House dust mites (HDMs) faeces are the main factor involved in respiratory disorder. The true HDMs, Dermatophagoides pteronyssinus and D. farinae, detected in the samples collected from the house dust are the most important causes of allergic disorders such as asthma.
ObjectiveThe aim of this investigation was to study the curcuma and karkade amelioration of the allergenic immunological disorder, especially some cytokines, IgE and ROS, caused by the faeces of the dominant true HDM, D. pteronyssinus and D. farinae in valley and desert houses in EL-Minia Governorate, respectively.
MethodsHDM cultures, faeces isolation, plant extraction and ELISA techniques were used. Male albino rats were classified into control, inhaled, and treated groups.
ResultsThe present immunological study on the dominant allergenic true HDMs, D. pteronyssinus and D. farinae, revealed that significantly higher serum levels of TNF-α, IL-1β, IL-4, IL-13 and IgE were found in rats treated with both D. pteronyssinus and D. farinae faeces than the other groups. In addition, statistical analysis of ROS data showed significant difference between the curcuma- and karkade-treated groups and either the control or the faeces-treated groups (P<0.05).
ConclusionsSome immunological disturbances caused by repeated exposure to the faeces of two dominant allergenic true HDM species (D. pteronyssinus and D. farinae) in the valley and desert houses could be ameliorated by curcuma and karkade.
Mite faeces are small airborne particles (about 20μm). They have great allergenic potential due to protein residues, and inhalation of faecal particles is the main way of exposure.1 Most allergens are biochemically active molecules and include enzymes, enzyme inhibitors, and proteins involved in molecular transport, regulation and cell and tissue structure.2 The majority of works on mite allergens referred to species of the family Pyroglyphidae.2 This family has five genera, of which the genus Dermatophagoides is the most important one from a medical point of view. The European house dust mite (HDM), D. pteronyssinus, and the American HDM, D. farinae, are distributed all over the world.3,4
The optimal environmental temperature for these mites is 18–27°C. Proper indoor relative humidity, temperature and enough food are the key factors determining their survival and development.5 Their major food source is skin scales, hair and wool of pets, fungi, plant pollen and organic debris. The highest HDM densities have been found in bedrooms and living rooms because these indoor spaces usually have large areas covered by textile materials.4 In recent years, lifestyle changes including central heating systems in homes provide suitable conditions for growth and reproduction of HDMs.1
Allergic diseases such as asthma are amongst the most common chronic diseases affecting people in developed countries.6 Asthma is a chronic and complex inflammatory disease of the airways with symptoms including excess mucus production, wheeze, dyspnoea, cough, fatigue, anxiety, tachycardia, and chest tightness.7
Relationships between HDMs and asthma are ambiguous. For example, atopy is not necessarily related to house dust mite allergens and also allergens may not be associated with atopy. Skin-prick test is the routine diagnostic method for atopy-related allergens. The population fraction of asthma attributable to atopy can be calculated when the proportion of people with asthma who have specific IgE or positive skin-prick tests is known.8
Many scientific reports suggest that asthma involves the activation of many inflammatory cells like mast cells, macrophages/monocytes, eosinophils, T-helper type-2 lymphocytes (Th2), dendritic cells, basophils, neutrophils and platelets.9 These cells may also be important sources of mediators in asthma. Cytokines, the main important one of these mediators, can synergise or antagonise the effects of other cytokines and regulated in a complex manner and function via cytokine cascade.10 The major groups of cytokines are lymphokines, pro-inflammatory cytokines, inhibitory cytokines, growth factors and chemokines. Asthmatic inflammatory cells are involved in the production of different cytokines such as Tumour necrosis factor (TNF)-α, Interleukin (IL)-1β, IL-4 and IL-13.
TNF-α and IL-1β are pro-inflammatory cytokines.9,10,12 Two major forms of TNF are TNF-α and TNF-β. TNF-α is produced by many cells including macrophages, T-lymphocytes, mast cells, and epithelial cells, but the principal source is the macrophage. The secretion of TNF-α by monocytes/macrophages is greatly enhanced by other cytokines such as IL-1, granulocyte macrophage-colony stimulating factor (GM-CSF) and interferon (IFN)-γ. Devalia et al. showed that human eosinophils are also capable of releasing TNF-α, together with airway epithelial cells.11 For IL-1, there are also two distinct forms (α and β). The major cellular sources of IL-1 are monocytes, macrophages, neutrophils, eosinophils, mast cells, platelets, lymphocytes, NK cells, endothelial cells, airway smooth muscle cells and vascular smooth muscle cells.12 Air pollutants like nitrogen dioxide can stimulate epithelial cells express IL-1β while eosinophils can produce IL-1α.13 A wide variety of stimuli including IL-1 itself TNF-α, GM-CSF, endotoxin and phagocytosis can increase the expression of IL-1 in monocytes/macrophages.10
IL-4 and IL-13 are anti-inflammatory lymphokines.9,10,12 IL-4, a type-2 T-helper cell derived cytokine, is thought to be an upstream cytokine that regulates allergic inflammation by promoting Th2 cell differentiation and IgE synthesis.14 Synthesis of IL-4 can be induced by stimulation of the antigen receptors on T-lymphocytes and by IgE-Fc receptor cross linking in mast cells and basophils. IL-13 is produced by activated T-lymphocytes, B-lymphocytes and mast cells. In the mouse, almost exclusively Th2 clones express IL-13, however, in humans it can be expressed in both Th1 and Th2 lymphocyte clones.15
Immunoglobulin (Ig) E is a class of antibody isotype that has been found only in mammals. It plays an essential role in type I hypersensitivity,16 which manifests various allergic diseases, such as allergic asthma. Diagnosis of allergy is most often done when a physician reviews a patient's history and finds a positive result for the presence of allergen specific IgE when conducting a skin or blood test.17
Oxidative stress plays a role in asthma aetiology due to activation of various inflammatory cells of the respiratory tract such as neutrophils, eosinophils, mast cells and lymphocytes.18 The continuous exposure of the respiratory tract to environmental oxidants and airway inflammatory cell-generated reactive oxygen species (ROS) creates a high level of oxidative stress in the lung.7 Many studies have shown that cells involved in an asthmatic inflammatory process have a capacity for producing ROS. Activated eosinophils, neutrophils, monocytes, and macrophages generate superoxide (O2−) via a membrane associated NADPH-dependent complex. The subsequent dismutation of O2− can result in the formation of hydrogen peroxide (H2O2). O2− and H2O2 are moderate oxidants and both of them are critical in the formation of potent cytotoxic free radicals in biological systems through their interactions with other molecules.19 This process is involved in asthmatic inflammation; moreover, the concentration of nitric oxide (NO) is increased in airways of asthmatic subjects.20 In addition to the recruited inflammatory cells, epithelial airway cells are potential sources of ROS production.21 Several asthma mediators, such as platelet activating factor, chemokines, adhesion molecules, and eosinophilic granule proteins22 are potential promoters of ROS production. In addition to these endogenous sources, environmental factors linked to asthma, such as air pollutants, are important.23 Increased production of ROS is deleterious because free radical-induced oxidation of proteins, DNA, and lipids can cause direct tissue damage and evoke cellular responses through the generation of secondary reactive species.24
Medicinal plants have been used to cure human illness since ancient times. Certain types of these plants are believed to promote positive health and maintain organism resistance against infection by re-establishing body equilibrium and conditioning the body tissues.25 Curcuma (Curcuma longa, Linn) is a rhizomatous perennial herb that belongs to the family Zingiberaceae. It is used as a herbal remedy due to the prevalent belief that the plant has medical properties. C. longa also acts as an antioxidant and used in the treatment of many diseases such as asthma.26 Karkade (Hibiscus sabdariffa, Linn), a member of the Malvaceae family, is one of these medicinal plant originated from Egypt. All parts of H. sabdariffa are used for medicinal purposes, especially in alternative medicine. H. sabdariffa is taken in many parts of the world for the treatment or management of many diseases. It is used as anti-inflammatory and antioxidant.27
Therefore, the present work aims to study the curcuma and karkade amelioration of the allergenic immunological disorder caused by the faeces of two mite species, D. pteronyssinus and D. farinae that were recorded as the dominant true HDM in the valley and desert houses of asthmatic patients in EL-Minia Governorate, respectively.28
Materials and methodsThe dominant allergenic mite species, D. pteronyssinus and D. farinae, recorded in the valley and desert houses were used for experiments.28 Mites were isolated from the collected dust samples by Berlese–Tullgren method, preserved and mounted on microscope slides for identification according to Colloff.2D. pteronyssinus photos were captured by light microscopy at 100× and 400× magnifications.
