Allergic asthma is a chronic inflammatory disorder of the airways. Th1, Th2 and Th17 cells are the main cells involved in the pathophysiology of asthma. The function of these cells is affected by T-bet, GATA3 and RORγt transcription factors (respectively). Therefore, the aim of this study was to evaluate the effect of ginger (officinal Roscoe) extract on the expression of T-bet, GATA-3 and ROR-γ in peripheral blood mononuclear cells (PBMC) of asthmatic patients, in comparison with healthy volunteers as controls.
Materials and methodsIn this case-control study, a total of 50 individuals including 25 patients with severe, moderate and mild allergic asthma and 25 unrelated healthy controls were involved. The PBMCs were isolated and divided into four groups: negative control, two positive controls (Budesonide and PHA) and ginger-extract treated group. After cell treatment and incubation for 48h, PBMCs were isolated and cDNA was synthesized. Gene expressions of T-bet, GATA3 and ROR-γt were evaluated by Real-time PCR.
ResultsAccording to the results of this study, hydroalcoholic extract of ginger could reduce the expression of GATA-3, ROR-γt, and T-bet in PBMCs of asthmatic patients in comparison with untreated PBMCs (P values=0.001, 0.001, and 0.002, respectively). It was also shown that the ginger extract could affect T-bet/GATA-3, T-bet/ROR-γt, and ROR-γt/GATA-3 expression ratios.
ConclusionsThis study showed that the use of ginger extract could control asthma and decrease the severity of this disease by affecting the main cells involving the symptoms of asthma in the airways.
Asthma is a chronic inflammatory disorder of the airways characterized by symptoms such as coughing, wheezing, tightness in chest, increased mucus secretion in the airways and increased airway responsiveness (AHR). Asthma is the most common non-communicable diseases in children and adults. According to WHO, the number of cases of the disease worldwide, is up to 300 million, and is expected to reach 400 million by 2025.1–3 The prevalence of asthma in patients younger than 18 years old, in girls and boys is approximately the same and the disease is more common in male adults.2,4 In the World Asthma report published in 2003, the prevalence of asthma in Iran is about 5.5% of all population.5 However, according to recent studies, the prevalence of asthma is approximately 13.14%, which is higher than its average in the world.6 Asthma is classified into two allergic and non-allergic forms, with the allergic form being more common than the other one.2 People with asthma have inflammation, swelling and tenderness in the airways and they may also show a strong reaction to inhalants. With reactions, muscles around the airways constrict and swell, which causes less infiltration of air into the lungs. The disease is seen in three forms clinically: mild, moderate, and severe, and according to disease severity, divided into four: borderline, mild, moderate, and severe forms.7
Inflammation plays an important role in the pathophysiology of asthma, and cellular elements such as mast cells, eosinophils, neutrophils, Th2, Th17 cells and their secreted cytokines, also genetic and environmental factors are involved in the diseases occurrence.8,9 Th2 cells producing interleukin (IL)-4, IL-5 and IL-13, as well as airway epithelial cells producing thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 have a very important role in creating inflammatory conditions.10,11 Most cells in the airway inflammation in asthmatic patients are Th17 and Th2.12,13 Therefore the major approaches to control and treatment of the disease are focused on controlling inflammation and reducing the airway responsiveness, using, for instance, long-acting beta-agonists and corticosteroids. However, these drugs have many side effects which limit their use in the long term. Therefore, paying attention to traditional medicine and herbal remedies that are used popularly can introduce solutions as an alternative treatment to chemical drugs.
Ginger (Zingiber officinale Roscoe) is one of the herbal medicines found in different parts of the world, especially Iran. Ginger is used as a food dispenser and also traditionally used to treat complications such as diarrhea, arthritis and inflammation of the gastrointestinal tract. It is also used as an anti-inflammatory drug.14–18 Phytochemical studies have shown that the ginger plant is rich in gingerol and shagol.19 These two compounds inhibit cyclooxygenase 2, reduce inflammation and inhibit Th2 responses.20,21 In addition, ginger has the ability to inhibit the synthesis of some pro-inflammatory cytokines such as interleukin-1, interleukin-18 and tumor necrosis factor (TNF-α).22,23 Ginger's ability to reduce inflammatory cytokines such as interleukin-6 and interferon gamma (IFN-γ) has also been proved in studies.24,25 Due to these anti-inflammatory effects of ginger and also the role of Th2, Th1 and Th17 responses in the pathophysiology of asthma, studying the effect of ginger on these responses can be effective in introducing alternative therapies for asthma. Therefore, the aim of this study was to evaluate the effect of ginger extract on the expression of transcription factor; T-bet, GATA-3 and ROR-γt, which respectively represent the activity of Th1, Th2, and Th17 cells, in peripheral blood mononuclear cells (PBMC) of asthmatic patients, in comparison with healthy volunteers as controls.
Materials and methodsStudy populationThis experimental clinical trial study was performed on peripheral blood mononuclear cells (PBMCs) of 25 asthmatic patients and 25 healthy controls referred to BuAli teaching Hospital affiliated to Mazandaran University of Medical Sciences, Sari, North of Iran, from March 2016 to September 2017. Patients with relevant clinical and laboratory findings based on the diagnostic criteria of Asthma Society of America were enrolled and verified by an asthma expert. Severity of asthma was determined according to Global Initiative for Asthma (GINA) criteria26 as mild, moderate and severe. Patients with emphysema, chronic obstructive pulmonary disease (COPD), autoimmune diseases, viral and bacterial infections were excluded from the study. The control group consisted of healthy volunteers without any underlying disease, including allergic diseases, autoimmune and infection. They were in good health and used no medication for at least two weeks prior to enrolment. The patients and controls were matched according to age, sex, localization, and ethnicity and had no history of past or current smoking. Before enrolment, the goals of the study were explained to all individuals and then written inform consent was given from each participant. The protocol of study was approved by the Ethics Research Committee of Mazandaran University of Medical Sciences (IR.Mazums.Rec.1394.1549).
