Juvenile systemic lupus erythematosus (JSLE) is a severe and chronic autoimmune disease of unknown origin. Inflammatory cytokines can play a pivotal role in the pathogenesis of JSLE, while their secretion is under genetic control. The current investigation was performed to analyse the associations of particular single nucleotide polymorphisms (SNPs) of interleukin-2 (IL-2) and interferon-gamma (IFN-γ) genes in a case control study.
Materials and methodsThe allele, genotype and haplotype frequencies of the polymorphic IL-2 (G/T at −330, rs2069762, and G/T at +166, rs2069763) and IFN-γ (A/T at +874, rs2430561) genes were estimated in 59 patients with JSLE by contrast with 140 healthy controls using polymerase chain reaction with sequence-specific primers method.
ResultsResults of the analysed data revealed a negative allelic association for JSLE in IL-2 −330/T (P=0.02), as well as a positive allelic association for IL-2 −330/G (P=0.02). IL-2 GG genotype (−330) in the patient group was also significantly overrepresented (P<0.001), while IL-2 GT genotype (−330) was notably decreased in the patients with JSLE (P<0.001). Additionally, the frequency of IL-2 (−330, +166) GT haplotype was significantly higher in the patient group (P<0.001).
ConclusionIL-2 cytokine gene polymorphisms could affect individual susceptibility to JSLE and can take on the role of possible genetic markers for vulnerability to JSLE.
Systemic lupus erythematosus (SLE) is a severe and chronic autoimmune disease of unknown origin, characterised by widespread inflammation and the production of a multitude of autoantibodies against native DNA and other cellular components. SLE is a prototypic autoimmune disease with a diverse spectrum of clinical manifestations that may affect virtually every organ in the human body.21,22 Ten to twenty percent of all SLE patients are categorised as patients with juvenile SLE (JSLE) with a disease onset prior to the age of 16 years.27,32 A plausible genetic predisposition along with environmental factors culminate in the expression of disease pathology.17,29 The earlier onset of JSLE, along with its more active entity at diagnosis and over time, compared to the adult-onset SLE, could be possibly rationalised by the presence of different SLE susceptibility loci in JSLE.
Given the pivotal role of cytokines in the pathogenesis of inflammatory and autoimmune disorders, it stands to reason that cytokines contribution to the initiation and progression of multiple diseases including SLE have been a topic of intensive research recently.31 Some such cytokines include interleukin-2 (IL-2) and interferon-gamma (IFN-γ). IL-2 is a multifunctional cytokine primarily secreted by T cells. This cytokine plays an important role in the processes leading to T cell activation, proliferation, and contraction. It has been speculated that production of IL-2 is decreased in patients with SLE and this deregulation exerts influence on several aspects of host immunity.12,13 IFN-γ is produced by the immune cells, particularly NK cells and T cells, which are involved in both innate and acquired immune systems. This cytokine is known to exert antiviral capacities, contribute to cytotoxic T-cell activity, activate macrophages, and be associated with T helper 1 responses. IFN-γ production has been demonstrated to be diminished in SLE.20,28
There is some evidence concerning the probable association of multiple cytokine gene polymorphisms with serum levels of the cytokines,1,3,4,14,24 but analyses of such loci have been limited to specific cytokines and few disorders, and as far as we know, only a few association studies have ever been established concerning Iranian paediatric patients with JSLE.15,16,23,30,34
The primary objective of this study was to determine the associations between certain SNPs in both IL-2 at positions −330 and +166 and IFN-γ at position +874 and juvenile SLE in a number of Iranian paediatric patients.
Patients and methodsStudy populationFifty-nine Iranian children diagnosed with juvenile SLE according to the revised criteria of the American College of Rheumatology (ACR) for classification of SLE,10 were recruited consecutively during routine visits at the Rheumatology Clinic of the Children's Medical Center Hospital, the Pediatrics Center of Excellence in Tehran, Iran. One hundred and forty healthy subjects who were randomly selected from blood donors at Iranian blood transfusion organisations were enrolled as the control group.4
The study was approved by the ethical committee of Tehran University of Medical Sciences. Written informed consent was obtained from all guardians and, as appropriate, assent was taken from the participants, before blood sampling.