Preparation of extractsMite faeces extractIsolated male and female individuals, in copulation state, of D. pteronyssinus and D. farinae were cultured and grown in small Petri dishes with paper tissues under their covers. Cultures were kept at 25°C, 75±5% relative humidity and fed on a mixture of Aquarium gold fish flakes (Mars Ireland, Dublin4, Mars Fishcare Europe) and dry yeast granules29 with 1:1 ratio in complete darkness. When the population density was sufficiently high, D. pteronyssinus and D. farinae crude faeces were isolated by the Berlese–Tullgren method. Five grams from these faeces pellets were used for each inhalation.
Medicinal plant extractsCurcuma and karkade were purchased from the local market in El-Minia Governorate, Egypt. A fresh sample of 50g plant species was sterilised, dried and ground into powder. Each sample (50g) was extracted twice with 300ml ethanol at room temperature for two days and filtered. The filtrate of each plant was concentrated by a rotary evaporation (Büchner rotary evaporate) at 40°C. The ethanol extract was weighed and stored at 4°C until used. Doses 250mg curcuma and 500mg karkade extracts per kg albino rat body weight (b.wt.) were used.26,30
Experimental animalsMale albino rats, Rattus norvegicus (6–8 weeks old, weight 100–120g) were purchased from the Biological Supply Center, Theodore Bilharz Research Institute, TBRI, Cairo, Egypt and housed under specific pathogen-free conditions and maintained on a 12-h light–dark cycle, with food and water ad libitum. Animals were classified into three groups (five animals each). The first group (control) was untreated. The second group was intranasal inhaled daily with either D. pteronyssinus or D. farinae faeces extract daily for 4 weeks. The third and the fourth groups were oral treated daily with curcuma and karkade extracts at a dose of 250 and 500mg/kg b.wt., respectively, and intranasal inhaled daily with faeces extract daily for the same period.
Cytokines, immunoglobulin and ROS in serumAfter 72h from the last treating, animals from each group were sacrificed under chloroform anaesthesia. Blood samples were taken from the heart and centrifuged at 3000rpm for 30min. Sera were removed and kept at −20°C for the assessments of TNF-α, IL-1β, IL-4, IL-13, IgE antibody levels by ELISA using kits purchased from R&D Systems (Minneapolis, MN, USA). ROS kit was obtained from Elaab (Wuhan, China).
Statistical analysisSPSS program version 18.0 was used to analyse the data. Statistical analysis of the obtained data was performed using one way analysis of variance (ANOVA) test followed by least square differences (LSD) analysis for comparison between means. Results were expressed as mean±standard error (SE). Values of P>0.05 were considered statistically non-significant, while P<0.05 value were statistically significant.
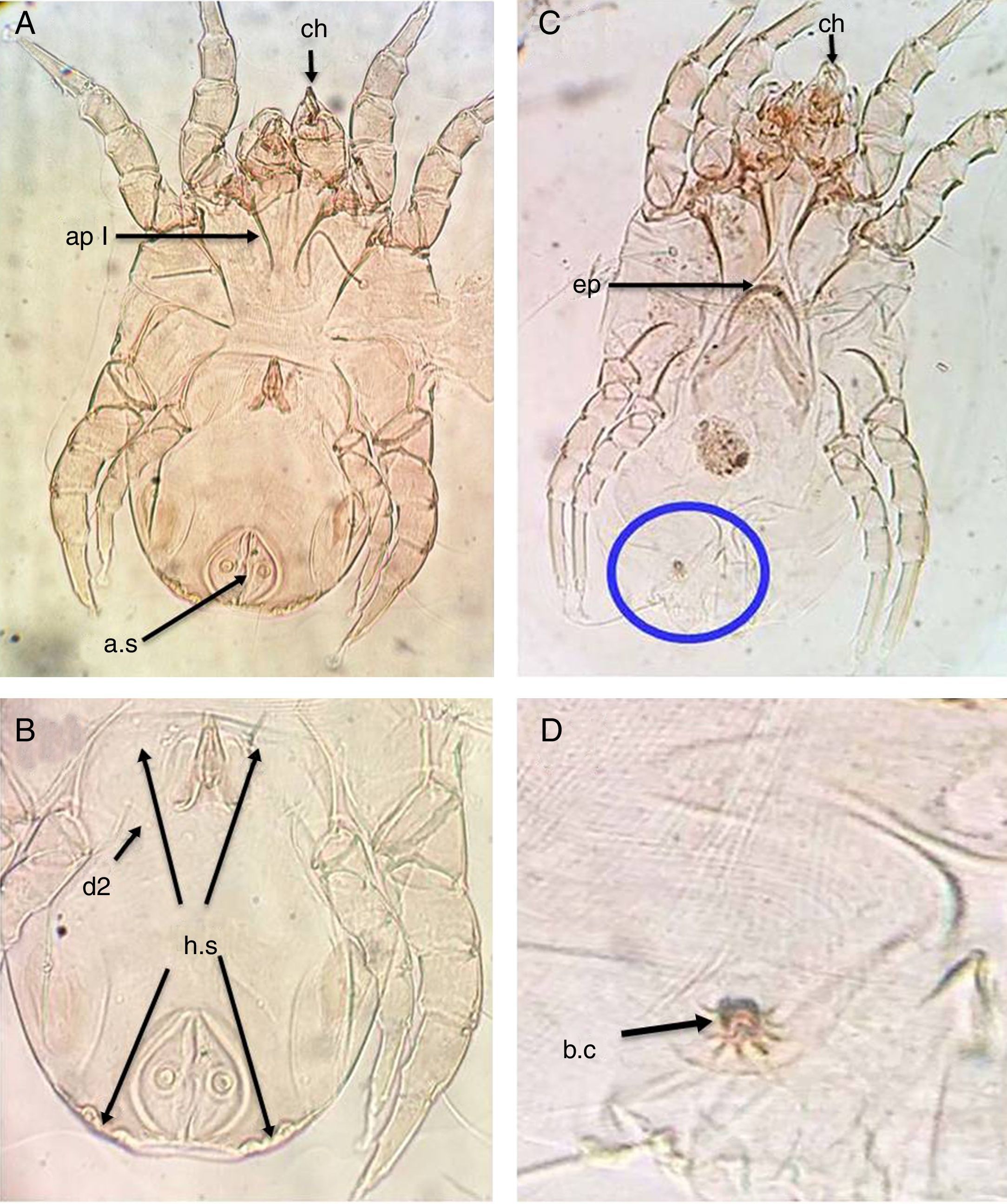
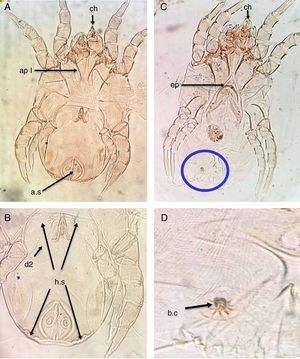
ResultsThe main characteristics of the dominant true house dust mite species in the valley and desert housesDermatophagoides pteronyssinusBoth sexes of D. pteronyssinus are feebly sclerotised mites, with striated cuticle and chelate chelicerae (ch) and without vertical setae.