PBMC isolation and cell cultureBlood samples were obtained from antecubital vein in test tubes containing 50mM EDTA anticoagulant. The blood sample was diluted with the same volume of PBS. The diluted blood sample was then carefully layered on Ficoll-Paque Plus (Biosra, France). The mixture was centrifuged at 400×g for 20min at 18–20°C. The undisturbed PBMC layer was carefully transferred out. The PBMCs were washed twice with three volumes of PBS and resuspended in RPMI-1640 media (Sigma Aldrich, USA) plus 10% FBS, 100IU/ml penicillin, and 100μg/ml streptomycin. The cells number was counted with a hematocytometer and the viability of the cells was checked by trypan blue exclusion assay. Only the isolated cells with 95% or above viability were resuspended to the target density, which was 2×106cells/ml, for experiments. The PBMCs were incubated at 37°C in a humidified atmosphere of 5% CO2. After isolation and culture, the cells were divided into four groups: untreated group; PHA-stimulated PBMC as positive controls; budesonide-treated, a corticosteroid type drug, as control drug; and the last group treated with hydro-alcoholic extracts of ginger. PHA and budesonide were used at 5μg/ml and 10−8M, respectively. The cells were treated with different components mentioned for 48h.
Cell viabilityCell viability was assessed by the MTT (methyl thiazol tetrazolium bromide) assay. Briefly, cells were plated in RPMI1640 medium in a 96 well microtiter plate in triplicate at a density of 2×105cells/well. The cells were treated with varying doses of ginger (50–800μg/ml) for 24 and 48h. After treatment, the cells were incubated with MTT dye at a concentration of 50mg/100ml for four hours at 37°C. The plates were then centrifuged at 300×g for 10min. The supernatant was then removed and 100μl of DMSO was added to each well to dissolve the purple formazan crystals. The absorbance at a wavelength of 540nm was measured with an ELISA reader (BioTek, USA). Results were expressed as the percentage of MTT absorbance with respect to control cells.
RNA isolation and cDNA synthesisTotal RNA from PBMCs was isolated using the RNA isolation kit (Favorgen Biotech Corp., Taiwan) according to the manufacturer's protocol. The resulting RNA was resuspended in 300μl DEPC water and stored at −80°C until use. The RNA yield was quantified with a Nanodrop ND 1000 spectrophotometer (GE Healthcare, UK). Each 0.8μg RNA was transcribed to cDNA using RevertAidTM First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc. USA) according to the manufacturer's protocol.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)The target-specific primers were designed on the basis of the reported cDNA sequences and synthesized by Biosearch Technologies. The sequences of GATA-3, ROR-γt and T-bet primers along with elongation factor-1 (EF-1) were obtained from the GenBank. Quantitative PCR was performed using SYBR Green PCR and primers for amplification of GATA-3, ROR-γt, T-bet and EF-1primes were designed using the Beacon designer 7 software and synthesized by TIBmol (Germany). Primer sequences of target genes including: (a) GATA-3 (F: 5′-GTCCTGTGCGAACTGTCA-3′ & R: 5′-GATGCCTTCCTTCTTCATAGTCA-3′), (b) ROR-γt (F: 5′-GAAGGACAGGGAGCCAAG-3′ & R: 5′-GTGATAACCCCGTAGTGGA-3′) and (c) T-bet (F: 5′-GATGCGCCAGGAAGTTTCAT-3′ & R: 5′-GCACAATCATCTGGGTCACATT-3′). The housekeeping gene EF-1 was used as an endogenous reference gene. Primer sequences of EF-1 were (F: 5′-CTGAACCATCCAGGCCAAAT-3′ & R: 5′-GCCGTGTGGCAATCCAAT-3′). All tests were performed in triplicate. qRT-PCR was performed in a 15μl final volume containing; 7.5μl SYBR Premix EX TaqII (2X) (Takara, Japan), 0.4μl each 10pM primer, 4.7μl distilled water and 2μl cDNA for each template. qRT-PCR was performed under the following conditions: 94°C, 1minutes; followed by 94°C, 30s; 58°C, 30s; 72°C, 45s; and cycled 40 times. EF-1 allows normalization of the expression level of the target gene (GATA-3, ROR-γt, T-bet) to the amount of input cDNA. ΔCT between the EF-1 and target genes (CTTarget gene−CTReference gene) was calculated for case and control group, and ΔΔCT was calculated by subtracting the average ΔCT of each ginger treated samples from untreated samples. Fold change expression was carried out using the 2−ΔΔCT method.
Statistical analysisAll experiments were performed in triplicate unless otherwise noted. Statistical analyses were performed using the SPSS software version 17.0. Quantitative variables were expressed as means±SD and any differences between patients and control subjects were assessed using independent t-test or one-way analysis of variance (ANOVA). Qualitative variables were assessed applying χ2 or Fisher's exact test. Differences between groups were considered statistically significant when P<0.05.
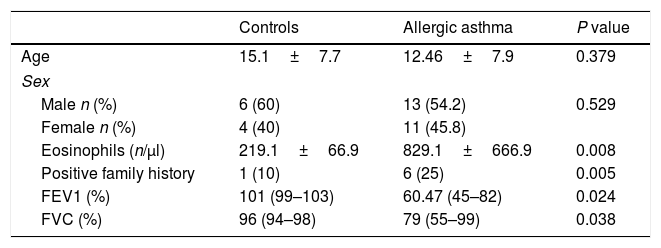
ResultsClinical findings and demographic characteristics of patientsDemographic and clinical characteristics of patients and healthy controls are presented in Table 1. In this study, 25 patients with allergic asthma and 25 healthy individuals as a control group were studied for evaluation of expression of T-bet, GATA-3, and RORγt genes that represent Th1, Th2, Th17 cells, respectively. The average age of the patients was 12.46±7.9 years, which was fully matched with control individuals (15.1±7.7 years). No significant difference was found between cases and controls with regard to sex, since 54.2% and 60% of the patients and controls were male, respectively (P=0.529). However, there was a significant difference in the two study populations with regard to the positive family history of allergic asthma (P=0.005). Additionally, peripheral blood eosinophilia was evaluated in the two populations to assess the allergic status of patients. In asthmatic patients, the number of eosinophils was significantly more than healthy subjects (P=0.008). Spirometry analysis also revealed significant differences between two study populations.
