There is limited evidence on the association between prenatal smoking exposure and the risk of asthma in children. The aim of this prebirth cohort study was to investigate the association between prenatal and postnatal tobacco smoke exposure and the risk of asthma in Japanese children.
MethodsStudy subjects were 1304 mother–child pairs. Information on the variables under study was obtained using repeated questionnaires that were completed by mothers, first prior to delivery, then shortly after birth and subsequently around 4, 12, 24, and 36 months after delivery. Ever asthma was defined as a maternal report of physician-diagnosed asthma at any time since birth. Current asthma was defined as the use of asthma medication at the time of the sixth survey.
ResultsLogistic regression models revealed that maternal active smoking, either before pregnancy or during pregnancy, was not associated with the risk of ever asthma or current asthma. Further, no association was observed between postnatally living with at least one household smoker and the risk of asthma. Among children whose mothers are never smokers, maternal second-hand smoke (SHS) exposure at work and/or at home during pregnancy increased the risk of ever asthma and current asthma in children; adjusted odds ratio (95% confidence intervals) for ever asthma and current asthma were 2.41 (1.13–5.05) and 4.82 (1.68–13.43), respectively.
ConclusionsOur findings suggest that maternal SHS exposure during pregnancy might be associated with an increased risk of ever asthma and current asthma in young children whose mothers have never smoked.
Exposure to second-hand smoke (SHS) in fetal life and early postnatal life may be a major risk factor for impaired lung function and development of asthma, given that children are particularly susceptible to environmental toxicants during lung development beginning from conception.1,2 A fetus is exposed to tobacco smoke via the umbilical cord blood when its mother smokes or is exposed to SHS.2,3
With regard to maternal active smoking during pregnancy, a meta-analysis of prospective studies in 2012 revealed that maternal active smoking during pregnancy increases the risk of developing asthma in children aged two years and younger, but not in children aged 3–4 years.4 However, pooled analysis in European children at 4–6 years of age demonstrated a positive association between maternal active smoking during pregnancy and the risk of asthma.5 Regarding maternal SHS exposure during pregnancy, there is limited evidence on its effects on asthma development. To date, only four epidemiological studies have addressed this relationship.6–9 One of those studies showed a positive association between maternal SHS exposure during pregnancy and asthma in children aged seven years,7 whereas the remaining three studies detected no association in children aged ≤14 years,6 aged 6 years,8 or aged 42 months.9 However, we should keep in mind the fact that a diagnosis of asthma in children is difficult, and there is potential variation in diagnostic practices across countries.10,11
Compared to the association between prenatal smoking exposure and asthma, more evidence is available regarding the association between postnatal SHS exposure and asthma, although most of this evidence is derived from cross-sectional studies rather than cohort studies. In recent years, several meta-analyses have been conducted based on prospective studies, but their results are inconsistent.4,5,12
In order to clarify the association between perinatal tobacco smoke exposure and the development of asthma, further evidence is needed. Here, using the data from fetal life to three years of age in the Kyushu Okinawa Maternal and Child Health Study (KOMCHS), we assessed the effect of maternal smoking status during pregnancy and postnatal living with smokers on the development of asthma in children. Furthermore, to assess the role of maternal SHS exposure during pregnancy on asthma development, we evaluated the association between maternal SHS exposure during pregnancy and asthma development in children whose mothers are never-smokers.
Materials and methodsStudy subjectsThe KOMCHS is an ongoing prospective birth cohort study that investigates risk and preventive factors for maternal and child health problems. The background and general procedure of the KOMCHS have been described previously.13 In brief, the KOMCHS requested that pregnant women complete a baseline survey, which was followed by several post-natal surveys. Eligible subjects were those women who became pregnant in one of seven prefectures on Kyushu Island in southern Japan or Okinawa Prefecture between April 2007 and March 2008. At 423 obstetric hospitals in the abovementioned eight prefectures, a set of leaflets explaining the KOMCHS, an application form to participate in the study, and a self-addressed and stamped return envelope were distributed to pregnant women by medical or clerical staff at each hospital, insofar as this was possible. Pregnant women who intended to participate in the KOMCHS returned the application form to the data management center. In the end, a total of 1757 pregnant women between the 5th and 39th week of pregnancy gave their fully informed written consent to participate and completed the baseline survey. Of the 1757 women, 1590, 1527, 1430, 1362, and 1305 mother–child pairs participated in the second (after delivery), third (approximately four months postpartum), fourth (approximately 12 months postpartum), fifth (approximately 24 months postpartum), and sixth (approximately 36 months postpartum) surveys, respectively. One pair with missing data on household income was excluded, leaving data on 1304 pairs available for analysis. The ethics committee of the Faculty of Medicine, Fukuoka University and Ehime University Graduate School of Medicine approved the KOMCHS.