In the male, the first and second pairs of legs are almost equal in length and width and the first apodemes (ap I) are always widely separated from one another and do not meet to form a sternum (Fig. 1A). The anus is encircled by an oval perianal ring which also encloses prominent anal suckers (a.s) (Fig. 1B).
D. pteronyssinus. (A) Ventral view of male. (B) Dorsal view of male. (C) Ventral view of female. (D) Flower-shaped apex of bursa copulatrix. Chelate chelicera (ch), apodem1 (ap1), anal suckers (a.s), oblong hysterosomal shield (h.s, arrows), dorsal seta (d2), crescentic epigynium (ep).
In the female, the genital opening is λ-shaped and bounded anteriorly by a crescent epigynium (ep) (Fig. 1C). The apex of bursa copulatrix is flower-shaped when viewed dorsally (Fig. 1D, arrow).
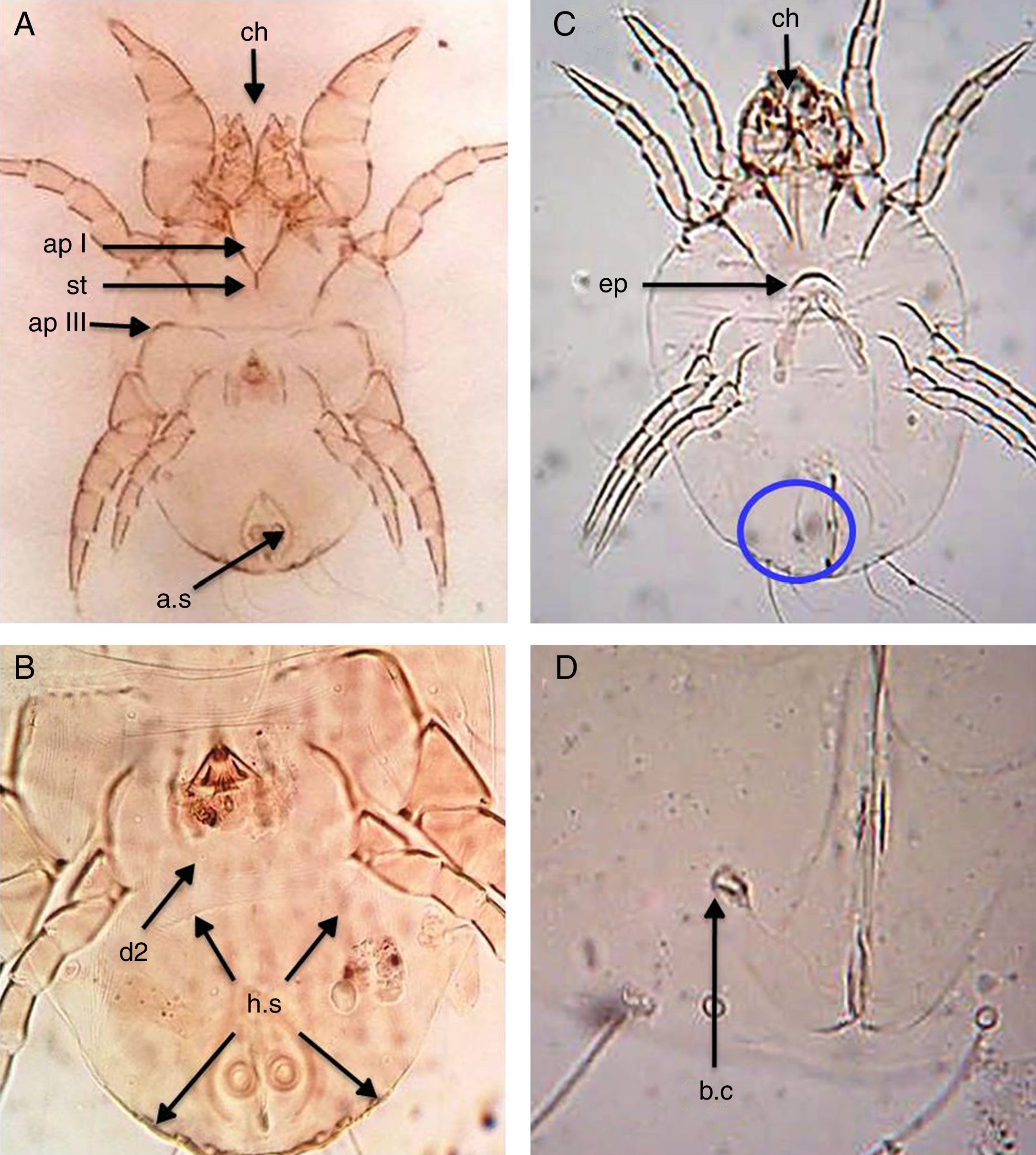
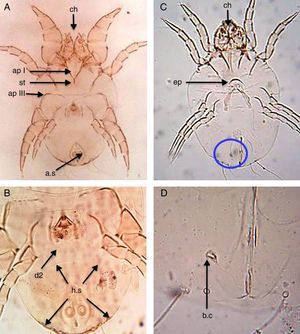
Dermatophagoides farinaeSimilar to D. pteronyssinus, both sexes of D. farinae are feebly sclerotised mites, with striated cuticle and chelate chelicerae and without vertical setae.
In the male, the first legs are thickened and the first apodemes (ap I) mostly fuse in the middle to form a short sternum (st), while the third apodemes (ap III) are long and bent sharply at a right angle. The anus is encircled by an oval perianal ring which also encloses prominent anal suckers (a.s) (Fig. 2A). The hysterosomal shield (h.s), as long as broad terminates posterior of the second dorsal setae (d2) (Fig. 2B).
