Ataxia-telangiectasia (AT) is a well-known primary immunodeficiency with recurrent sinopulmonary infections and variable abnormalities in both the humoral and cellular immune system. Dysfunctions in immunoglobulin production, reduced number of B cells, and B-cell receptor excision circles copies have been reported. We aimed to understand the immunological mechanisms involving the humoral compartment in AT patients by analysing peripheral blood B cells subsets, B-T lymphocyte cooperation through the expression of CD40 and CD40 ligand (CD40L), and cytokines involved in class-switch recombination production.
MethodsWe compared the proportion of B-cell subsets, the expression of CD40/CD40L, and the plasma levels of IL-6 and IFN-γ of 18 AT patients and 15 healthy age-sex-matched controls using flow cytometry.
ResultsWe found that some steps in peripheral B cell development were altered in AT with a pronounced reduction of cell-surface CD40 expression. The proportions of transitional and naïve-mature B cells were reduced, whereas CD21-low, natural effector memory, IgM-only memory, and IgG atypical memory B cells were present in a higher proportion.
ConclusionsThese findings revealed a disturbed B-cell homeostasis with unconventional maturation of B lymphocyte memory cells, which can explain the consequent impairment of humoral immunity.
Ataxia-telangiectasia (AT) is a multisystem disorder1–3 where patients either lack the ATM protein or the ATM kinase activity, which belong to a family of proteins involved in cell cycle control, DNA repair, and signal transduction.4–6
AT is recognised as a primary immunodeficiency due to recurrent viral and bacterial infections, which cause significant morbidity and are a frequent factor or cause of death in the AT population.6
Immunological disorders in AT are very diverse7,8 and laboratory findings indicate that in both humoral and cellular immune system impairment is present. Schubert et al. found that naïve CD4+ and CD8+ lymphocytes are decreased with diminished migration of these cells from the thymus coupled to increased apoptosis.9 Natural Killer (NK) cells are significantly elevated in AT patients.10 Among the reported dysfunctions in immunoglobulin production, raised levels of IgM, IgA deficiency, and a lack of response to polysaccharide antigens are the most frequent.10,11 Reduced numbers of B cells have also been reported, although signal transduction by the B cell receptor has shown adequate function.12 Recently, low copy numbers of T-cell and B-cell receptor excision circles (TREC and KREC) were found.13–15
ATM is critically important for processes in lymphocyte development that rely on double-strand break repair, such as V(D)J recombination and class-switch recombination (CSR) in immunoglobulin genes resulting in inadequate immunoglobulin function.16–20 Also, the absence of ATM activity has been associated with a reduction in T lymphocytes.20 Although the effects of ATM mutations on the V(D)J recombination and CSR processes are present, little is known about the consequences of ATM mutations on B lymphocyte maturation and antibody production.18,19 In this study, we aimed to understand the immunological mechanisms involving the humoral compartment from patients with AT by analysing peripheral blood B cells subsets, B-T lymphocyte cooperation through the expression of CD40 and CD40 ligand (CD40L), and cytokines involved in CSR production.
Material and methodsStudy centre and clinical samplesThis single centre study was conducted in a Brazilian referral centre for patients with primary immunodeficiency diseases after approval by the Ethics Committees of the Federal University of Sao Paulo. Eighteen patients with AT and 15 healthy age-sex-matched control subjects were enrolled after informed consent was obtained. All patients included in the study were diagnosed with AT based on the (probable/definitive) criteria of the European Society for Immunodeficiency.21 Healthy volunteers were selected based on the absence of exclusion criteria (such as chronic and degenerative diseases, not using immunosuppressive medications, and absence of acute infection). Fifteen millilitres of peripheral blood samples was collected in EDTA vacuum tubes (BD Biosciences, Franklin Lakes, NJ, USA) from patients and healthy volunteers. Clinical data and immunoglobulin levels for patients at the time of AT diagnosis were obtained from patients and their medical files.
Absolute T, B, and NK cell countsT, B, and NK cell counting were performed using BD Multitest™ CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC and CD3-FITC/CD16+CD56-PE/CD45-PerCP/CD19-APC, both with BD Trucount™ Tubes (BD Biosciences, San Jose, CA, USA). Samples were prepared following the manufacturer's instructions. Briefly, 20μL of each pool of antibodies was added to BD Trucount™ Tubes, followed by 50μL of whole blood treated with EDTA, which was well-mixed, homogenised and incubated for 15min in the dark at room temperature. Afterwards, 450μL of 1× BD FACS lysing solution was added to each tube and incubated for a further 15min in the dark at room temperature. Data acquisition and analysis were performed using Multiset™ software and a 4 colours FACSCalibur™ flow cytometer.