MeasurementsEach survey consisted of a self-administered questionnaire. Participants filled out the questionnaires and then mailed them to the data management center at the time of each survey. Research technicians completed missing or illogical data by telephone interview.
The baseline survey questionnaire elicited information on region of residence; number of children; maternal and paternal education levels; household income; maternal and paternal history of asthma, atopic eczema, and allergic rhinitis; and maternal SHS exposure at home and at work. Maternal or paternal history of asthma, atopic eczema, or allergic rhinitis was defined as positive if the respective parent had been diagnosed by a physician as having any of these allergic diseases.
The second survey (after delivery) included questions about the baby's sex, birth weight, and maternal active smoking during pregnancy. The third (approximately four months postpartum) and fourth (approximately 12 months postpartum) surveys asked about the smoking habits of the adult household members and breastfeeding duration. Maternal smoking during pregnancy was defined as positive if mothers smoked at any time during the first, second, or third trimesters. Postnatal living with at least one household smoker was defined as positive if the child had lived with at least one smoker at any point up to the date of the fourth survey. Breastfeeding duration was defined as the length of the period during which infants received breast milk, regardless of exclusivity. Maternal reports on asthma among the children were obtained in the sixth survey via questionnaire. Ever asthma was considered present if the child had been diagnosed by a physician as having asthma at any time since birth. As a result, 94 children were identified as having ever asthma. Among these 94 children, 49 children were also classified as current asthma when a mother reported the use of asthma medication at the time of the sixth survey.
Statistical analysisRegion of residence at baseline; number of children at baseline; maternal and paternal education levels; household income; maternal and paternal history of asthma, atopic eczema, and allergic rhinitis; infant's sex; infant's birth weight; and breastfeeding duration were selected a priori as potential confounding factors.14–17
Multiple logistic regression analysis was performed to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of ever asthma or current asthma relative to categories of perinatal smoking exposure. All statistical analyses were performed using the SAS software package version 9.4 (SAS Institute, Inc., Cary, NC, USA).