D. farinae. (A) Ventral view of male. (B) Dorsal view of male. (C) Ventral view of female. (D) Flask-shape bursa copulatrix (b.c). Chelate chelicera (ch), apodem I (apI), apodem III (apIII), anal suckers (a.s), hysterosomal shield (h.s, arrows), dorsal seta (d2), crescentic epigynium (ep), sternum (st).
In the female, the genital opening is λ-shaped and bounded anteriorly by a short crescent epigynium (ep) (Fig. 2C). Bursa copulatrix (b.c) has a flask-shape (Fig. 2D).
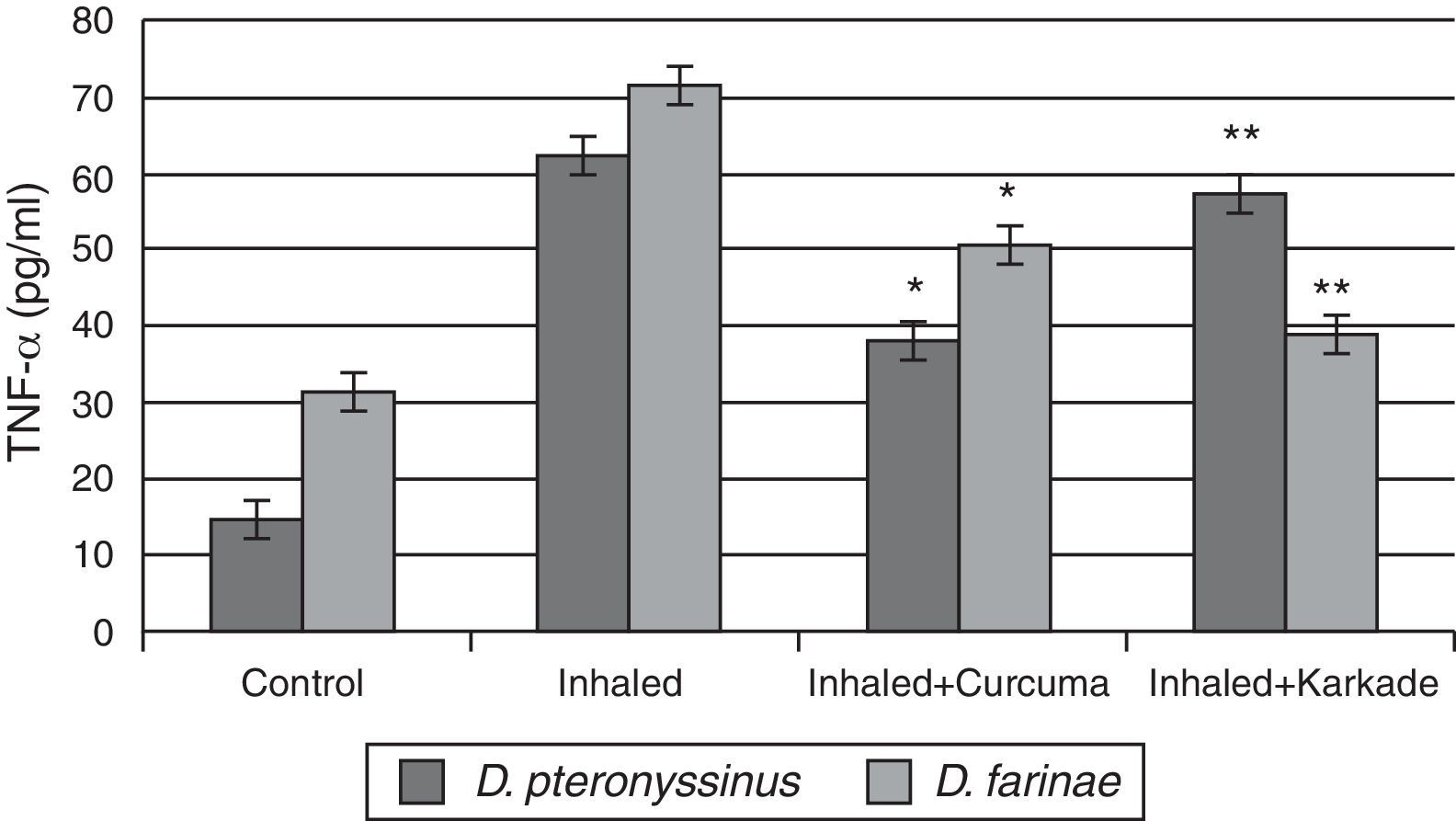
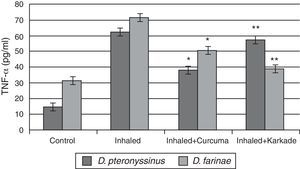
Immunological studiesAssessments of TNF-αResults of ELISA in Fig. 3 showed that TNF-α in control groups of D. pteronyssinus and D. farinae recorded 14.4±1.27pg/ml and 31.08±1.359pg/ml, respectively. Inhaled groups with D. pteronyssinus and D. farinae faeces counted higher level of TNF-α (61.9±2.973pg/ml and 71.55±4.074pg/ml, respectively) than those recorded in the inhaled groups treated with the curcuma (38.2±2.247pg/ml and 50.5±3.147pg/ml, respectively) and the karkade (57.3±4.343pg/ml and 38.73±3.338pg/ml, respectively). Statistical analysis showed a significant difference in TNF-α levels between the curcuma- and karkade-treated groups and either control or faeces-treated groups, P<0.05.
TNF-α levels in male albino rats untreated (control), inhaled with D. pteronyssinus and D. farinae mite faeces and treated with either curcuma or karkade. * shows the significance of treated rates with curcuma in comparison to the inhaled rates. ** shows the significance of treated rates with karkade in comparison to the inhaled rates.