Plasma and peripheral blood mononuclear cells (PBMCs) isolation, cryopreservation, and thawingAfter performing T, B, and NK cell counts, the remainder of each blood sample was subsequently centrifuged. Plasma was separated and immediately frozen at −80°C. PBMCs were obtained using the Ficoll-gradient method (Ficoll-Paque PLUS; GE Healthcare Bio-Sciences AB, Uppsala, Sweden), frozen in foetal calf serum (FCS; Invitrogen-Gibco, Gaithersburg, MD, USA) with 10% dimethyl sulfoxide (DMSO; Calbiochem, La Jolla, CA, USA), and stored in liquid nitrogen until use. Upon thawing, the cells were washed and suspended in R10 (RPMI 1640 medium supplemented with 10% foetal bovine serum, 1% 100-mM HEPES buffer solution, 1% 200-mM l-glutamine, 1% 100-mM sodium pyruvate, 1% 100× penicillin/streptomycin, and 0.1% 55-mM 2-mercaptoethanol (all from Invitrogen)) to a density of 3×105cells/mL. Lymphocyte viability was assessed with seven-aminoactinomycin (7-AAD; BD Biosciences) and found to be over 90%.
B-cell subset immunophenotypingFor B-cell subset identification, PBMCs were surface-stained with CD19-PerCP (clone 4G7), CD38-phycoerythrin-cyanine 7-conjugate (PE-Cy7; clone HB7), CD3-allophycocyanin-cyanine 7 conjugate (APC-Cy7; clone SK7), CD24-PE (clone ML5), CD21-APC (clone B-ly4), CD27-PE-Cy7 (clone M-T271), IgM-APC (clone G20-127), IgD-FITC (clone IA6-2), IgG-brilliant violet 510™ (BV510, clone B18-145, Becton Dickinson, Pharmingen, San Diego, CA, USA) and IgA-PE (Southern Biotechnology Associates, Birmingham, AL, USA) for 30min on ice in FACS buffer (phosphate-buffered saline [PBS] 0.1% BSA; Sigma, Laboratories, St. Louis, MO, USA, with 2.0mM EDTA, Sigma). Then, stained cells were fixed in PBS containing 1% paraformaldehyde for 30min on ice and rewashed. Forty thousand events were acquired from a gate, based on total lymphocyte staining and forward-scatter versus side-scatter parameters. Acquisitions and analyses were performed using FACSDiva™ software in a LSRFortessa™ flow cytometer and FlowJo™ software (Tree Star, Ashland, OR, USA), respectively. During the analysis, transitional B cells were characterised as CD3−CD19+CD24hiCD38hi, CD21low B cells as CD3−CD19+CD21loCD38lo, and naïve B cells as CD3−CD19+CD27−IgM+IgD+. Seven memory B-cell subsets were characterised, as described previously22: CD3−CD19+CD27+IgM+IgD+ (natural effector), CD3−CD19+CD27+IgM+IgD− (IgM-only), CD3−CD19+CD27+IgM−IgG+(IgG B-cell), CD3−CD19+CD27+IgM−IgA+(IgA B-cell) CD3−CD19+CD27−IgM−IgG+ (atypical IgG B-cell), CD3−CD19+CD27−IgM−IgA+ (atypical IgA B-cell), and plasmoblast (CD3−CD19+CD27hiIgD−). Fluorescence minus one and isotype-antibody controls were used when necessary.
Expression of CD40L and CD40To evaluate CD40 expression on the surface of B lymphocytes and CD40-ligand on the surface of T lymphocytes, PBMCs were cultured for 3h at 37°C in 5% CO2 in six-well plates in a final volume of 2mL of cell suspension in R10 medium, with and without phorbol 12-myristate 13-acetate (PMA, 20ng/mL, Sigma) and ionomycin (2ng/mL, Sigma). Upon incubation, cells were washed with FACS buffer; stained with specific monoclonal antibodies, as previously described23: CD3-APC-Cy7, CD8-FITC (clone SK1), CD19-PerCP, CD40-APC (clone 5C3), CD69-PE-Cy7 (clone FN50), and CD154-PE (clone TRAP1) (Becton Dickinson, Pharmingen, San Diego, CA, USA); and fixed in PBS with 1% paraformaldehyde. Forty thousand events were acquired from a gate, based on total lymphocyte staining and forward-scatter versus side-scatter parameters. CD3+CD8−CD154+ cells were analysed in both conditions and CD3−CD19+CD40+ cells were analysed only without stimulus. Cell activation was confirmed by staining CD3+CD4− T cells with an antibody specific for CD69.
Plasmatic levels of IL-6 and IFN-γCytokines were measured using Cytometric Bead Array (CBA) kits (BD Biosciences, USA), which allows simultaneous quantitation of proteins by flow cytometry. The CBA test results were acquired using LSRFortessa™ and analysed with FCAP Array™ software (Becton Dickinson Immunocytometry).
StatisticsStatistical analysis was performed with the SPSS software package version 20.0 (IBM, Tokyo, Japan) and Stata version 12 (STATA Corp., College Station, TX, USA). The linear association between IgM and CD40 was measured via Pearson's correlation. A mixed-effect model was used to compare data from patients with AT and healthy age-sex-matched control subjects. Normal data distribution was checked using the Kolmogorov–Smirnov test. Statistical significance was set at a P value of less than 0.05.