Sampling and genotypingAmount of five millilitres of peripheral blood was collected from all of the entrants to this study and kept with ethylenediaminetetraacetic acid (EDTA) as anticoagulant, at −20°C until investigation. Genomic DNA was extracted from blood samples using the “salting out” technique.18 Genotyping of the polymorphisms in cytokine genes was carried out using polymerase chain reaction with sequence-specific primers (PCR-SSP) assay (PCR-SSP kit, Heidelberg University, Heidelberg, Germany), as discussed previously.4 Amplification of the isolated DNA was performed by a thermal cycler Techne Flexigene apparatus (Rosche, Cambridge, UK) under the following conditions: initial denaturation at 94°C for 2min; denaturation at 94°C for 10s; annealing+extension at 65°C for 1min (10 cycles); denaturation at 94°C for 10s; annealing at 61°C for 50s; extension at 72°C for 30s (20 cycles). The availability of polymerase chain reaction (PCR) products was visualised by 2% agarose gel electrophoresis and subsequent ultraviolet transilluminator. The frequencies of alleles, genotypes, and haplotypes of IL-2 at positions −330 and +166 and IFN-γ at position +874 were assessed.
Statistical analysisWe evaluated allele, genotype, and haplotype frequencies for all cytokine gene polymorphisms by direct counting. Using the chi-square test, frequencies of alleles, genotypes, and haplotypes were compared between the case and control groups. The odds ratio and 95% confidence intervals for the influence of the aforementioned SNPs on juvenile SLE risk were calculated. A P-value less than 0.05 was regarded as statistically significant. Compliance with the Hardy–Weinberg equilibrium constant was established using chi-square test.
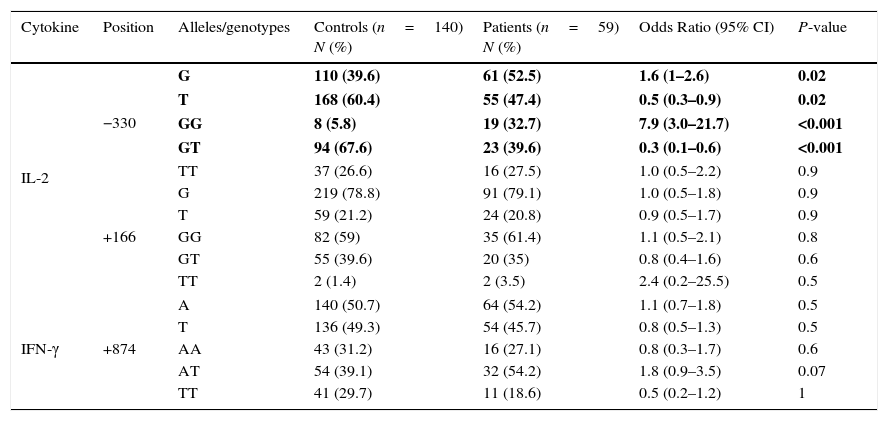
ResultsAllele frequenciesA negative allelic association for JSLE was observed in IL-2 −330/T (47.4% in patients vs. 60.4% in controls, P=0.02). We also noted a positive allelic association which makes the patient prone to the juvenile SLE for IL-2 −330/G (52.5% in patients vs. 39.6% in controls, P=0.02).
No significant differences were observed between the groups for IFN-γ at +874 position.
Allele frequencies of both patients with juvenile SLE and healthy control subjects are presented in Table 1.
IL-2 and IFN-γ allele and genotype polymorphisms in Iranian patients with juvenile SLE, and controls.