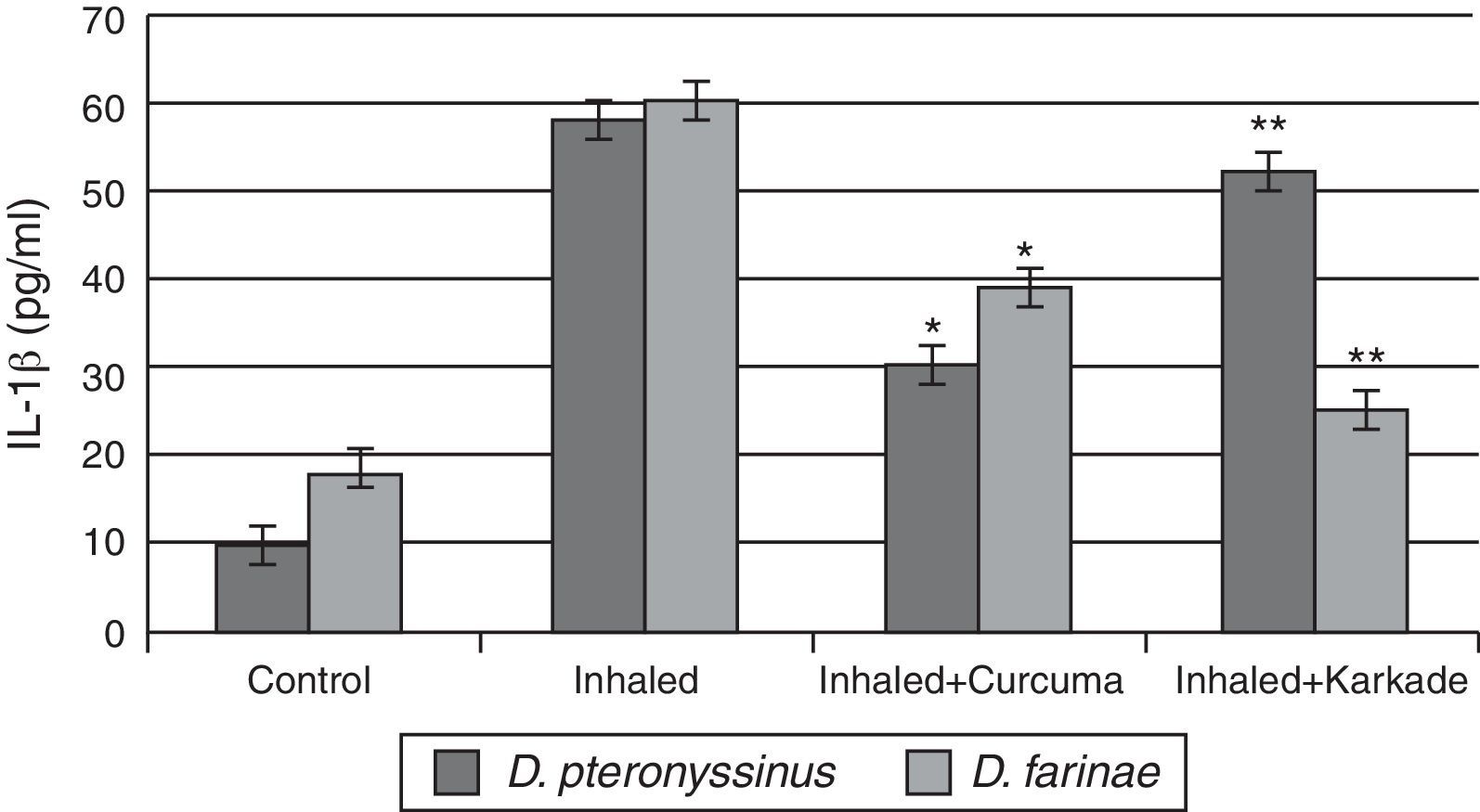
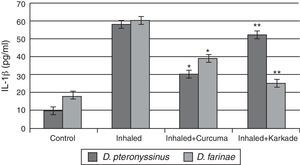
The IL-1β ELISA values demonstrated that the curcuma-treated groups for D. pteronyssinus and D. farinae (30±1.767pg/ml and 38.55±3.403pg/ml, respectively), the karkade-treated groups (52.3±4.206pg/ml and 25.53±3.446pg/ml, respectively) and the control groups (10.46±0.751pg/ml and 18±1.357pg/ml, respectively) are lower than the groups inhaled with D. pteronyssinus and D. farinae faeces (58.3±4.907pg/ml and 59.63±2.593pg/ml, respectively) (Fig. 4). This study also showed that there is a significant difference in IL-1β level between the curcuma-and karkade-treated group and either native or faeces-treated group (P<0.05).
IL-1β levels in male albino rats untreated (control), inhaled with D. pteronyssinus and D. farinae mite faeces and treated with either curcuma or karkade. * shows the significance of treated rates with curcuma in comparison to the inhaled rates. ** shows the significance of treated rates with karkade in comparison to the inhaled rates.
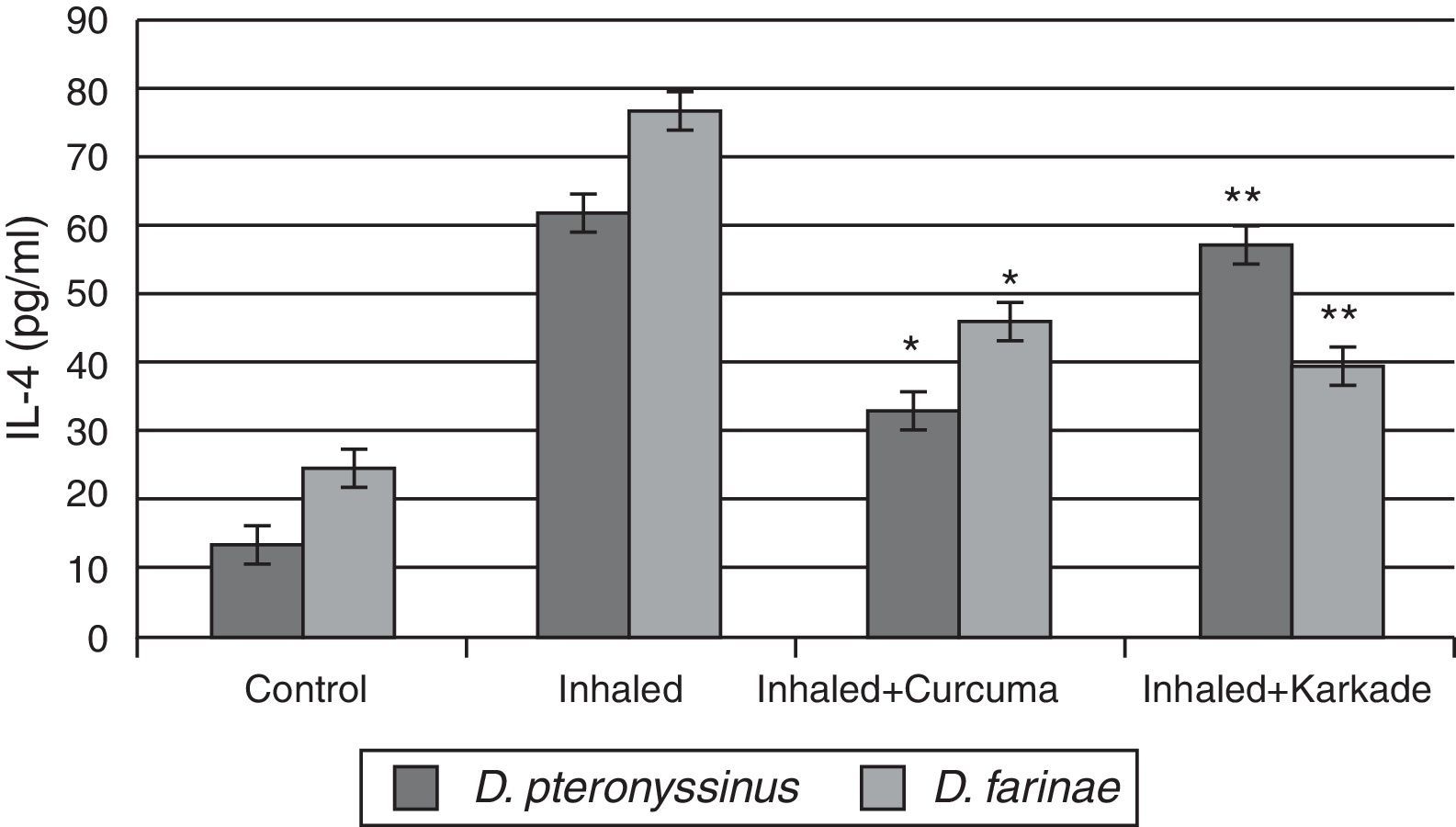
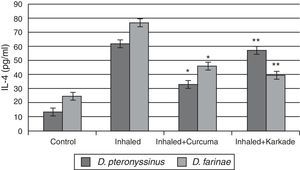
In parallel to TNF-α and IL-1β, results of IL-4 indicated that the groups inhaled with D. pteronyssinus and D. farinae faeces (61.3±3.572pg/ml and 76.45±4.074pg/ml, respectively) are higher than the curcuma-treated groups (32.5±2.313pg/ml and 45.48±3.708pg/ml, respectively) and the karkade-treated groups (56.6±4.108pg/ml and 39.1±3.58pg/ml, respectively) and the control groups (13.1±0.873pg/ml and 24.5±1.357pg/ml, respectively) (Fig. 5). In addition, a significant difference in IL-4 levels between the curcuma- and the karkade-treated groups and either control or faeces-treated group was monitored (P<0.05).