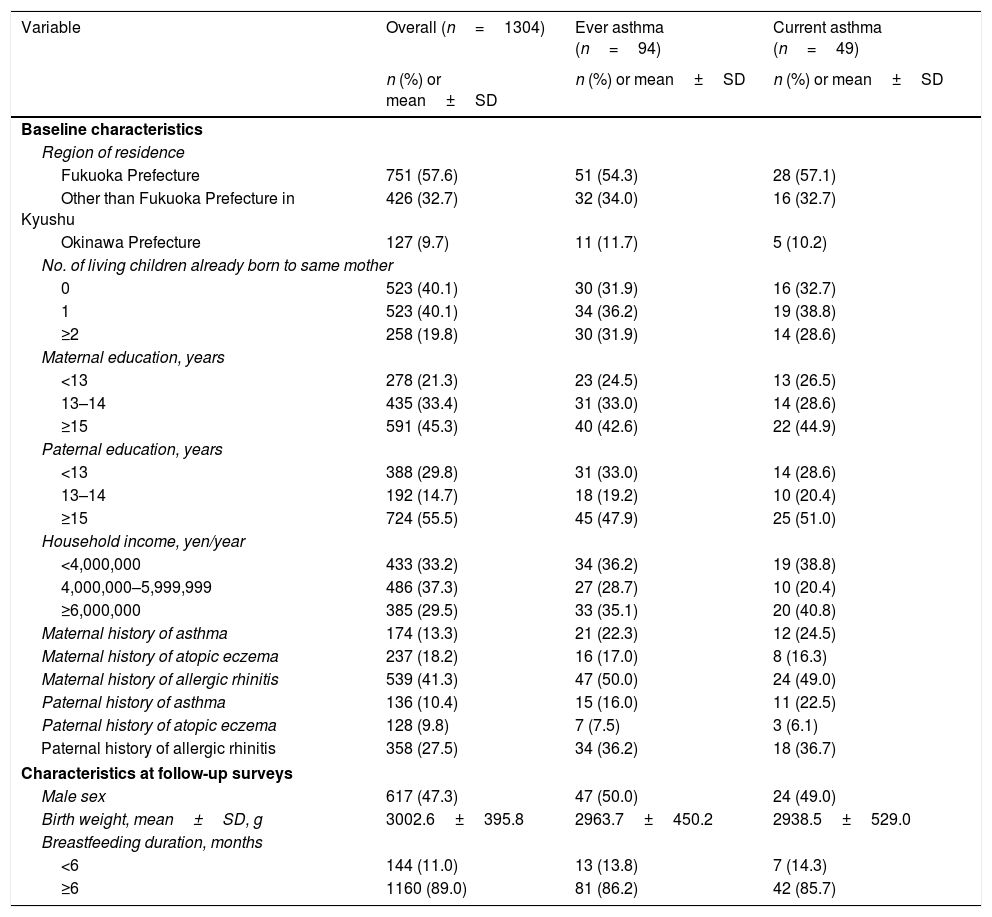
ResultsOf 1304 children, 94 (7.2%) and 49 (3.8%) children were classified as ever and current asthma, respectively. Table 1 shows the distribution of characteristics of the study subjects. About 40% of the subjects had no siblings. The mean birth weight of our study subjects was 3003g. Approximately 89% of the children were breastfed for six months or longer. About 8% of children were exposed to maternal active smoking during pregnancy, and 44% of children had lived postnatally with at least one household smoker.
Distribution of selected characteristics in 1304 parent-child pairs, Kyushu Okinawa Maternal and Child Health Study, Japan.
| Variable | Overall (n=1304) | Ever asthma (n=94) | Current asthma (n=49) |
|---|---|---|---|
| n (%) or mean±SD | n (%) or mean±SD | n (%) or mean±SD | |
| Baseline characteristics | |||
| Region of residence | |||
| Fukuoka Prefecture | 751 (57.6) | 51 (54.3) | 28 (57.1) |
| Other than Fukuoka Prefecture in Kyushu | 426 (32.7) | 32 (34.0) | 16 (32.7) |
| Okinawa Prefecture | 127 (9.7) | 11 (11.7) | 5 (10.2) |
| No. of living children already born to same mother | |||
| 0 | 523 (40.1) | 30 (31.9) | 16 (32.7) |
| 1 | 523 (40.1) | 34 (36.2) | 19 (38.8) |
| ≥2 | 258 (19.8) | 30 (31.9) | 14 (28.6) |
| Maternal education, years | |||
| <13 | 278 (21.3) | 23 (24.5) | 13 (26.5) |
| 13–14 | 435 (33.4) | 31 (33.0) | 14 (28.6) |
| ≥15 | 591 (45.3) | 40 (42.6) | 22 (44.9) |
| Paternal education, years | |||
| <13 | 388 (29.8) | 31 (33.0) | 14 (28.6) |
| 13–14 | 192 (14.7) | 18 (19.2) | 10 (20.4) |
| ≥15 | 724 (55.5) | 45 (47.9) | 25 (51.0) |
| Household income, yen/year | |||
| <4,000,000 | 433 (33.2) | 34 (36.2) | 19 (38.8) |
| 4,000,000–5,999,999 | 486 (37.3) | 27 (28.7) | 10 (20.4) |
| ≥6,000,000 | 385 (29.5) | 33 (35.1) | 20 (40.8) |
| Maternal history of asthma | 174 (13.3) | 21 (22.3) | 12 (24.5) |
| Maternal history of atopic eczema | 237 (18.2) | 16 (17.0) | 8 (16.3) |
| Maternal history of allergic rhinitis | 539 (41.3) | 47 (50.0) | 24 (49.0) |
| Paternal history of asthma | 136 (10.4) | 15 (16.0) | 11 (22.5) |
| Paternal history of atopic eczema | 128 (9.8) | 7 (7.5) | 3 (6.1) |
| Paternal history of allergic rhinitis | 358 (27.5) | 34 (36.2) | 18 (36.7) |
| Characteristics at follow-up surveys | |||
| Male sex | 617 (47.3) | 47 (50.0) | 24 (49.0) |
| Birth weight, mean±SD, g | 3002.6±395.8 | 2963.7±450.2 | 2938.5±529.0 |
| Breastfeeding duration, months | |||
| <6 | 144 (11.0) | 13 (13.8) | 7 (14.3) |
| ≥6 | 1160 (89.0) | 81 (86.2) | 42 (85.7) |
SD: standard deviation.