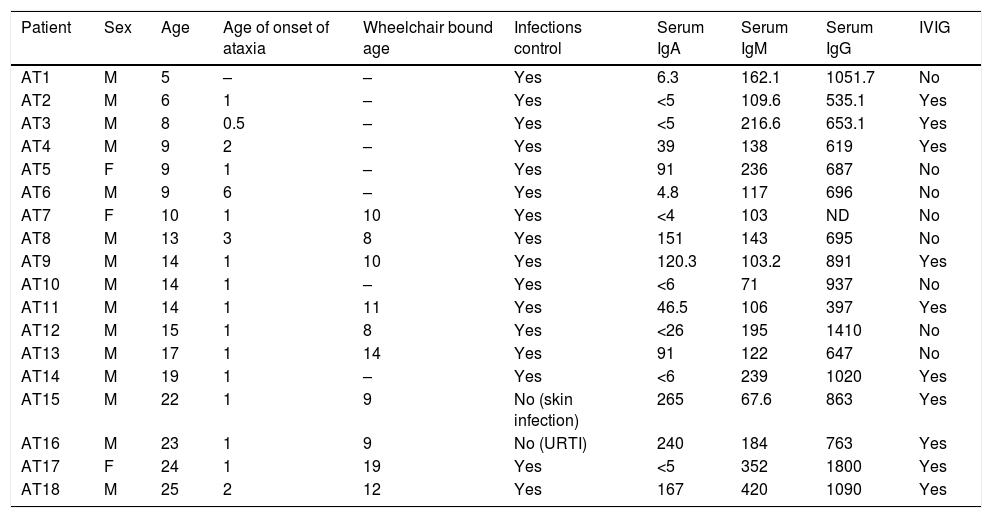
ResultsThe patients’ characteristics are summarised in Table 1. Consanguinity was present in 16.7% (three cases), and three brother-brother pairs were included in this study (AT1 and AT6; AT2 and AT14; AT11 and AT16). One patient, AT10, had completely recovered from Lymphoma prior to enrolment in this study and his results were similar to the other patients (TCD4+=205cells/mm3; TCD8+=463; CD19+=39; CD16/56+=403). During the follow-up period, patient AT7 died four days after an appendectomy. One patient presented with sarcoidosis and another patient had diabetes. The patients’ serum immunoglobulin levels at the time of diagnosis showed that 53.8% and 23% of them had low IgA and IgG respectively, while 46% of patients had high IgM levels.
Ataxia-telangiectasia patients’ characteristics.
| Patient | Sex | Age | Age of onset of ataxia | Wheelchair bound age | Infections control | Serum IgA | Serum IgM | Serum IgG | IVIG |
|---|---|---|---|---|---|---|---|---|---|
| AT1 | M | 5 | – | – | Yes | 6.3 | 162.1 | 1051.7 | No |
| AT2 | M | 6 | 1 | – | Yes | <5 | 109.6 | 535.1 | Yes |
| AT3 | M | 8 | 0.5 | – | Yes | <5 | 216.6 | 653.1 | Yes |
| AT4 | M | 9 | 2 | – | Yes | 39 | 138 | 619 | Yes |
| AT5 | F | 9 | 1 | – | Yes | 91 | 236 | 687 | No |
| AT6 | M | 9 | 6 | – | Yes | 4.8 | 117 | 696 | No |
| AT7 | F | 10 | 1 | 10 | Yes | <4 | 103 | ND | No |
| AT8 | M | 13 | 3 | 8 | Yes | 151 | 143 | 695 | No |
| AT9 | M | 14 | 1 | 10 | Yes | 120.3 | 103.2 | 891 | Yes |
| AT10 | M | 14 | 1 | – | Yes | <6 | 71 | 937 | No |
| AT11 | M | 14 | 1 | 11 | Yes | 46.5 | 106 | 397 | Yes |
| AT12 | M | 15 | 1 | 8 | Yes | <26 | 195 | 1410 | No |
| AT13 | M | 17 | 1 | 14 | Yes | 91 | 122 | 647 | No |
| AT14 | M | 19 | 1 | – | Yes | <6 | 239 | 1020 | Yes |
| AT15 | M | 22 | 1 | 9 | No (skin infection) | 265 | 67.6 | 863 | Yes |
| AT16 | M | 23 | 1 | 9 | No (URTI) | 240 | 184 | 763 | Yes |
| AT17 | F | 24 | 1 | 19 | Yes | <5 | 352 | 1800 | Yes |
| AT18 | M | 25 | 2 | 12 | Yes | 167 | 420 | 1090 | Yes |
F, female; M, male; IVIG, intravenous immunoglobulin replacement; ND, not determined; URTI, recurrent respiratory tract infection.
Age, age of onset of ataxia and wheelchair bound age are expressed as years. Immunoglobulin levels are measured in mg/dL.
The total number of lymphocytes was low in 11/18 subjects (61.1%), and subsequent analysis also revealed low numbers of TCD4+ and TCD8+ cells, compared to age-matched controls according to the Brazilian reference.24 The opposite was observed regarding the total number of NK cells. Absolute numbers of B-cells were significantly reduced in all patients (Table 2).