IL-4 levels in male albino rats untreated (control), inhaled with D. pteronyssinus and D. farinae mite faeces and treated with either curcuma or karkade. * shows the significance of treated rates with curcuma in comparison to the inhaled rates. ** shows the significance of treated rates with karkade in comparison to the inhaled rates.
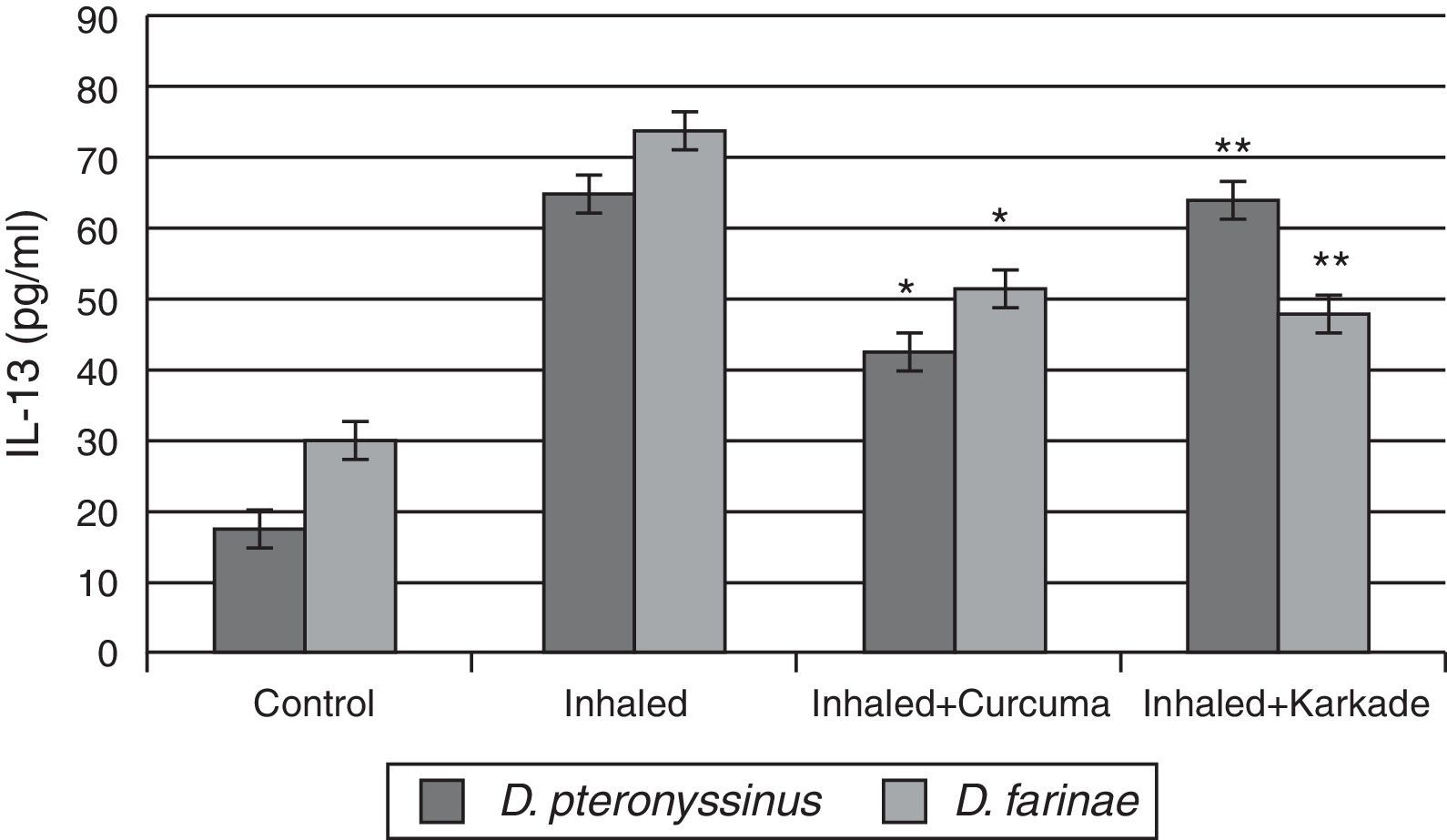
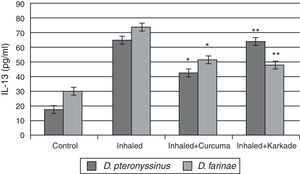
ELISA data of the fourth cytokine, IL-13, showed that both the control groups for D. pteronyssinus and D. farinae (16.73±1.121pg/ml and 30.13±1.125pg/ml, respectively) and the inhaled groups treated with either the curcuma (42±1.514pg/ml and 51.43±3.704pg/ml, respectively) or the karkade (63.2±4.252pg/ml and 47.43±3pg/ml, respectively) (Fig. 6) are lower than the inhaled groups with D. pteronyssinus and D. farinae faeces (64.3±2.325pg/ml and 74.13±2.858pg/ml, respectively). Statistical analysis showed a significant difference in IL-13 between the curcuma- and the karkade-treated groups and either control or faeces-treated groups, P<0.05.
IL-13 levels in male albino rats untreated (control), inhaled with D. pteronyssinus and D. farinae mite faeces and treated with either curcuma or karkade. * shows the significance of treated rates with curcuma in comparison to the inhaled rates. ** shows the significance of treated rates with karkade in comparison to the inhaled rates.