Comparison of demographic and clinical characteristics of asthmatic and control groups.
| Controls | Allergic asthma | P value | |
|---|---|---|---|
| Age | 15.1±7.7 | 12.46±7.9 | 0.379 |
| Sex | |||
| Male n (%) | 6 (60) | 13 (54.2) | 0.529 |
| Female n (%) | 4 (40) | 11 (45.8) | |
| Eosinophils (n/μl) | 219.1±66.9 | 829.1±666.9 | 0.008 |
| Positive family history | 1 (10) | 6 (25) | 0.005 |
| FEV1 (%) | 101 (99–103) | 60.47 (45–82) | 0.024 |
| FVC (%) | 96 (94–98) | 79 (55–99) | 0.038 |
In order to have a better image regarding the efficacy of ginger on the disease course, the asthmatic patients were categorized into mild, moderate, and severe asthma, based on GINA recommendation. In individuals with severe asthma, FEV1 percentage was significantly lower than those with mild to moderate asthma (P<0.0001). Also, evaluation of the FEV1/FVC ratio showed that the rate of cramping of the respiratory tract in people with severe asthma was significantly higher than those with moderate asthma (P=0.005). Although family history in patients with mild asthma was higher than in the other two groups, it was not statistically significant (P=0.431). There was no statistically significant difference in age and sex among the three groups (P=0.37), and (P=0.67) respectively.
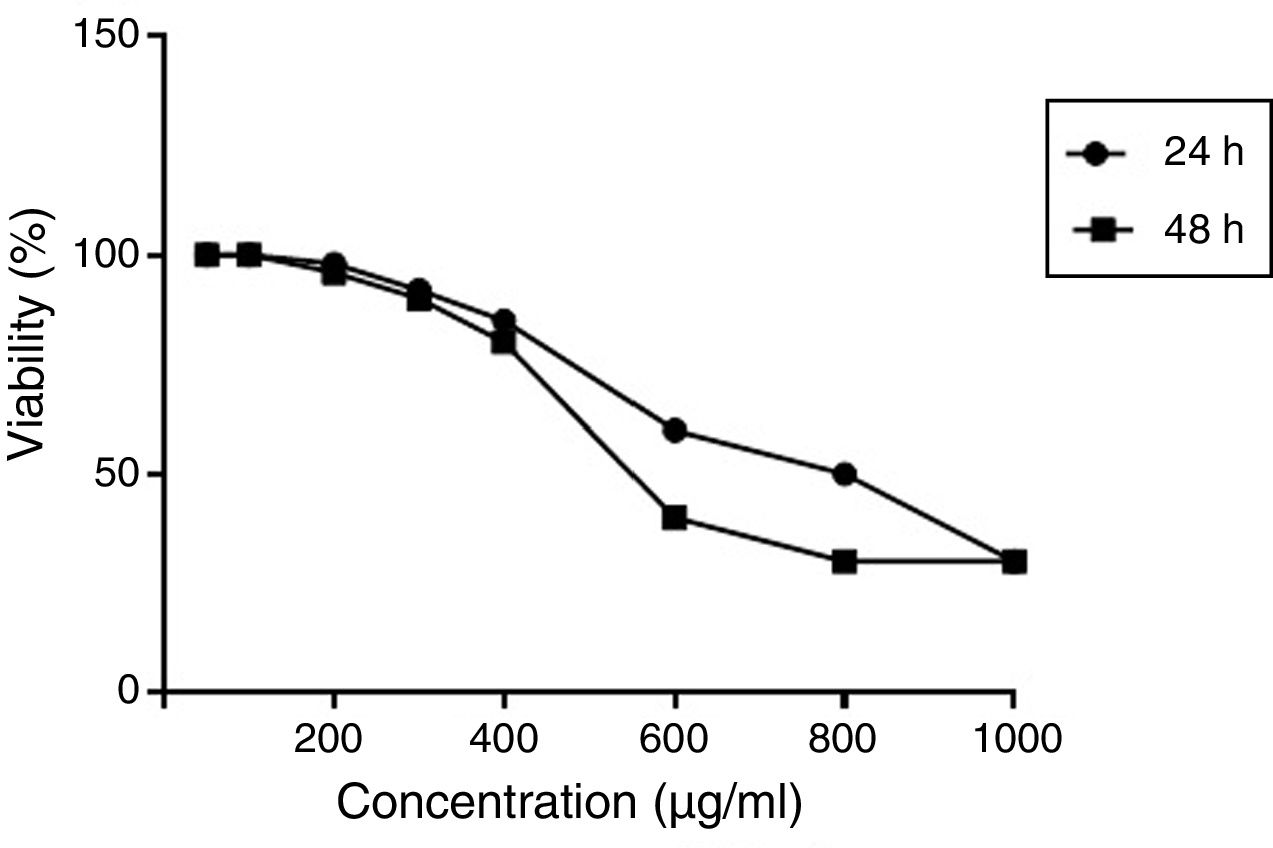
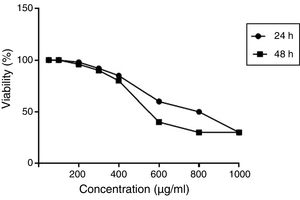
Optimum dose of ginger effectiveness on the proliferation and gene expression of cellsCell viability was assessed by the MTT (methyl thiazol tetrazolium bromide) assay. Results were expressed as the percentage of MTT absorbance with respect to control cells. An effective dose was considered as a concentration that had the least toxicity and at the same time had the best response in expressing the intended genes (unpublished gene expression data). According to the results, the optimal dose of 350 μ)g/ml) was obtained. The effect of different concentrations of hydroalcoholic extract of ginger extract on viability (%) of PBMCs at 24 and 48h is shown in Fig. 1.
The effect of different concentrations of hydroalcoholic extract of ginger extract on viability (%) of PBMCs at 24 and 48h. Results were expressed as the percentage of MTT absorbance with respect to control cells. MTT results demonstrated that the optimal dose of ginger was 350μ)g/ml).