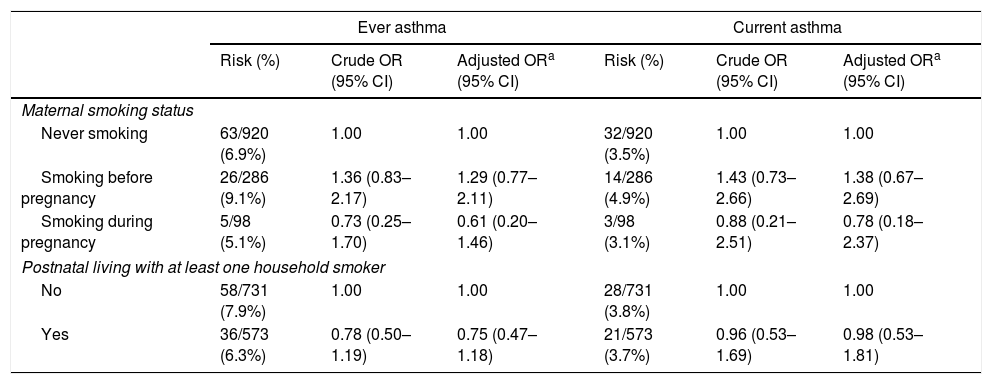
Table 2 shows ORs and 95% CIs for asthma in relation to perinatal smoking exposure. Maternal active smoking, either before pregnancy or during pregnancy, was not associated with the risk of ever asthma or current asthma. In addition, no association was observed between postnatal living with at least one household smoker and the risk of ever asthma or current asthma.
Adjusted ORs and 95% CIs for asthma according to perinatal tobacco smoke exposure, Kyushu Okinawa Maternal and Child Health Study, Japan.
| Ever asthma | Current asthma | |||||
|---|---|---|---|---|---|---|
| Risk (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Risk (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | |
| Maternal smoking status | ||||||
| Never smoking | 63/920 (6.9%) | 1.00 | 1.00 | 32/920 (3.5%) | 1.00 | 1.00 |
| Smoking before pregnancy | 26/286 (9.1%) | 1.36 (0.83–2.17) | 1.29 (0.77–2.11) | 14/286 (4.9%) | 1.43 (0.73–2.66) | 1.38 (0.67–2.69) |
| Smoking during pregnancy | 5/98 (5.1%) | 0.73 (0.25–1.70) | 0.61 (0.20–1.46) | 3/98 (3.1%) | 0.88 (0.21–2.51) | 0.78 (0.18–2.37) |
| Postnatal living with at least one household smoker | ||||||
| No | 58/731 (7.9%) | 1.00 | 1.00 | 28/731 (3.8%) | 1.00 | 1.00 |
| Yes | 36/573 (6.3%) | 0.78 (0.50–1.19) | 0.75 (0.47–1.18) | 21/573 (3.7%) | 0.96 (0.53–1.69) | 0.98 (0.53–1.81) |
Adjustment for region of residence at baseline; number of children at baseline; maternal and paternal education levels; household income; maternal and paternal history of asthma, atopic eczema, and allergic rhinitis; infant's birth weight; infant's sex; and breastfeeding duration.