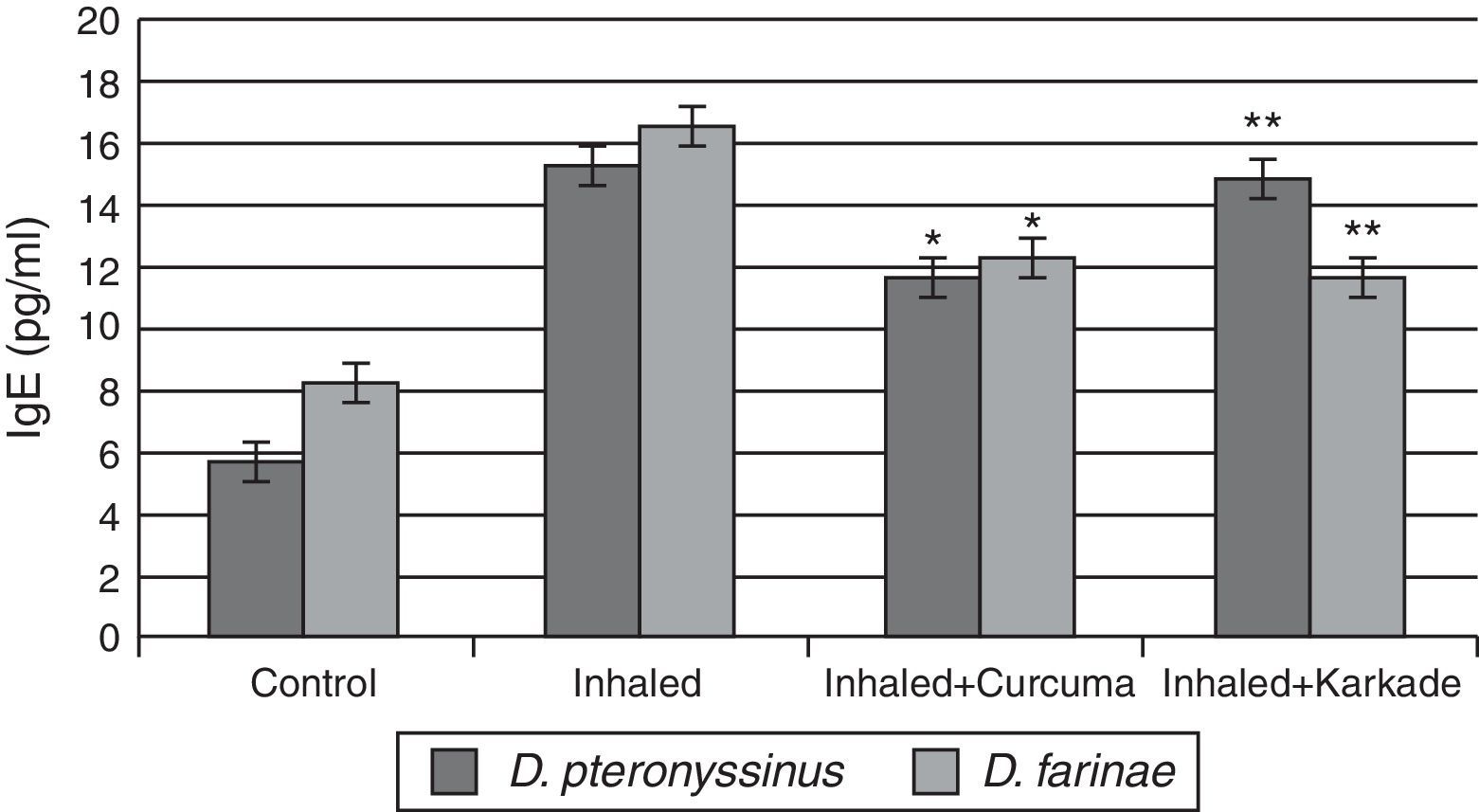
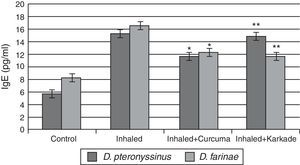
Total IgE ELISA data indicated that both the treated groups with curcuma for D. pteronyssinus and D. farinae (11.5±0.435pg/ml and 12.23±0.351pg/ml, respectively) and the karkade-treated groups for the same two species (14.8±1.183pg/ml and 11.6±0.461pg/ml, respectively) are lower than the groups inhaled with D. pteronyssinus and D. farinae faeces (15.2±0.851pg/ml and 16.46±1.702pg/ml, respectively), while the control groups counted 5.63±0.433pg/ml for D. pteronyssinus and 8.13±1.563pg/ml for D. farinae (Fig. 7). This study also showed that there is a significant difference in IgE levels between the curcuma- and the karkade-treated groups and the faeces-inhaled groups (P<0.05).
IgE levels in male albino rats untreated (control), inhaled with D. pteronyssinus and D. farinae mite faeces and treated with either curcuma or karkade. * shows the significance of treated rates with curcuma in comparison to the inhaled rates. ** shows the significance of treated rates with karkade in comparison to the inhaled rates.
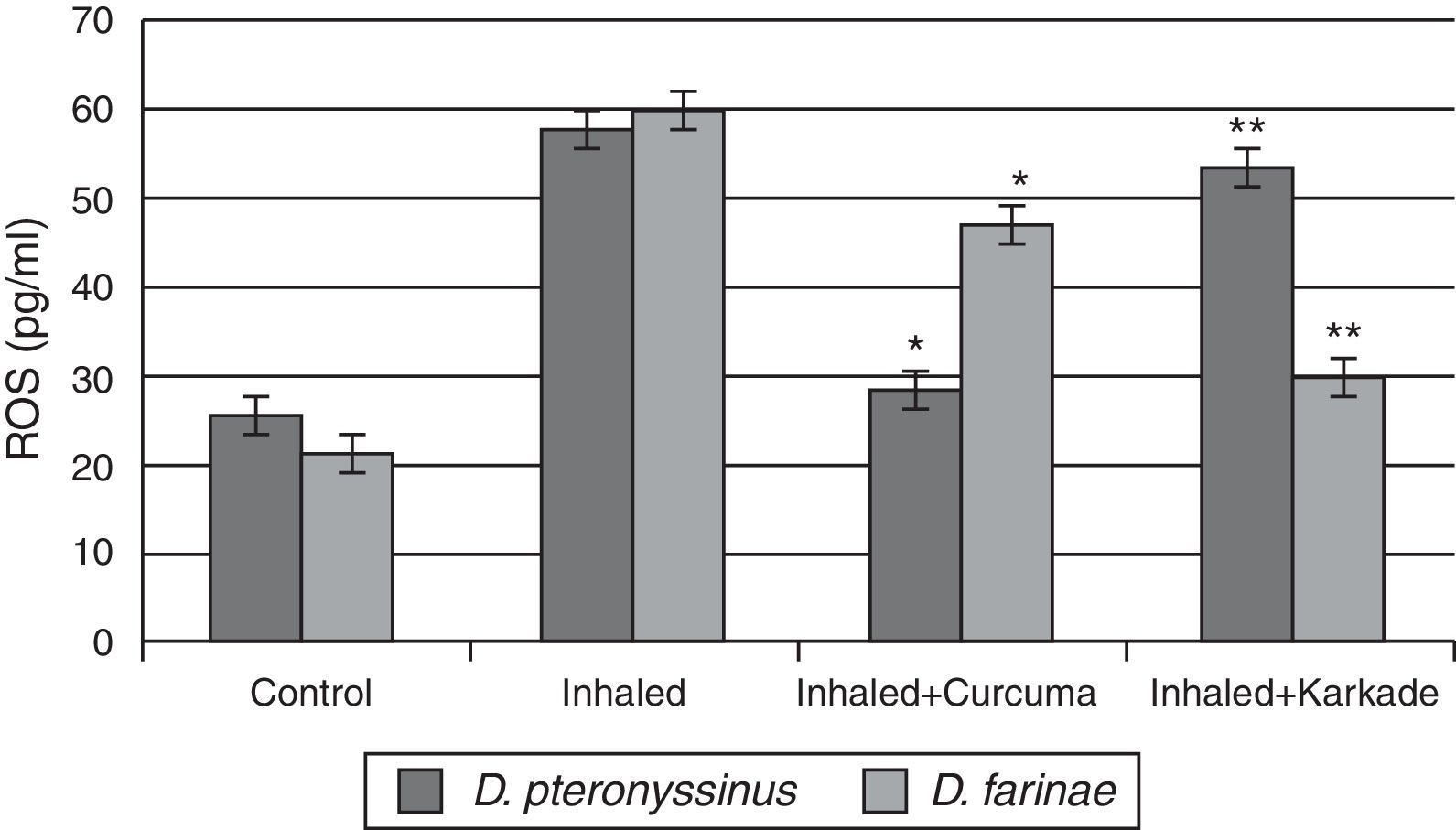
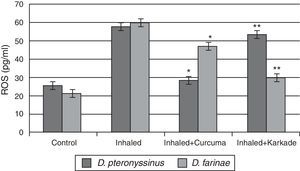
The ROS in serum had high levels in group inhaled with D. pteronyssinus and D. farinae faeces (57.6±2.764pg/ml and 59.78±1.492pg/ml, respectively) than the curcuma- (28.5±2.285pg/ml and 46.80±2.285pg/ml, respectively) and karkade-treated groups (53.2±5.217pg/ml and 29.76±3.447pg/ml, respectively) and the control groups (25.2±1.738pg/ml and 21.52±1.131pg/ml, respectively) (Fig. 8). In addition, a significant difference in ROS levels between the curcuma- and the karkade-treated group and either control or faeces group was detected (P<0.05).
ROS levels in male albino rats untreated (control), inhaled with D. pteronyssinus and D. farinae mite faeces and treated either curcuma or karkade. * shows the significance of treated rates with curcuma in comparison to the inhaled rates. ** shows the significance of treated rates with karkade in comparison to the inhaled rates.
Asthmatic disorders have been rising in recent years. So, the first factor to be considered related to these disorders is the cytokine production. Cytokines are small, extracellular signalling mediators playing an important role in the co-ordination and persistence of inflammation in asthma.12 They possess overlapping biological activities, exert different effects at different concentrations, can synergise or antagonise the effects of other cytokines and regulate in a complex manner and function via cytokine cascade.10
The high level of TNF-α in our results in the inhaled group with D. pteronyssinus and D. farinae faeces confirms the previous experiments, where TNF-α is present abundantly in asthmatic airways. There is evidence that IgE triggering in sensitised lungs leads to increased TNF-α expression in epithelial cells in both rat and human.31 It is reported that TNF-α is also released from alveolar macrophages of asthmatic patients after allergen challenge.32 Furthermore, both monocytes and alveolar macrophages show increased gene expression of TNF-α after IgE triggering in vitro that is enhanced by INF-γ. In vitro studies also indicate that TNF-α plays a role in bronchial hyperresponsiveness and airway remodelling in asthma.33
Asthmatic patients show an increased level of IL-1β.34 In addition, increased expression of IL-1β in asthmatic airway epithelium has been reported, together with an increased number of macrophages expressing IL-1β.35 The high level of IL-1β is parallel to our measured results.