Total counts of lymphocytes and subpopulations in AT patients (n=18) and controls (n=15).
| AT median (range) | Controls median (range) | Mixed-effect P values | |
|---|---|---|---|
| Lymphocytes | 1670 (928–4579) | 2897 (1646–6.601) | 0.001* |
| T cells (CD3) | 871.5 (483–3039) | 1934 (1143–3957) | <0.001* |
| CD4 | 459 (205–1535) | 1101.5 (576–2352) | <0.001* |
| CD8 | 353 (141–1834) | 791 (463–2090) | 0.007* |
| B cells | 82.5 (26–402) | 424 (221–2038) | <0.001* |
| NK cells | 400.5 (174–1535) | 311 (68–811) | 0.04* |
Absolute cell numbers are expressed as cells/mm3. Range is mentioned in brackets.
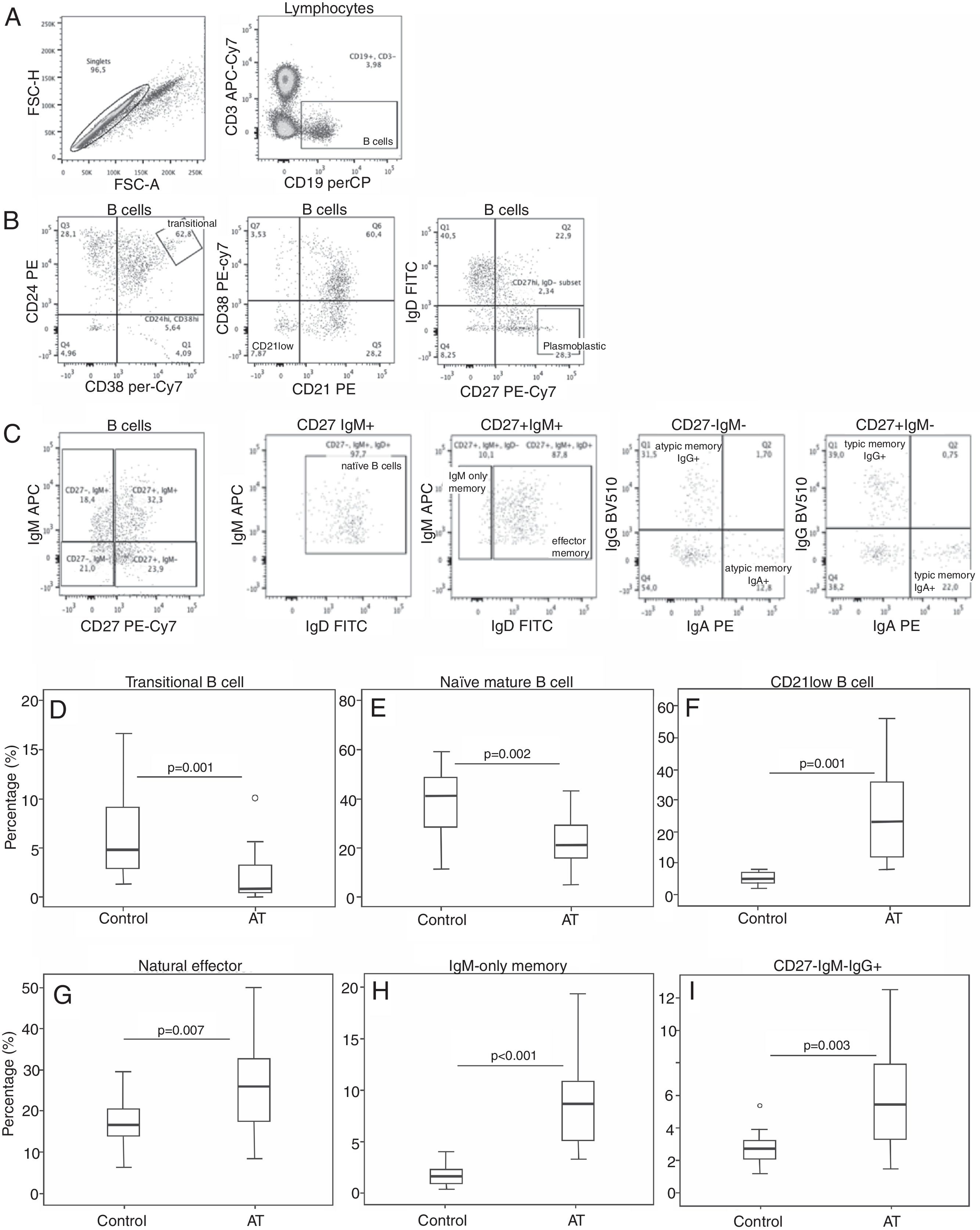
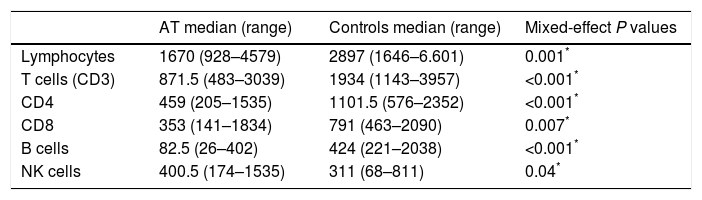
A detailed analysis of the B-cell subsets was performed to investigate B-cell maturation in patients with AT. Circulating transitional B-cells (i.e. early emigrant B-cells from the bone marrow) showed a reduced proportion compared with those seen in controls (r: 0–10.1%; median: 0.9; Fig. 1D). Naïve, mature B-cell populations were also reduced in patients with AT (r: 5.1–70.7%; median 21.5; Fig. 1E). A distinct B-cell population containing mostly autoreactive unresponsive clones that might represent anergic B-cells (CD21lowCD38low B-cells) was found to be increased in patients (r: 7.9–55.9%; median 23.2; Fig. 1F). Subsequently, we investigated seven memory B-cell subsets. Patients showed an increased proportion in three of these subsets: natural effector B-cells (range: 8.2–50%; median 25.9; Fig. 1G), IgM-only B-cells (r: 3.3–19.4%; median 8.7; Fig. 1H) and atypical IgG B-cells (r: 1.5–12.5%; median 5.4; Fig. 1I). For other memory cells analysed, the proportion was similar between patients and control subjects: atypical IgA B-cells, (P=0.163), IgG B-cells (P=0.883), IgA B-cells (P=0.182), and plasmoblasts (P=0.934).