CI: confidence interval; OR: odds ratio
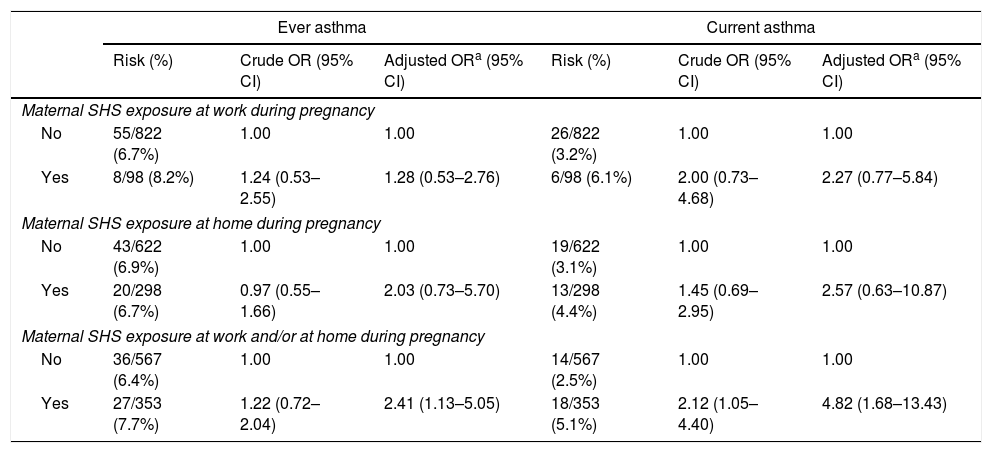
To assess the effect of maternal SHS exposure during pregnancy on asthma development, we performed a sensitivity analysis among children whose mothers were never smokers (n=920), in which further adjustment was made for postnatal living with at least one household smoker. Among these children, the prevalence values of maternal SHS exposure during pregnancy at work and at home were 10.7% and 32.4%, respectively. No association was observed between the risk of ever asthma or current asthma and maternal SHS exposure during pregnancy at home or at work when those two settings were evaluated separately, but there was a significant increase in risk associated with maternal SHS exposure during pregnancy at work and/or at home when the two settings were considered together; adjusted odds ratio (95% CIs) for ever asthma and current asthma were 2.41 (1.13–5.05) and 4.82 (1.68–13.43), respectively (Table 3).
Adjusted ORs and 95% CIs for asthma according to maternal SHS exposure during pregnancy among 920 children whose mothers were never smokers, Kyushu Okinawa Maternal and Child Health Study, Japan.
| Ever asthma | Current asthma | |||||
|---|---|---|---|---|---|---|
| Risk (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Risk (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | |
| Maternal SHS exposure at work during pregnancy | ||||||
| No | 55/822 (6.7%) | 1.00 | 1.00 | 26/822 (3.2%) | 1.00 | 1.00 |
| Yes | 8/98 (8.2%) | 1.24 (0.53–2.55) | 1.28 (0.53–2.76) | 6/98 (6.1%) | 2.00 (0.73–4.68) | 2.27 (0.77–5.84) |
| Maternal SHS exposure at home during pregnancy | ||||||
| No | 43/622 (6.9%) | 1.00 | 1.00 | 19/622 (3.1%) | 1.00 | 1.00 |
| Yes | 20/298 (6.7%) | 0.97 (0.55–1.66) | 2.03 (0.73–5.70) | 13/298 (4.4%) | 1.45 (0.69–2.95) | 2.57 (0.63–10.87) |
| Maternal SHS exposure at work and/or at home during pregnancy | ||||||
| No | 36/567 (6.4%) | 1.00 | 1.00 | 14/567 (2.5%) | 1.00 | 1.00 |
| Yes | 27/353 (7.7%) | 1.22 (0.72–2.04) | 2.41 (1.13–5.05) | 18/353 (5.1%) | 2.12 (1.05–4.40) | 4.82 (1.68–13.43) |
Adjustment for region of residence at baseline; number of children at baseline; maternal and paternal education levels; household income; maternal and paternal history of asthma, atopic eczema, and allergic rhinitis; infant's sex; infant's birth weight; breastfeeding duration; and postnatally living with at least one household smoker.
CI: confidence interval; OR: odds ratio; SHS: second-hand smoke.