Another cytokine (IL-4) plays an important role in the pathogenesis of allergic disorders.36 Additional effects that seem of particular importance for asthma include stimulation of mucus producing cells and fibroblast, thus also implicating IL-4 in the pathogenesis of airway remodelling.37 Numerous in vivo studies highlighted its role in IgE production.38 Over expression of IL-4 in lungs leads to a lymphocytic and eosinophilic inflammation, but without airway hyperreactivity.39 IL-4 appears to play an important role in Th2 cell development and recruitment to the airways.40 These properties of IL-4 ensure our results, where we found that high levels of IL-4 were detected in inhaled groups.
The fourth cytokine, IL-13, has a very similar biological activity to IL-4. The levels of IL-13 together with IL-4 are increased after allergen challenge of patients with asthma.41 In allergen-induced airway changes, it has been hypothesised that IL-4 is crucial for the initial Th2 development during primary sensitisation but IL-13 release might prove more important during secondary antigen exposure.42 Our data of IL-13 are in accordance with the above reviews, where we recorded a lower level in the control group and groups treated with medicinal plants than the inhaled group.
In addition to the above cytokines, IgE antibodies play an important role in mediating type I hypersensitivity in humans. In both food and inhalant allergy it is accepted that HDM-specific IgE binds to high-affinity Fc RI on mast cells, basophils, macrophages, and dendritic cells, as well as to low-affinity Fc RII on macrophages, monocytes, lymphocytes, eosinophils, and platelets.43 When mite allergens penetrate mucosal barriers of the respiratory tract and contact IgE antibodies bound to mast cells or basophils, histamine and other mediators that induce symptoms of immediate hypersensitivity are released.44
The other factor to be considered related to asthma disorder is the reactive oxygen species (ROS). The lungs have endogenous antioxidant mechanisms to combat the damaging effects of ROS.8 Many studies have reported increased indices of oxidative stress in the blood and airways of asthmatic subjects, where airway inflammation in asthmatic patients is associated with increased production of ROS (superoxide anion, hydrogen peroxide and hydroxyl radicals) by peripheral blood eosinophils, neutrophils, and alveolar macrophages.45
Curcuma is used as a food additive, while karkade is a herbal tea consumed both hot and cold by people around the world. Oral administration of curcumin in instances of acute inflammation was found to be as effective as cortisone or phenylbutazone, and one-half as effective in cases of chronic inflammation.46 In this study we found that some allergenic disorder could be highly modulated in some cytokines, IgE and ROS by curcuma than karkade. The low amelioration caused by karkade might be due to the interplay effect of the karkade extract between humoral (B-cells) and cell-mediated (T-cells) immunity since the administration of the extracts led to changes in the production of cytokines (cell mediated immunity) and antibodies titre (humoral immunity).27
The data conclusion showed that repeated exposure to D. pteronyssinus or D. farinae faeces caused an increase in some cytokines (TNF-α, IL-1β, IL-4 and IL-13), immunoglobulin IgE and ROS. The high level of these mediators may participate in airway disorder. These disorders were ameliorated with a medicinal plant (curcuma and karkade) treatment. This ensures that curcuma and karkade may play an important role in the amelioration of the airway immune response.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration
Confidentiality of dataNo patient data appears in this article.
Right to privacy and informed consentNo patient data appears in this article.
We would like to thank all family members who collaborated in the collection of house dust samples.