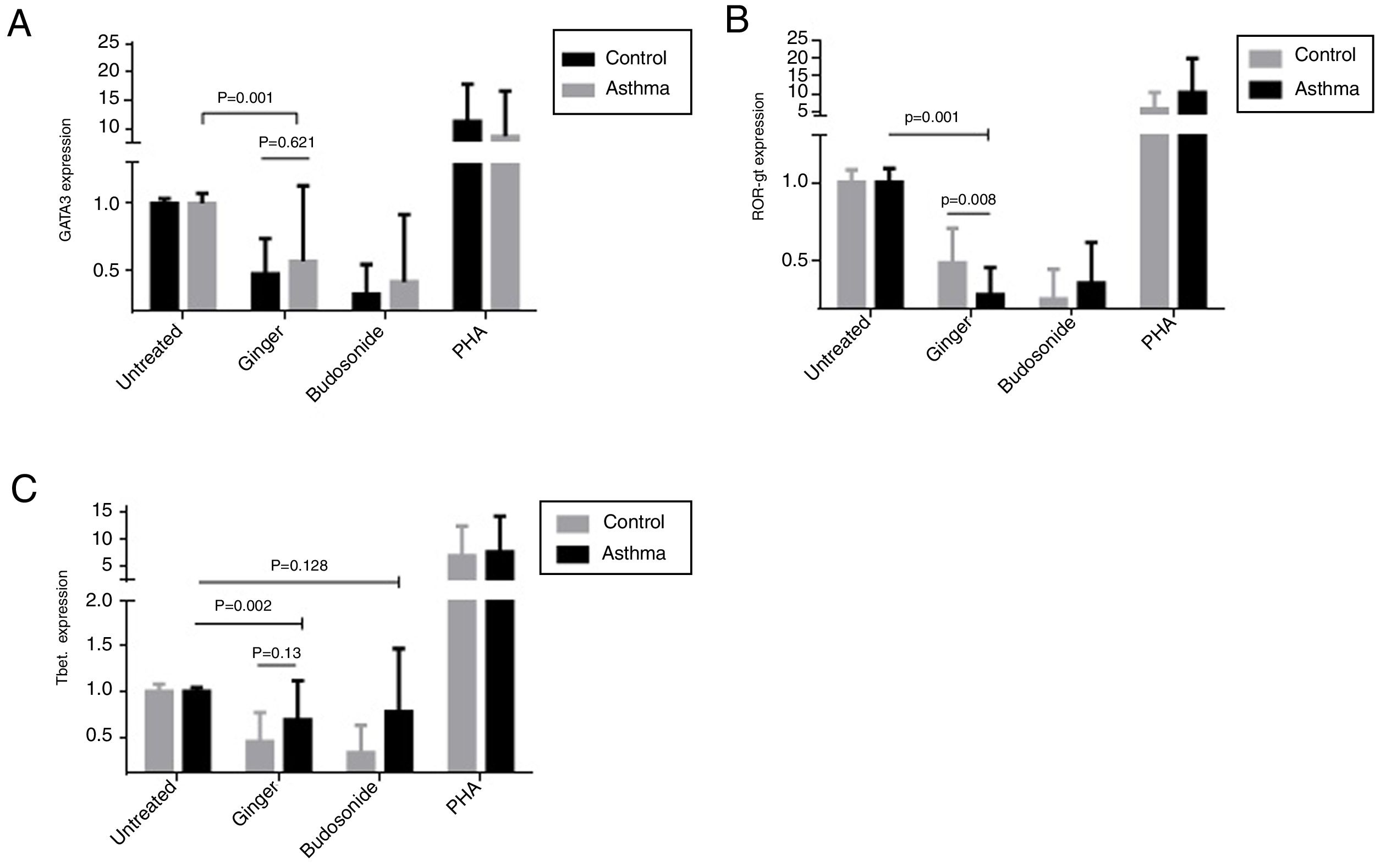
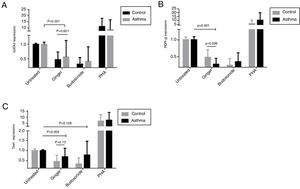
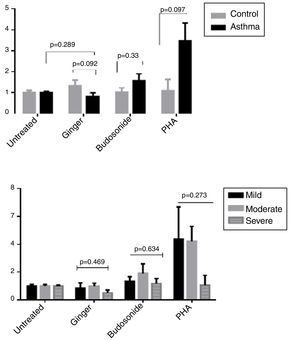
To evaluate the effect of ginger extract on immune responses, expression of GATA-3, ROR-γt, and T-bet transcription factors in PBMCs of asthmatic patients were evaluated in comparison to external and internal controls. In the PBMCs group treated with PHA, the expression level of the three genes, T-bet, ROR-γt, and GATA-3 had a significant increase, suggesting that the study groups were normal in expressing these genes and PHA induced the expression positively. As was shown in Fig. 2 part A, the expression of GATA-3 gene significantly decreased in the group of patients’ PBMCs treated with ginger extract compared with the group not treated with ginger (P=0.001). But budesonide did not have a significant effect on this gene between the two groups. There was no significant difference between the asthmatic patients’ and healthy individuals’ PBMCs treated with ginger or budesonide in the GATA-3 expression. The expression of GATA-3 gene in both healthy and patient individuals affected by ginger is respectively=0.48±0.26 and 0.57±0.56. The expression of GATA-3 gene on control and asthma group affected by budesonide is respectively, 0.33±0.22 and 0.42±0.5). Although the same as budesonide, ginger extract was significantly decreased mRNA expression of GATA-3 compared with untreated condition. This shows the fact that treatment with ginger was effective enough in a way that it could reduce the GATA-3 gene in patients’ group to reach the same level as the control group treated with ginger.
The expression of (A) GATA-3, (B) ROR-γt, and (C) T-bet genes in both healthy and patient PBMCs. Hydroalcoholic extract of ginger (350μg/ml), budesonide (10−8 M), and PHA as a positive control were added to PBMCs separated from patients (n=24) and healthy controls for 48h (n=10). There was also an untreated group as a control. The gene expression was measured by quantitative PCR and the results were expressed as the mean±1 SD calculated from triplicated experiments. EF-1 gene expression was used as a control for normalization. Data are shown as relative expression levels of the mentioned genes under the effect of ginger extract compared with budesonide or PHA or untreated group. P value <0.05 was considered significant.
Similarly, ginger extract and budesonide reduced the expression of ROR-γt gene compared with the untreated groups, and this difference was significant for the ginger treated group (Fig. 2B) (P=0.001). There was also a statistically significant reduction in the ginger treated group between patients and healthy individuals (P=0.008). The expression of ROR-γt gene in both healthy and patient individuals affected by ginger is respectively=0.49±0.22 and 0.29±0.17; the expression of ROR-γt gene on control and asthma group affected by budesonide is respectively=0.26±0.19 and 0.36±0.26. Moreover, ginger and budesonide made a reduction in T-bet gene expression in both healthy and patients groups compared with the untreated group. This was the case although the difference was only significant for the ginger treated group (Fig. 2C) (P=0.002). There was no significant difference between the asthmatic patients’ and healthy individuals’ PBMCs treated with ginger or budesonide in the T-bet expression. The expression of T-bet gene in both healthy and patient individuals affected by ginger is respectively=0.45±0.32 and 0.69±0.43. The expression of T-bet gene on control and asthma group affected by budesonide was, respectively, 0.26±0.3 and 0.78±0.69.