| Cytokine | Position | Alleles/genotypes | Controls (n=140) N (%) | Patients (n=59) N (%) | Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| IL-2 | −330 | G | 110 (39.6) | 61 (52.5) | 1.6 (1–2.6) | 0.02 |
| T | 168 (60.4) | 55 (47.4) | 0.5 (0.3–0.9) | 0.02 | ||
| GG | 8 (5.8) | 19 (32.7) | 7.9 (3.0–21.7) | <0.001 | ||
| GT | 94 (67.6) | 23 (39.6) | 0.3 (0.1–0.6) | <0.001 | ||
| TT | 37 (26.6) | 16 (27.5) | 1.0 (0.5–2.2) | 0.9 | ||
| +166 | G | 219 (78.8) | 91 (79.1) | 1.0 (0.5–1.8) | 0.9 | |
| T | 59 (21.2) | 24 (20.8) | 0.9 (0.5–1.7) | 0.9 | ||
| GG | 82 (59) | 35 (61.4) | 1.1 (0.5–2.1) | 0.8 | ||
| GT | 55 (39.6) | 20 (35) | 0.8 (0.4–1.6) | 0.6 | ||
| TT | 2 (1.4) | 2 (3.5) | 2.4 (0.2–25.5) | 0.5 | ||
| IFN-γ | +874 | A | 140 (50.7) | 64 (54.2) | 1.1 (0.7–1.8) | 0.5 |
| T | 136 (49.3) | 54 (45.7) | 0.8 (0.5–1.3) | 0.5 | ||
| AA | 43 (31.2) | 16 (27.1) | 0.8 (0.3–1.7) | 0.6 | ||
| AT | 54 (39.1) | 32 (54.2) | 1.8 (0.9–3.5) | 0.07 | ||
| TT | 41 (29.7) | 11 (18.6) | 0.5 (0.2–1.2) | 1 | ||
Values in bold indicate significant P values.
IL-2 GG genotype at position −330 (32.7% in patients vs. 5.8% in controls, P<0.001) was remarkably overrepresented in the patient group, while IL-2 GT genotype at position −330 (39.6% in patients vs. 67.6% in controls, P<0.001), was significantly decreased in the patients with JSLE, compared to the healthy controls. No significant differences were detected between the two groups of patients and controls for neither TT genotype at the aforesaid position nor for GG, TG, and TT genotypes at position +166 in IL-2 gene.
We found no significant differences between the groups for IFN-γ at +874 position.
Genotype frequencies of both patients with juvenile SLE and healthy control subjects are depicted in Table 1.
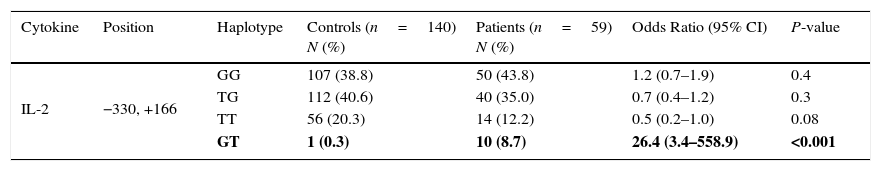
Haplotype frequenciesThe frequency of the IL-2 (−330, +166) GT haplotype (8.7% in patients vs. 0.3% in controls, P<0.001) was significantly higher than controls. There were no significant differences between the two groups for GG, TG, and TT haplotypes at the same positions.
Haplotype frequencies of both patients with juvenile SLE and healthy control subjects are shown in Table 2.
IL-2 haplotype polymorphism in Iranian patients with juvenile SLE, and controls.
| Cytokine | Position | Haplotype | Controls (n=140) N (%) | Patients (n=59) N (%) | Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| IL-2 | −330, +166 | GG | 107 (38.8) | 50 (43.8) | 1.2 (0.7–1.9) | 0.4 |
| TG | 112 (40.6) | 40 (35.0) | 0.7 (0.4–1.2) | 0.3 | ||
| TT | 56 (20.3) | 14 (12.2) | 0.5 (0.2–1.0) | 0.08 | ||
| GT | 1 (0.3) | 10 (8.7) | 26.4 (3.4–558.9) | <0.001 |
Values in bold indicate significant P values.
Systemic lupus erythematosus is a multisystem autoimmune disease caused by an intricate interplay between environmental events and genetic risk factors that include cytokines genes abnormalities.25,26 Reports from several investigations involving adults and some paediatric cohorts indicate that serological, immunological and clinical abnormalities of paediatric patients with JSLE are more pronounced than those of adults. Additionally, previous studies infer that SLE in adults is less active and is associated with less perpetual damage than JSLE is.5 The reasons for more aggressive SLE in children than in adults remain to be elucidated; however, this could be probably attributed to the greater genetic background of SLE predisposing genetic variations in childhood-onset SLE. As with any other type of genetic predisposition, SNPs can exert influence on the expression pattern of associated genes. The role of particular cytokine gene polymorphisms in individual susceptibility to a multitude of disorders have been documented thus far.3,7,8,14,19 However, there is a paucity of data concerning cytokines SNPs which may affect individual vulnerability to juvenile systemic lupus erythematosus. In the present study, we have evaluated a sample of Iranian paediatric patients with SLE for the SNPs at positions −330 and +166 in IL-2 gene together with IFN-γ polymorphisms at position +874.
