is related with persistence and exacerbations of asthma symptoms.24
The associations between the type and consumption frequency of dietary products and current asthma symptoms in childhood is not well-defined at present and findings from different studies are sometimes contradictory.25,26 Ellwood et al.27 in a large international study at global scale, found that frequent consumption of fruits and vegetables was protective for current and severe asthma in adolescents; and conversely, frequent consumption of fast food (≥3 times per week) was a risk for asthma. Nevertheless, this is not a consistent finding and Wickens et al.28 found no protective effect for fruits or vegetables but reported that hamburgers and take-away foods consumption was associated with asthma symptoms and increased airway responsiveness in children. Thus, the interpretation of the associations found between the frequency of consumption and type of dietary products with asthma symptoms, airway responsiveness or body mass index should be cautious; for instance, in a recent analysis of ISAAC III data, Braithwaite et al.29 found that frequent and very frequent intake of fast-foods was related with a lower body mass index in adolescents, although other studies have reported the contrary.30 We found no risk for frequent consumption of fast foods and no protective effect against asthma for fruits or vegetables; although the proportion of adolescents who reported frequent intake of fruits decreased in 2015, it still was high (around 59%). Noticeably, frequent consumption of sea-foods was remarkable low (about 3%) in both surveys, probably because they are expensive products in this country, as compared with frequent consumption of fast foods that remained similar between 2002 and 2015, 19% and 18%, respectively. The type of diet, as well as the frequency of some dietary product consumption has been related to asthma prevalence, either as risk or protective factors, as occurs with Western diet (pro-inflammatory effects) versus Mediterranean diet (anti-inflammatory effects).25,26,31,32 However, at present it appears as unrealistic to categorize the reported frequency of food consumption because the wide diversity of food types and cooking styles available everywhere which are low-cost, “fashionable” and easily accessed by adolescents, even by those from low-resourced populations. These complexities should be considered when interpreting the effects of diet and other ecological factors on the prevalence and severity of asthma in children. The possible mechanisms to explain the relationship between diet and asthma are not fully understood, but the available evidence would indicate that increasing the consumption of fruits and vegetables is healthy and could reduce the risk of asthma and other diseases, whereas fast foods could increase the risk, particularly in children.25–32
Apart from diet, there are several other factors that have been found to be associated with asthma32; among them, outdoor air pollution24 and tobacco exposure (passive or active) which is one of the most harmful for children.33 The present study found an increasing trend in the prevalence of adolescents reporting current active tobacco smoking from 16.2% in 1994, to 17.5% in 2002 and to 28.9% in 2015. Recently, Urrutia-Pereira et al.34 in Brazil, found that 29.3% of adolescents have tried tobacco smoking, 14.5% started smoking before 12 years of age and 13.0% smoked at least one cigarette per day in the previous month. In a cross-sectional study performed in the same locality as the present study, we found that about 25% of current symptoms of asthma in adolescents could be explained by active tobacco smoking, with a predominance of smoking girls.35 In a prospective cohort study of Canadian adolescents, female gender and exposure to passive smoking was associated with the development of asthma16; which agrees with our findings from 2015 survey, where female gender and parental tobacco smoking were positively associated with asthma symptoms. Tobacco smoking in adolescent females is worrying because it is a known risk for continuing smoking in adulthood and smoking during pregnancy, with all the well-known deleterious effects for mother and child.36,37
LimitationsThis study has several limitations which are inherent to its cross-sectional design which do not allow for concluding on causality; however, they are good for determining prevalence and temporal trends of asthma and other allergies in populations (adolescents) living at same area. Another limitation is that adolescents could not be fully aware on their symptoms or about the food type and frequency of consumption, introducing a recall bias and limiting interpretations. However, it is likely that they can better recall frequent consumption of some types of food than moderate or infrequent intake per week. The interpretation of these findings could also be limited by several other confounders that were not assessed in the study, such as those related with changes at individual and country socioeconomic status and life style. According to UN Development Report 2016,38 Chile's Human Development Index and GNI had an important increase between 1994 and 2015. It is likely that our study illustrates changes related with an improvement in some socioeconomic aspects of low-resourced populations as that from this study, which may affect the conventional relationship between asthma and socioeconomic level. This could alter adolescents’ life-style in countries with transitional economies allowing them for an easier access to a high variety of non-healthy and processed foods, tobacco cigarettes, alcoholic beverages, among others, while other environmental factors such as poor housing and living conditions, high indoor and outdoor air pollution, high active and passive tobacco exposure, among others, persist inadmissibly high. This would be reflected by the high percentage of our unprivileged children (about 60%) that owned smartphones in 2015, as compared with well-resourced countries as Spain where about 90% of adolescents owned a smartphone by 2015.39 The decrease we found in TV-watching hours per day may be indicating a change in adolescents’ preferences toward spending more time using their smart phones.
ConclusionIn the last 21 years the prevalence of asthma in adolescents still shows an overall trend to increase; however, it could be ceasing as supported by the decrease observed between 2002 and 2015; female gender was consistently associated with current asthma in the three surveys. Parental tobacco smoking was associated to current asthma; overall, active and passive exposure to tobacco, remained unacceptably high in this group of adolescents. Additionally, our study suggests that asthma could be underdiagnosed and undertreated in the studied population. More attention should be given to female gender, outdoor pollution (smog) and active and passive tobacco exposure and to local diagnosis and treatment preferences when studying and interpreting changes in the prevalence of asthma and associated factors in adolescents from unprivileged localities in developing countries with a transition economy.
Conflict of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appear in this manuscript.
Right to privacy and informed consentThe authors declare that no patient data appear in this manuscript.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Author contributionsAll authors participated in the conception and design of the study; collection, analysis, and interpretation of the data; drafting and revision of the manuscript; and approval of final version of the manuscript.
FundingNo funding was granted for this study.
The authors thank to the children and parents who participated in the study and the valuable collaboration provided by Dr. Alfredo Alfaro and Dr. Gonzalo Espinoza. We express our special thanks to the directors and teachers of the participating schools.