Imbalance of B-cell subsets. (A–C) Analysis of B-cell populations in the PBMCs of patients with AT. (A) The left plot shows the gate of single cells, and the right plot shows the identification of B-cells based on CD19 expression. (B) Plots showing the analytical strategy used for studying the transitional, CD21low, and plasmoblast subpopulations, based on CD19+ cells. (C) Plots showing gating selection of IgM versus CD27, and identification of naïve B, memory effector, IgM-only, atypical memory IgG (CD27−IgM−IgG+) and IgA (CD27−IgM−IgA+), and typical memory IgG (CD27+IgM−IgG+), and IgA (CD27+IgM−IgA+) cells. (D–I) Box plots for transitional (D), naïve (E), CD21low (F), natural effector (G), IgM-only (H), and CD27-IgM-IgG+ (I) B-cells are shown. The mixed-effect model was used to calculate statistical significances.
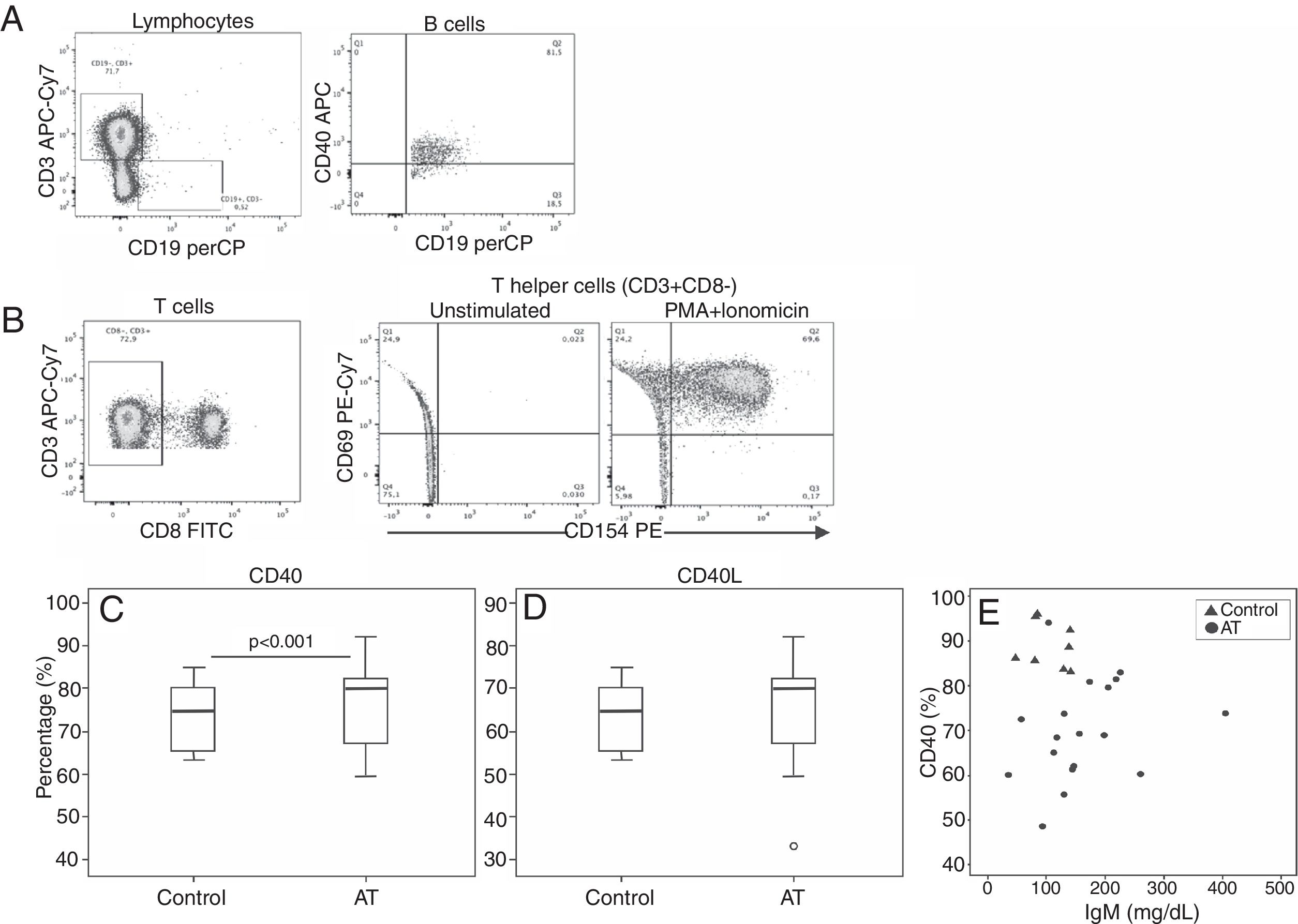
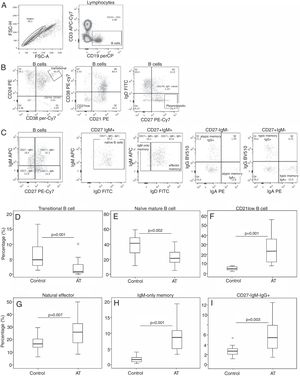
Comparative analysis of CD40 and CD40L expression is shown in Fig. 2. Expression of CD40 in patients with AT was reduced compared with controls (AT=69.9%; range: 48.6–94; C=87.4%; range: 83.1–96; P<0.001). No significant statistical difference was found in CD40L expression between patients and controls (P=0.616). Correlation between CD40 and IgM levels showed a tendency to be inversely correlated (r=0.423; P=0.091), although it was not significant (Fig. 2).