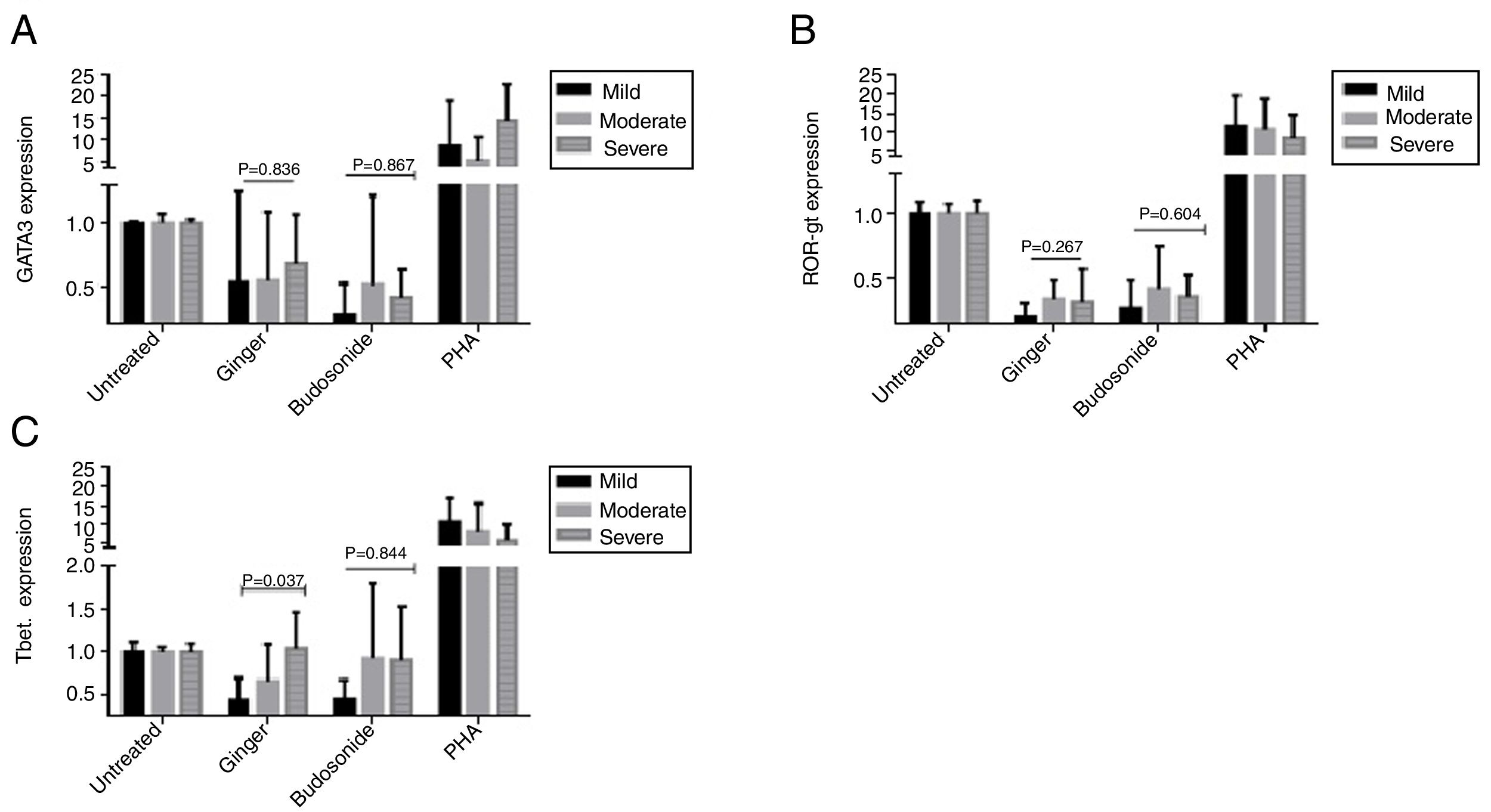
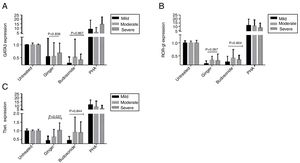
Expression of GATA-3, ROR-γt, and T-bet genes in the severe, moderate, and mild asthmatic patientsThere is no statistically significant difference in GATA-3 gene expression between the three forms of asthma under the treatment of ginger and budesonide (Fig. 3A). The expression of GATA-3 gene in the severe, moderate, and mild asthmatic patients under the treatment of ginger was respectively, 0.68±0.39, 0.54±0.55, and 0.53±0.72. Additionally, the expression of GATA-3 gene in the severe, moderate, and mild asthmatic patients under the treatment of budesonide was respectively, 0.41±0.22, 0.51±0.71, and 0.27±0.25.
The expression of (A) GATA-3, (B) ROR-γt, and (C) T-bet genes in the severe, moderate, and mild asthmatic patients’ PBMCs. Hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control were added to PBMCs separated from three groups of patients (n=24, severe (6), moderate (11), and mild (7) allergic asthma, according to GINA criteria) for 48h. There was also an untreated group as a control. The gene expression was measured by quantitative PCR and the results were expressed as the mean±1 SD calculated from triplicated experiments. EF-1 gene expression was used as a control for normalization. Data are shown as relative expression levels of the mentioned genes under the effect of ginger extract compared with budesonide or PHA or the untreated group. P value <0.05 was considered significant.
The result was the same for ROR-γt gene expression (Fig. 3B). The expression of ROR-γt gene in the severe, moderate, and mild asthmatic patients under the treatment of ginger was respectively, 0.32±0.25, 0.34±0.14, and 0.2±0.11. Furthermore, the expression of ROR-γt gene in the severe, moderate, and mild asthmatic patients under the treatment of budesonide was respectively, 0.36±0.16, 0.42±0.33, and 0.27±0.21.
In contrast, ginger could significantly decrease the expression of T-bet gene in the mild asthmatic group compared with the severe and moderate groups (Fig. 3C) (P=0.037). The expression of T-bet gene in the severe, moderate, and mild asthmatic patients under the treatment of ginger is respectively 1.04±0.42, 0.66±0.43, and 0.44±0.25.