IL-2 is categorised as a typical T helper 1 cytokine, and acts as a crucial factor to impede the formation of autoimmunity.20 It is currently believed that T cells show defective IL-2 production in patients with SLE.2,6,13 The decreased IL-2 expression is known to contribute to multiple immune alterations comprising reduced activation-induced cell death (AICD), diminished numbers and function of regulatory T cells, up-regulation of IL-17 production, and reduced cytotoxic T cells (CTL) responses.12,20 Two single base substitutions have been determined at +166 G/T and −330 G/T positions from the transcription start site in IL-2 gene.11 In the current study, we noticed an increased presence of the G allele at position −330 (known to be associated with increased levels of cytokine expression)4 in IL-2 gene while the T allele in the same position showed an under-expression with a possible protective effect. Our findings also revealed that the IL-2 (−330) GG genotype (identified as a polymorphism with a high level of cytokine production following anti-CD3/CD28 stimulation of lymphocytes)4 frequency in patients with JSLE was remarkably higher, while the GT genotype at the same position (acknowledged as a genotype with an intermediate level of IL-2 gene expression)4 was notably lower than controls. Moreover, the frequency of IL-2 (−330, +166) GT haplotype was higher in patients compared to controls. On the other hand, our data did not convey any association between IL-2 (−330) TT genotype (recognised to cause low IL-2 levels)4 and IL-2 (+166) GG, GT, and TT genotypes and susceptibility to JSLE. To our best of knowledge, this is the first study delineating an association between IL-2 (−330) G allele, IL-2 (−330) GG genotype and IL-2 (−330, +166) GT haplotype with juvenile systemic lupus erythematosus in Iranian patients. Compared to other studies, our results with regard to both allelic and genotypic differences in IL-2 gene at position −330 are in favour of the enhanced level of the above-stated cytokine, although serum level of IL-2 was not measured in this investigation.
It has long been speculated that the production of IFN-γ, a cytokine which is predominantly expressed in T helper 1 cells, was decreased in SLE patients.33 Nevertheless, over-expression of IFN-γ has been recently demonstrated in SLE subjects, which might conduce to SLE pathogenesis by inducing B-cell-activating factor (BAFF) production.9 In the present investigation, we did not recognise any association between polymorphisms in IFN-γ at position +874 and individuals’ vulnerability to JSLE.
Our study has certain limitations that should be acknowledged. Firstly, the relatively small number of patients in the patient group reduces the statistical power and prevents us from obtaining more conclusive data regarding the roles of IL-2 and IFN-γ in disease severity and various clinical manifestations. Moreover, serum levels of IL-2 and IFN-γ were not evaluated, as discussed in advance.
ConclusionIn conclusion, this study demonstrates the association between specific alleles [IL-2 −330 (T and G)], genotypes [IL-2 −330 (GG and GT)], and haplotypes [IL-2 (−330, +166) in GT] with juvenile systemic lupus erythematosus. These associations may help us define both some novel genetic predisposing as well as protective factors in regard to JSLE. In order to delineate the role of IL-2 and IFN-γ genotypes in the pathogenesis of juvenile systemic lupus erythematosus and influence on IL-2 and IFN-γ levels, further investigation using a larger sample size is recommended.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingThis study was funded by Tehran University of Medical Sciences and Health Services (grant number: 18261).
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of Tehran University of Medical Sciences and with the 1964 Helsinki declaration and its later amendments.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflict of interestThe authors declare no conflicts of interest.
Disclaimer: It should be noted that there is no ethical problem (approved by the research ethics committee of Tehran University of Medical Sciences) or conflict of interest in our research. There was no honorarium, grant, or other form of payment to authors to produce the manuscript.