The present study did not demonstrate a statistically significant effect of maternal active smoking during pregnancy or of postnatal living with at least one household smoker on the development of asthma in children. Our results are consistent with those of several previous studies that also observed no association between maternal smoking during pregnancy18,19 or postnatal SHS exposure18–23 and asthma in children. Other studies have reported a positive association between maternal smoking during pregnancy24,25 and postnatal SHS exposure24–27 and asthma. Thus, the available reports on the association between perinatal smoking exposure and asthma are inconsistent. A meta-analysis based on five studies showed a positive association between maternal smoking during pregnancy and the risk of asthma, while no association was observed between postnatal household SHS exposure and the risk of asthma in an analysis based on three studies, among children aged two years or younger.4 It is difficult to separate out the effects of prenatal and postnatal smoking exposure because women who smoke during pregnancy are likely to continue after delivery. In the present study, we were not able to assess the independent effects of pre- and postnatal smoking exposure on the development of asthma due to the small sample size of children whose mothers smoked during pregnancy but who did not live postnatally with at least one household smoker.
In the sensitivity analysis, we found that maternal SHS exposure during pregnancy was associated with an increased risk of ever asthma and current asthma at three years of age in children whose mothers were never smokers. Our findings are partially consistent with those of two previous studies. In a Canadian retrospective cohort study among children whose mothers were not active smokers during pregnancy, maternal in-home SHS exposure during pregnancy increased the risk of physician-diagnosed childhood asthma at seven years old, and this positive association remained significant after adjusting for the child's postnatal SHS exposure from birth until age seven.7 A cross-sectional study of children aged one to six years in Greece demonstrated that maternal exposure to SHS during the third trimester of pregnancy was marginally significantly associated with the prevalence of physician-diagnosed asthma among children who were not exposed to postnatal SHS and whose mothers did not smoke during pregnancy.9 In a cross-sectional study of Hong Kong Chinese children aged ≤14 years, no association was observed between SHS exposure during pregnancy in non-smoking mothers and the prevalence of ever having had asthma based on the International Study of Asthma and Allergies in Childhood criteria.6 A prospective cohort study in the Netherlands also showed no association between paternal smoking during pregnancy and a risk of ever having had physician-diagnosed asthma at age six, among children of mothers who did not smoke during pregnancy.8 These results differ from our results. Several methodological differences including the study population and design, definition of outcome, classification of exposure status, and the confounding factors considered may have contributed to these discrepant findings.
The underlying mechanisms by which maternal SHS exposure during pregnancy may affect asthma in children are unknown. Circulating nicotine from the blood of a non-smoking mother exposed to SHS has been shown to reach the fetus by crossing the placenta.3 In utero exposure to nicotine might alter lung development in the fetus, thereby leading to impaired lung function and an increased risk of respiratory illnesses such as asthma.28 Nicotine is also associated with epigenetic changes such as DNA methylation or altered microRNA expression, which modulate epithelial differentiation, immune cell differentiation, or both, in a long-lasting manner.29,30 Through such immune dysregulation, prenatal tobacco smoking exposure is likely to be linked to the development of asthma. Alternatively, because non-smokers are more aware of the health risk of SHS than smokers are,31 non-smokers, particularly during pregnancy, might be highly sensitive to their own smoking exposure, resulting in an overestimation of exposure.
Our study has the advantage of utilizing a prospective design, in which subjects were followed from the fetal period onward, minimizing the effect of recall bias. In addition, a number of potential confounding factors were controlled for in the analysis, although it is possible that our results remain confounded by other potentially important factors.
Several limitations of this study deserve mention. First, of the 1757 participants at baseline, only 1304 (74.2%) children were evaluated in this study. Non-participation in follow-up surveys or the exclusion of subjects due to incomplete information could have led to biased effect estimates. Compared with children who were excluded, study subjects were more likely to live in Fukuoka Prefecture, to live in families with relatively high incomes, and to have parents with relatively high educational levels, and their mothers were more likely to have never smoked. There were no material differences between included and excluded children regarding the distribution of the number of children at baseline or parental history of asthma, atopic eczema or allergic rhinitis. Moreover, selection bias might have occurred at the baseline survey. The participation rate could not be calculated because the exact number of eligible pregnant women who were provided with a set of leaflets explaining the KOMCHS, an application form, and a self-addressed and stamped return envelope by the 423 collaborating obstetric hospitals is not available. Our subjects were probably not a representative sample of the Japanese population. In fact, the educational levels of the parents of the current study population were higher than those of the general population.32 Thus, our study subjects were more educated and may therefore have had a greater awareness about health than the general population would.