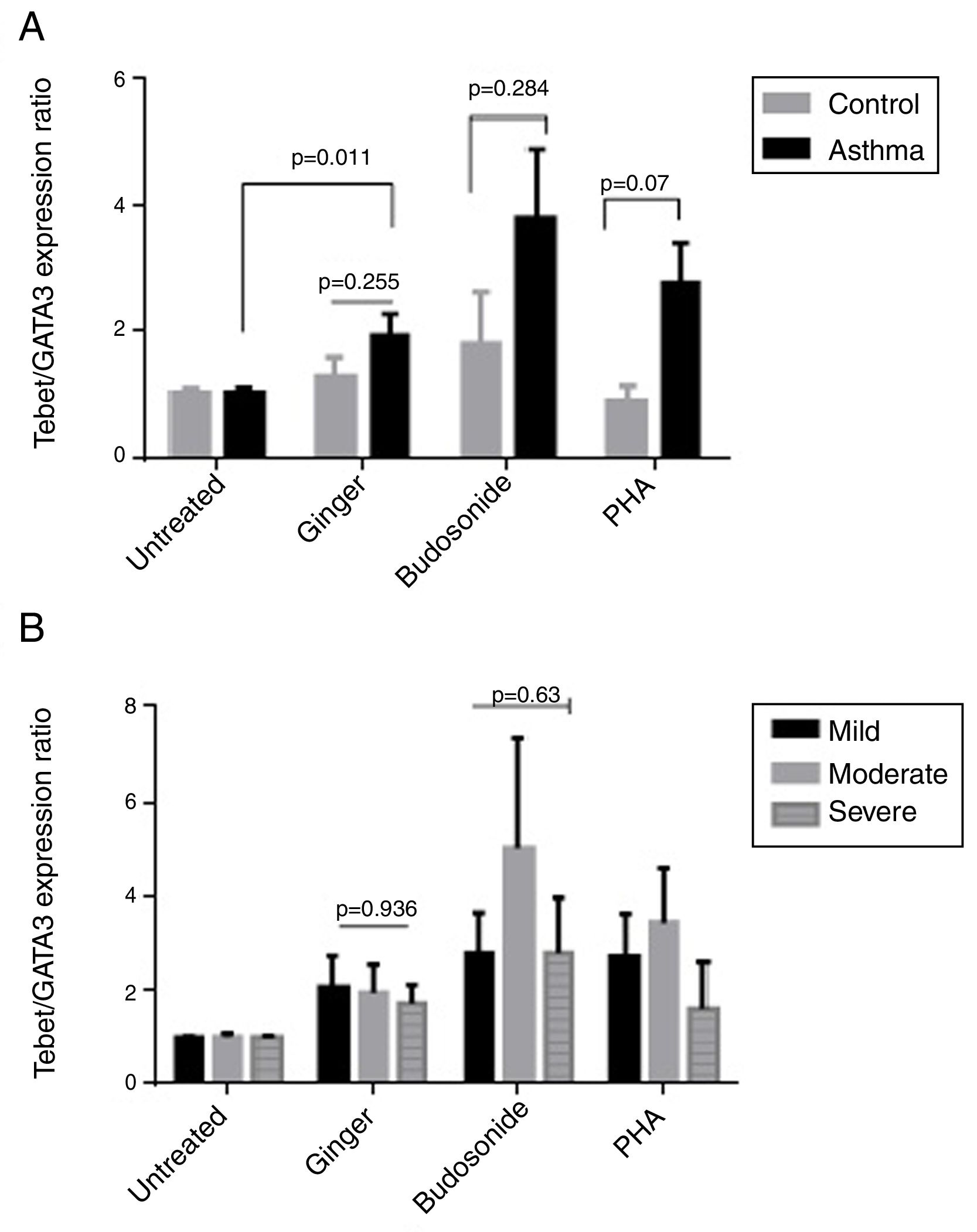
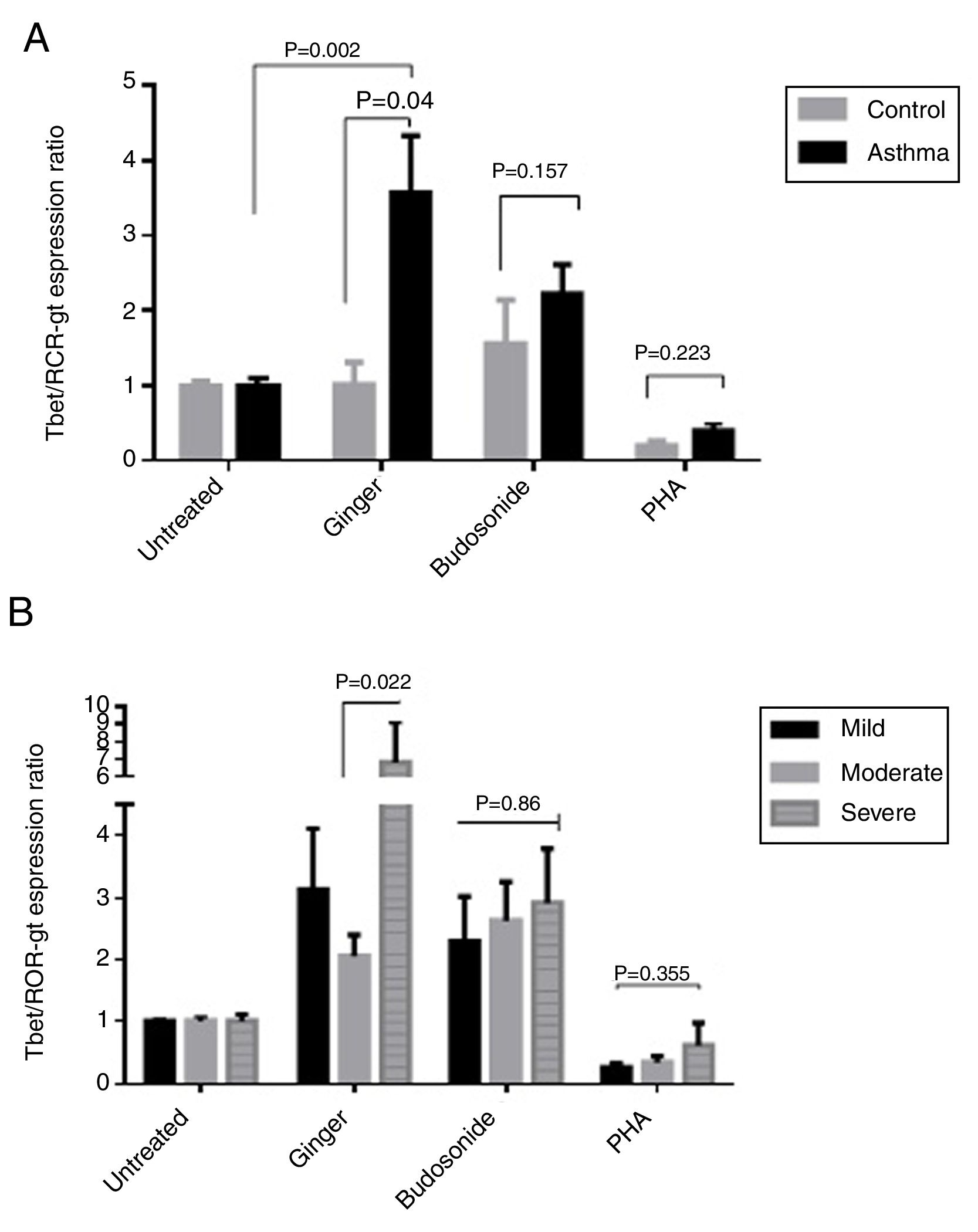
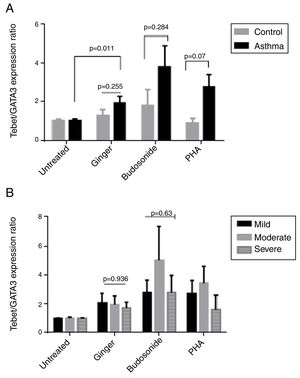
Effect of ginger extract on T-bet/GATA-3 expression ratio in healthy controls and patientsSince the polarity of T cell subtypes is effective on the prognosis of asthma, we therefore investigated the ratios of the transcription factors, T-bet, ROR-γt, and GATA-3, which are representatives of T cell subtypes. The results indicated that the expression ratio of T-bet/GATA-3 was significantly higher in asthmatic individuals affected by ginger extract than in the untreated asthmatic group (Fig. 4A) (P=0.011). But there was no significant difference between the three forms of asthma in T-bet/GATA-3 expression ratio under the effect of ginger extract (Fig. 4B).
(A) T-bet/GATA-3 expression ratio in a comparison between healthy controls and patients under the treatment of hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control. There was also a group of untreated PBMCs as control for treatment. (B) T-bet/GATA-3 expression ratio in a comparison between the three groups of asthmatic patients (severe, moderate, mild) PBMCs under the treatment of hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control. P values <0.05 were considered as significant.
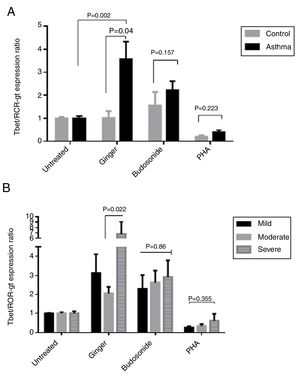