Reduced CD40 and CD154 expression in patients with AT. (A, B) Flow-cytometric analysis of T and B-cell populations in the PBMCs. (A) Plot showing gate selection of CD40-positive expression on CD19+ B-cells. (B) TCD4+ cells were identified as CD3+CD8− cells. In sequence, CD69 versus CD154 (CD40L) expression on T helper cells are shown without stimulation and after PMA and ionomycin stimulation, respectively. Expression of CD69 was used to confirm cell activation. (C, D). Box plots for CD40 and CD40L expression are shown. The mixed-effect model was used to calculate the statistical significance. (E) Correlation between CD40 and IgM levels in patients. The dispersion between CD40 and IgM levels is shown. Pearson's correlation coefficient (rP) was used to calculate the statistical significance (r=0.423; P=0.091).
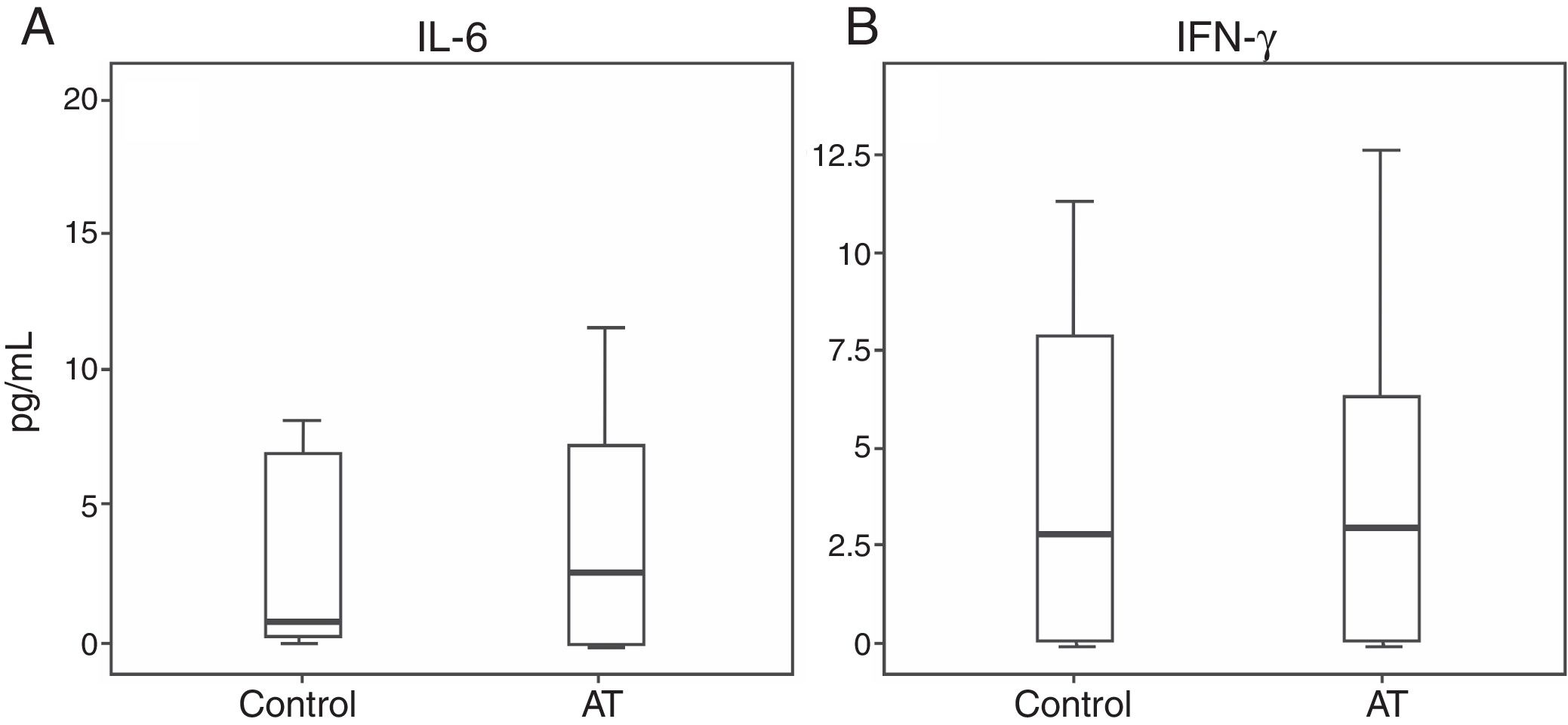
Regarding cytokine levels, no significant statistical difference was observed between patients and controls: IL-6 (P=0.487) and IFN-γ (P=0.922) (Fig. 3).