Second, because our definition of asthma relies entirely on maternal reporting, misclassification of outcome could result from maternal recall errors or from variation in diagnostic practices among physicians. Additionally, we should keep in mind the fact that a diagnosis of asthma in children, especially at a young age, is difficult to make, and differentiating asthma from other viral-induced respiratory diseases is problematic.10 Data on spirometry and serum IgE levels, the preferred metric in the assessment of asthma, were not available in the present study.
Third, data on smoking exposure were self-reported and were not validated by objective measurements such as salivary, serum, or hair cotinine levels. However, a validation study in Canada demonstrated that self-reported active smoking status during pregnancy was fairly accurate, with sensitivity and specificity levels for self-reported active smoking status, using a cut-off of 5.21ng/mL plasma cotinine, of 85.4 and 99.5, respectively.33 Regarding self-reported SHS exposure status, a study of non-smoking pregnant women in Iran showed that maternal reported SHS exposure was significantly correlated with the cotinine level of maternal urine and umbilical cord serum.34 On the other hand, a validation study in Japan on the association between self-reported SHS exposure and plasma cotinine levels during pregnancy demonstrated a low validity of self-reported SHS exposure.35 We cannot therefore exclude the possibility of exposure misclassification, especially of SHS exposure. Additionally, the time period for gathering information on maternal SHS exposure during pregnancy was quite wide (i.e., between the 5th and 39th weeks of gestation). Thus, reporting and information bias due to underreporting by the participants might have occurred and might have led to misclassification of exposure, resulting in underestimation of the true associations.
Fourth, we were not able to take into consideration postnatal exposure to SHS in settings other than the home. Postnatal exposure to SHS may therefore be underestimated. Finally, the current study might not have substantial statistical power. The lack of association between maternal SHS exposure at work or at home during pregnancy and the risk of asthma might be due to insufficient statistical power.
ConclusionsOur results failed to show any significant association between maternal smoking during pregnancy or postnatal living with at least one household smoker and the risk of asthma. However, in a sensitivity analysis of children with never-smoking mothers, maternal SHS exposure at work and/or at home during pregnancy was associated with an increased risk of asthma in children. In order to determine whether maternal exposure to SHS during pregnancy has an adverse effect on asthma, further epidemiological studies are needed in order to accumulate more evidence. Additionally, cohort studies with long follow-up periods are also needed to determine whether the effects of smoking exposure on asthma diminishes as the age of the child increases. Follow-up surveys constituting the remainder of the KOMCHS will reveal the long-term effects of smoking exposure on asthma.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to acknowledge the Kyushu Branch of the Japan Allergy Foundation, the Fukuoka Association of Obstetricians & Gynecologists, the Okinawa Association of Obstetricians & Gynecologists, the Miyazaki Association of Obstetricians & Gynecologists, the Oita Association of Obstetricians & Gynecologists, the Kumamoto Association of Obstetricians & Gynecologists, the Nagasaki Association of Obstetricians & Gynecologists, the Kagoshima Association of Obstetricians & Gynecologists, the Saga Association of Obstetricians & Gynecologists, the Fukuoka Society of Obstetrics and Gynecology, the Okinawa Society of Obstetrics and Gynecology, the Fukuoka City Government, and the Fukuoka City Medical Association for their valuable support.
The Kyushu Okinawa Maternal and Child Health Study is funded by JSPS KAKENHI Grant Numbers JP19590606, JP20791654, JP21590673, JP22592355, JP22119507, JP24390158, JP25463275, JP25670305, 17H04135 and 17K12011; Health and Labour Sciences Research Grants for Research on Allergic Disease and Immunology; Health Research on Children, Youth and Families from the Ministry of Health, Labour, and Welfare, Japan; Meiji Co. Ltd.; and the Food Science Institute Foundation. These organizations did not have any influence on the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.