According to the results, the expression ratio of T-bet/RORγt in patients’ PBMCs affected by ginger extract was significantly increased compared with healthy individuals’ PBMCs affected by ginger (P=0.04). There is also a significant difference between the untreated group and the group treated with ginger extract in asthmatic patients (Fig. 5A) (P=0.002). The difference in the ginger-treated group was significant between the moderate form and the severe form. The ginger extract could decrease the ratio of T-bet/RORγt expression in the moderate form significantly (Fig. 5B) (P=0.022).
(A) T-bet/ROR-γt expression ratio in a comparison between healthy controls and patients under the treatment of hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control. A group of untreated PBMCs was considered as control for treatment. (B) T-bet/ROR-γt expression ratio in a comparison between the three groups of asthmatic patients (severe, moderate, mild) PBMCs under the treatment of hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control. P values <0.05 were considered as significant.
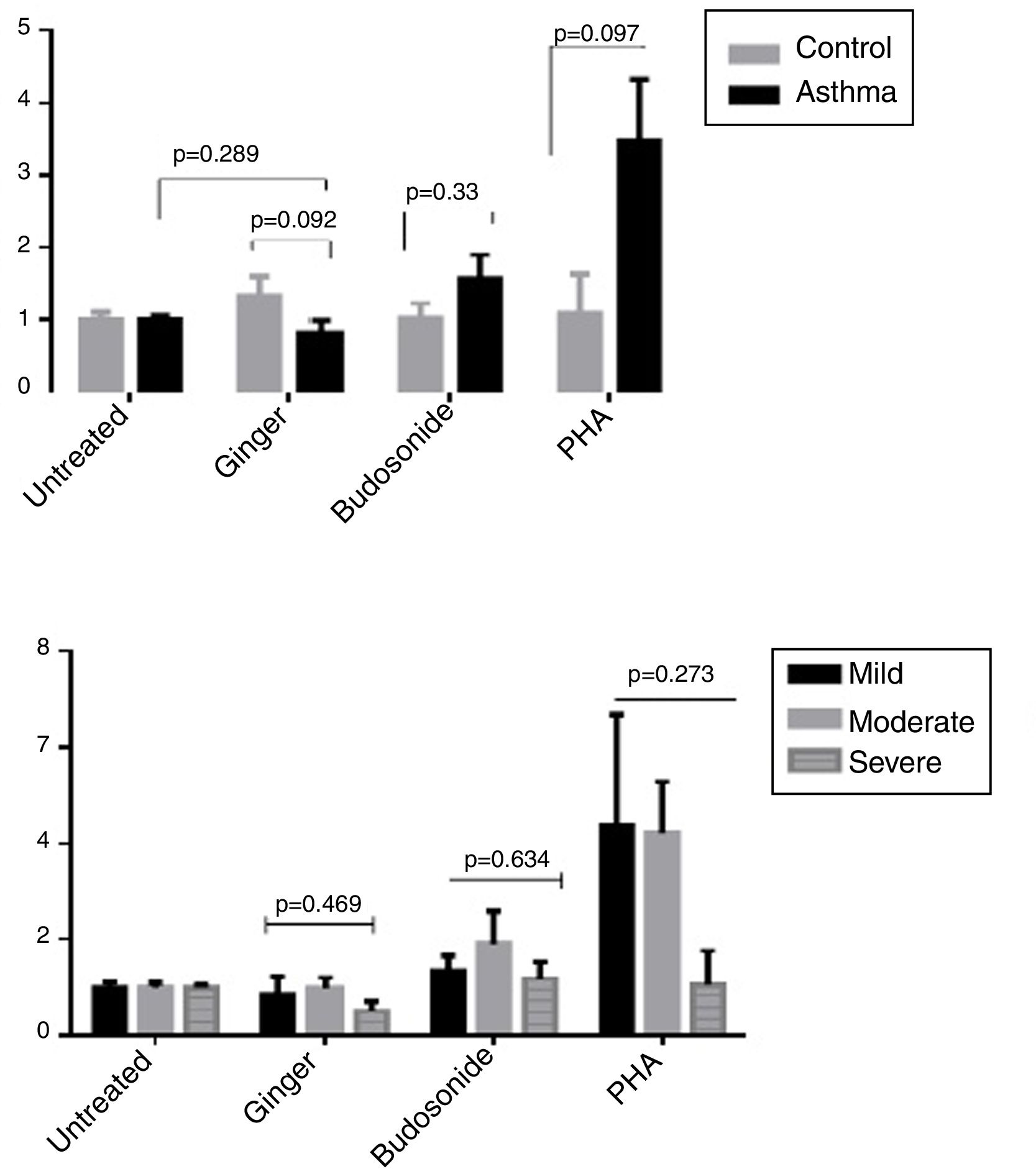
As shown in Fig. 6A, there was no significant difference between the group influenced by the ginger extract compared with the untreated group and also between the healthy controls and patients in the expression ratio of RORγt/GATA-3. The difference was not significant in the three forms of asthma under the effect of ginger extract either (Fig. 6B).