DiscussionAT is a well-defined combined immunodeficiency involving both T and B-cell immunity. Although the immune system of patients with AT has been extensively studied, attention has been focused on T cell compartment. Our aim was to study the B-cell branch in AT patients, evaluating the distribution of subsets, CD40-CD40L signalling, and cytokine production. We showed that B-cells in patients with AT displayed a variety of abnormalities, including impaired expression of key co-stimulatory pathways and perturbations in developmental subsets.
Patients with AT are prone to a high frequency of infections.25 Respiratory tract infections and chronic lung disease are important causes of morbidity and mortality in these patients.1,26,27 Indeed, 55% of our patients displayed recurrent infections and were under immunoglobulin-replacement therapy.
All of our patients, irrespective of age or infection frequency, showed a markedly decreased number of CD4+, CD8+, and CD19+ lymphocytes. Another peculiar finding in these patients was that opportunistic infections were uncommon, despite a very low number of CD4+ T lymphocytes.28 None of our patients had suffered from such infections. NK cells appear to serve as an important link between the adaptive and innate immune systems, playing a central role against intracellular pathogens and tumour cells. This factor could explain their increased abundance in AT.29 These patients, however, are under constant risk for developing malignancies.
The absence of ATM activity has been associated with an inadequate production of immunoglobulins and, therefore, the effects of ATM on V(D)J recombination and CSR processes have been extensively studied. Systematic analysis of B lymphocyte maturation in common variable immunodeficiency by characterisation of circulating B-cell subpopulations became an important tool to classify patients according to pathogenic aspects.30–32 We analysed the distribution of peripheral blood B-cell subpopulations in a representative set of patients with AT from a unique centre of care and found surprising abnormalities: a decreased proportion of transitional and naïve B-cells and increased levels of CD21low, natural effector, IgM-only memory and atypical IgG B-cells. In addition, another unexpected finding was low CD40 expression on the surface of B-cells in general. Taken together, our findings suggested a pattern of ageing in the humoral compartment of the immune system from patients with AT, reinforcing the excellent review by Shiloh and Lederman.33 This has also been suggested for the T cell profile, with the observation of a selective deficiency of CD4+ naïve and CD8+ naïve cells, and a prevalence of gamma delta (γδ) receptors.34,35
Although T cell alterations play a significant role in age-related immune changes, alterations in B-cells also occur and advanced age are accompanied by substantial changes in all B-cell compartments and, consequently, in humoral immune function.36–38 As previously reported, in the elderly a significant decrease was observed in early B-cell populations such as transitional and mature naïve B-cells, without significant reciprocal increase in CD27+ memory B-cells.38–40 However, the results of a more recent study in elderly people demonstrated the expansion of a B memory subpopulation lacking the classic memory marker CD27, namely double-negative (IgG+IgD−CD27−) CD19+ cells.40 Moreover, the same lab found a significant reduction in CD40 expression in CD27− B-cells compared to CD27+ cells and suggested that these cells might develop outside of the germinal centre or independently of T cell help.40 Some authors suggested that these cells might reflect an age-related manifestation of a chronic stimulation or dysregulation of the immune system.41,42
During the development of B-cell lineages, some un-switched cells also express IgM and have been designated “IgM memory”, “innate-like B cells,” or “natural effector B-cells” (CD27+IgM+IgD+), while some CD27+IgD− (memory switched) cells are IgM+ and have been designated “IgM-only memory B-cells”.43 In contrast to CD27+IgM+IgD-CD27+IgM+IgD+ “natural effector” B-cells are present in patients with CD40 or CD40L deficiency, indicating that at least part of this subset can be generated independently of T cell help.44–46 It has been proposed that “natural effector” B-cells represent an innate-like repertoire, equivalent to splenic marginal zone cells that can be mobilised from the marginal zone into the peripheral blood in response to a T cell-independent challenge.44 However, recent results from one study aimed at a comparative analysis of the replication history and somatic hypermutation levels in different memory B-cells demonstrated high levels of resemblance between “IgM-only” and CD27−IgG+ B-cells, distinguishing them as being derived from primary germinal centre-dependent responses.22 Our findings showed that all of these cells with similar origins in the B-cell-differentiation pathway were proportionally increased in the peripheral blood of patients, suggestive of chronic antigen stimulation and a hallmark response of CD40-CD40L independent signalling. Despite the increased proportion of IgM-only memory B-cells, patients with AT usually have defective polysaccharide antibody responses.11
Transitional B-cells which are considered an intermediate state between immature and mature B-cells with reduced proliferative and functional capabilities30 were reduced in our patients similar to Down's syndrome, which is also an early ageing syndrome.47 The size of the mature-naive pool depends on the bone marrow input (i.e. number of transitional B-cells) and on the availability of space and survival factors in the secondary lymphoid tissues.48
Conclusions regarding the origin of CD21low B-cells differ markedly, since these cells have been described as anergic naïve B-cells, as well as resembling exhausted tissue-like memory B-cells.49–52 The AT patients showed a significant increase in the percentage of these cells, reflecting an immune dysregulation that was previously associated with the presence of autoimmune cytopenias and splenomegaly.53 None of our patients, however, presented these clinical features, but presented with metabolic syndrome. CD21(low) B-cells express increased levels of inflammatory chemokine receptors, which could explain their preferential homing to peripheral tissues and has been suggested to represent a human innate-like B-cell population.50
Defective central tolerance would lead to the development of anergic naïve B-cells, whereas exhausted memory-like B-cells would be a consequence of activation-driven peripheral exhaustion. Thorarinsdottir et al. proposed that a possible explanation for this discrepancy could be that the CD21low B-cells consist of more than one subset, and variation among patients could be another explanation.54 As in human immunodeficiency virus and common variable immunodeficiency, we believe that CD21low B-cells as in AT disorder could reflect chronic activation by disease-specific antigens, leading to an expanded CD21low B-cell memory subset.54
Our results strengthen the recent data of Driessen et al. obtained in patients with AT, suggesting that antibody deficiency in these patients is caused by disturbed naïve B-homeostasis, leading to abnormal memory B-cell formation.55 These authors also found a reduction of transitional and naïve B-cell counts and increased CD21lowCD38low B-cell numbers in heterogeneous patient samples, including those with both the classic and variant AT phenotypes. However, our data disagree in respect to the abnormalities in memory B-cell subsets. The abundances of five out of six memory B cell subsets decreased in patients with classical AT plus hypogammaglobulinaemia, whereas in patients with classical and variant AT, only T cell-dependent germinal-centre reactions were affected. We believe that such differences could be explained by the fact that all of our patients were considered to have the classical disease, and the majority of them were found to have normal or high IgG and IgM levels. Our findings are supported by the importance of CD40 in B-cell proliferation and differentiation, immunoglobulin isotype switching, cell-surface molecule upregulation, cytokine production, resistance to apoptosis and development of humoral memory.56 The expression of CD40 was lower in AT patients resulting in the impairment of all its functions.
Importantly, cells with ATM defects are under a permanent oxidative-stress state, which along with other findings (high incidence of cardiovascular disease, insulin-resistance, elevated serum levels of inflammatory cytokines) suggest that patients with AT suffer from pathological inflammation driven by innate immune dysfunctions.57–61 Furthermore, it was recently reported that treatment with the anti-inflammatory steroid betamethasone improved neurological symptoms in those patients.62 Thus, although the exact mechanism is not completely clear, the absence of ATM activity appears to disrupt immune regulation and potentially tip the scales in the direction of chronic inflammation.62
Although pro-inflammatory effects in AT have been strongly suggested, our previous data did not demonstrate a difference in the production of the important inflammatory cytokine IL-6, which may reflect a poor CBA sensitivity to plasma samples.63 The results might have been better if we had stimulated samples. The normal level of the T lymphocyte cytokine IFN-γ is in accordance with the absence of opportunist or severe infections in patients with AT, despite having T cell lymphopenia.10
ConclusionsIn conclusion, ATM appears to play a central role in the mechanism underlying the antibody immunodeficiency of AT. Exhaustive stimulation of B-cells could lead to their premature ageing, resembling what happens with the skin appearance and what has also been proposed for their cellular abnormalities. In addition, we cannot exclude the hypothesis implicating impaired germinal-centre formation because patients have demonstrated prominent responses in a CD40-independent manner.
FundingThis work was funded by São Paulo Research Foundation – FAPESP (grant number #2013/00786-4). The funding source had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, in the decision to submit the article for publication.
Conflict of interestThe authors declare that they have no conflict of interest.
We would like to thank the patients with AT for their participation in this study and the AT-Brasil Project. This work was supported by grant #2013/00786-4 from the São Paulo Research Foundation (FAPESP). D.C.B.S and C.T.M.P designed and performed the experiments, analysed the data, and wrote the manuscript; N.V.S.F. helped with the experiments; R.S. provided facilities for the experiments; M.K.C.B. and B.T.C.C. directed the study and supervised the writing of the manuscript.