(A) ROR-γt/GATA-3 expression ratio in healthy controls and patients under the administration of hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control. A group of untreated PBMCs was studied as a control for treatment. (B) ROR-γt/GATA-3 expression ratio in a comparison between the three groups of asthmatic patients (severe, moderate, mild) PBMCs under the treatment of hydroalcoholic extract of ginger (350μg/mL), budesonide (10−8M), and PHA as a positive control. P values <0.05 were considered as significant.
As it has been proved that ginger can inhibit Th2 responses27 and synthesis of some pro-inflammatory cytokines such as IL-1, IL-18 and TNF-α,28 and reduces inflammatory cytokines such as IL-6 and IFN-γ, our aim was to investigate the effect of hydroalcoholic extract of ginger on the expression of T-bet, GATA-3, and ROR-γ as transcription factors of Th1, Th2, and Th17, respectively, in peripheral blood mononuclear cells of asthma patients compared with healthy individuals. Inflammation as one of the most important parts of the asthma pathophysiology involves inflammatory cells such as mast cells, eosinophils, airway epithelial cells, and Th2 and Th17 cells. Th2 cells by secreting IL-4, IL-5 and IL-13, and airway epithelial cells by producing TSLP, IL-25 and IL-33 have important roles in asthma's inflammatory conditions.29 Because of its chronic nature, the major approaches in controlling the symptoms and treatment of asthma are focused on reducing the inflammation and airway responsiveness, such as long-acting beta-agonists and corticosteroids. However, these drugs have many side effects that limit their use in the long term. Therefore, searching for safe food-derived anti-inflammatory compounds can introduce solutions for alternative treatments for chemical drugs. Ginger (Zingiber officinal Roscoe) as one of the herbal medications in traditional medicine of Iran is used to treat complications such as diarrhea, arthritis and inflammation of the gastrointestinal tract. It is also used as an anti-inflammatory drug.30
GATA-3 is the main transcription factor of Th2 cells and ROR-γt is the main transcription factor of Th17. Since these two cells have major roles in the pathophysiology of asthma, most of the medications target their responses in the airways. As shown in our results, treatment with ginger can reduce the expression of GATA-3 and ROR-γt in both asthmatic patients’ and healthy controls’ PBMCs compared with untreated PBMCs. This reduction was significant in patients’ PBMCs. There was also a significant reduction in ROR-γt expression between healthy controls and asthmatic patients. In addition, we observed that T-bet as Th1 transcription factor declined significantly in the PBMCs of asthmatic patients compared with untreated cells. The results showed that ginger's effects on the expression of the three genes, T-bet, ROR-γt, and GATA-3, is somewhat similar to that of budesonide, considering that ginger is a natural component and does not have the harmful side effects of chemical drugs. Kawamato et al. investigated the effect of 6-gingerole on the Th1 and Th2 cytokines in allergic mice and proposed that ginger can affect Th1 and Th2 functions and effectively reduces their cytokine production.20,21 But other studies showed that ginger can induce the production of IFN-γ and ameliorates the production of IL-4, IL-13, and IL-10.31 Ahui et al. also proposed that ginger can prevent Th2-mediated immune responses in a mouse model of airway inflammation. Putting this together we concluded that ginger can have an effect on these three T helper cells by reducing the expression of their transcription factors and ameliorating their activation and function.
To understand if there are any differences in the expression of GATA-3, ROR-γ and T-bet in severe, moderate, and mild forms of asthma under the effect of ginger, we treated the three groups with or without ginger extract. Our findings showed no significant difference between mild, moderate, and severe forms of asthma in the expression of GATA-3 and ROR-γ, but T-bet expression increased significantly in the severe form of asthma compared with the mild form. Considering that T-bet has an important role in the equilibrium of immune responses, it can be concluded that ginger can positively affect severe allergic asthma and improve the equilibrium of immune responses. One of the reasons that we could not find any differences between the mild, moderate, and severe forms of asthma in the expression of GATA-3 and ROR-γ may probably be the non-uniformity of the populations and also the small size of the severe group.
According to the importance of the balance between Th1 and Th2 in asthma, our results showed that T-bet/GATA-3 and T-bet/RORγ ratios were increased significantly in asthmatic PBMCs treated with ginger comparing with untreated group. This is a promising result suggesting that by using ginger extract, the balance between Th1 and Th2 and Th17 in allergic asthma and also in the severe form of the disease in comparison with the mild form can improve. Ginger can also augment the T-bet/ROR-γ ratio in asthmatic patients’ PMBCs compare with healthy controls.
Altogether, the results of this study validated the use of ginger extract in controlling asthma and decreasing the severity of this disease via affecting the main cells involving the symptoms in the airways.
Author's contributionsAll authors contributed to the study.
R.A. and V.R.: conceived and designed the experiments; G.J.: Patients selection and clinical evaluation; K.M., M.Z. and T.S.: Performed the experiments; K. M. and R.A.: Analyzed the data; R.A. K.M. and T.S.: Wrote the draft. All authors read and approved the final manuscript.
Conflict of interestThe authors declare that they have no conflict of interests.
We would like to thank all the patients and also the laboratory staff of Bu-ali sina Hospital in Sari for their cooperation in the collecting of samples and information.